Abstract
Neurocysticercosis, caused by infestation of the nervous system by the larval form of Taenia solium, continues to baffle the neurologist, because of varied clinical manifestations. A large body of the literature related to this disease is clinically oriented, enough attention has not been given to parasite related factors modulating the host response. Using immunohistochemical techniques, three features related to the biology of the Cysticercus cellulosa e were studied. Firstly, to the question as to which part of the worm is recognised by the host immune system, the surface glycoprotein is found to be immunolabelled by the CSF from patients of neurocysticercosis. This surface protein is depleted following specific antihelmenthic therapy, thus accounting for a fall in anticysticercal antibosy level in the CSF. Secondly, the cysticercal cyst, by immunochemical and histochemical methods, is found to have “ACTH like” molecule in the body wall and has neurotransmitter and mitochondrial metabolic pathways similar to the host, facilitating the immune evasion and successful parasitisation. Finally, Cysticercus cellulosae is found to contain a “peptide” opening the blood brain barrier at the arteriolar level when injected into mice intravenously. Similar phenomenon may be functional in the patients as well, resulting in cerebral oedema, especially following praziquintel therapy.
KEY WORDS: Cystericercus cellulosae, I mmunohistochemistry, Blood Brain Barrier, Immune evasion, Parasite metabolism
Introduction
Neurocysticercosis (NCC) caused by larval form of Taenia solium, is the most common parasitic disease of the nervous system. It is common in many of the developing countries, mainly affecting individuals of low socio-economic status, accounting for 1.2 – 25% [1, 2, 3] of all intracranial space occupying lesions seen in specialised centres in India and 2.2 – 18.6% [2] of unselected cases of seizures from the epilepsy clinics in the country. The invasion of the brain by this larval form of cestode occurs in 60% of cases, of skeletal muscle in 5% and of the eye in nearly 3% of cases [4]. The coincidence of taeniasis (infestations by the adult worm) and the cysticercosis in the same patient is rare.
The parasite appears to have a predilection to brain and spinal cord. The larval stage also infests other tissues, especially those with pulsatile or contractile property like skeletal muscle, diaphragm, heart and tissues and cavities subjected to contractile, rhythmic, pulsatile movement by the internal organs like peritoneum, pleura and subcutaneous tissue. These tissues in addition have rich lymphatic and vascular plexus with high degree of permeability. Solid organs like liver, kidney, spleen and lymphnode are rarely affected, except probably when the oncosphere gets trapped during its journey in the body. Based on the above topographic distribution and tissue predilection, it appears that this parasite prefers area rich in cholinergic innervation.
Nearly 5–10% of patients of NCC can be asymptomatic [2]. Among the symptomatic group, epilepsy forms the most common clinical presentation. In some of these patients, the CSF examination may reveal no cell response [1, 2, 4]. Patients of cerebral parenchyma cysticercosis may present with signs of intracranial hypertension/cerebral oedema resembling encephalitic picture or can be even asymptomatic [1]. The co-existence of this cestode infestation is found to enhance the degree of pathology, clinical morbidity and mortality of arboviral encephalitis like Japanese encephalitis in endemic area [5, 6, 7, 8].
The intensity of the host inflammatory reaction to the parasite is highly variable, some show a remarkable tolerance to the parasite with minimal or no inflammation, while others develop a dramatic reaction with intense cell reaction and oedema [1, 9, 10, 11].
How the Taenia oncosphere, measuring approximately 40–45 µm in diameter at the 32 cell stage, gains an entry into brain negotiating the cerebral capillaries of 6–7 µm diameter, endowed additionally with tight junctions is still an enigma. Though it is generally stated that the cysts enter the brain via circulation, so far no convincing evidence of this hexacanth embryo emerging out of the cerebral vessels, is found. The cysticercal cysts, once formed are known to remain alive in the brain for years, with no host reaction.
Several immunodiagnosis tests have been developed for the detection of anticysticercal antibodies in CSF of patients, using either crude preparation of the antigen or mixture of relatively purified antigens with variable specificity and sensitivity [12, 13, 14].
It is evident from some of these “facts”, that majority of the studies concentrated on the clinical and diagnostic aspects of neurocysticercosis. Enough attention however, had not been bestowed upon the biology of the worm, hence in the present study, following questions were raised, hitherto unaddressed, keeping in view the “known facts” to gain insight into the pathogenesis of neurocysticercosis :-
-
(a)
Which are the structural locations of the antigens in Cysticercus cellulosae, recognised by the human immune system? What are the alternations that take place in these structures, following host reaction and antihelmenthic therapy?
-
(b)
How does the host develop tolerance to the presence of the cyst?
-
(c)
Does the oncosphere and the cyst have a capacity to open the blood brain barrier or alter vascular permeability, by secreting soluble factors, thus facilitate the entry of the oncosphere into the brain/other tissues?
Material and Methods
The methodology used to answer each question are mentioned separately and briefly.
(a) To answer the first question regarding the location of immuno reactive antigens in the cysticercal cyst, immunocytochemistry was used as the technique. The CSF collected from nine confirmed cases of neurocysticercosis (3 proved at postmortem, 1 neurosurgically resected and 5 had evidence of multiple cysts in the brain scan in addition to histologically proven cysts in skeletal muscle. These served as a source of primary antibody. The control CSF samples were obtained from 2 cases of culture proven tuberculous meningitis and 2 cases of disc prolapse with no known infective or neurological illness. None of the control cases had either clinical or radiological evidence of systemic or cerebral cysticercosis. A polyclonal antibody to total homogenate of the porcine cysticercal cyst raised in rabbit, was also used as the positive control serum. The cysticercal cysts were collected from the following sources: (i) infested human brain, the cysts collected at autopsy (ii) Pork muscles infested with the cysts, both with or without host reaction. (iii) cysts from pork muscle maintained in vitro for 12 hours in serum-free medium (Eagles MEM) to remove the adsorbed host proteins, from the surface of the parasite. (iv) Cysts from the brain at autopsy, from a case treated with praziquantel/albendazole. The cysts were maintained in vitro in serum-free medium for 48 hours, to establish the viability of the cysts. Paraffin sections from the cysts were stained by immunoperoxidase technique, with CSF (both test and control) and rabbit antiserum, as the primary antibody and HRP tagged appropriate secondary antibody.
(b) To look for some of the common metabolic pathways in the cysticercal cyst and the host, facilitating the immune evasion and host tolerance porcine muscle with cysticercal cyst embedded in it, was snap frozen. 15 µm thick frozen sections were stained for NADH & SDH, the mitochondrial enzymes [15]. Simultaneously, frozen sections of porcine and human skeletal muscle were also processed. Another set of frozen and formalin fixed sections were stained for acetyl choline esterase by Karnovsky and Roots method [16]. The stained sections were examined for the similarity topographic distribution and intensity of histochemical reaction, between the parasite nervous system and host muscle motor end plates.
To verify the hypothesis that the cysticercal cyst bears an antiinflammatory, steroid producing “ACTH like” molecule on the surface, facilitating host tolerance, paraffin sections of in vitro maintained (for 72 hours) formalin fixed cyst were immunocytochemically stained using rabbit antisera against procine ACTH. The antibodies were kindly provided by Prof J Ramachandran (UCSF, USA). The immunoreaction was visualised by the colour DAB-H2O2 reaction product. Simultaneously, whole mount preparations of the evaginated and invaginated cysticercal cysts were immunolabelled with the same antibody, incubating for 24 hours, and visualising the reaction with FITC conjugated secondary antibody. Flat mounted cysts were viewed under an epi-illuminating fluorescent microscope. The topographic distribution of the immuno staining, both in the whole mount and paraffin sections were examined.
(c) To address the question regarding the alteration on blood brain barrier (BBB), as described by Reese and Karnovsky [17] 10 mg of horse radish peroxidase (Sigma) along with 15 µl of delipidated crude extract of the whole cysticercal cyst was injected intravenously into the albino mice. The HRP, because of its molecular size, is not permeable across the intact blood brain barrier. The enzyme was allowed to circulate for 0, 15, 30 and 60 mins. 16–30 µm thick frozen sections were cut and collected on poly-lysine coated slides. The enzyme was visualised by diamino-benzidine-tertrahydrochloride – H2O2 reaction. Similarly, a group of animals received a crude extract mixed with trypan blue intravenously. The brain was sliced coronally to examine grossly the staining of various anatomical structures of the brain. The trypan blue, bound to albumin, escapes to the brain parenchyma, after opening of the BBB only.
The control animals received saline of equal volume, instead of the crude extract of the cyst in addition to HRP. The experiment was repeated multiple times, each set having the control and test. As a positive control, mannitol, a therapeutic agent known to transiently open the BBB was injected along with trypan blue. The slides were read blind coded and the anatomical locations where the disruption of the BBB had occurred, were mapped.
Results
-
(a)
Immunoreactive areas of the cysticercal cysts, with human CSF as the source of antibody: The control CSF failed to react with any of the components of the cyst, derived either from human or porcine sources (Fig. 1A). On the contrary, the most intense immunostaining was observed on the external surface, the glycocalyx with CSF from the cases of neuro cysticercosis (Fig. 1B). This zone is PAS positive, suggesting the presence of a glycocomponent in the antigen. This finding was invariable in all the nine CSF samples from the cases of neurocysticercosis. The tegument, just beneath the fuzzy glycocalyx remained nonreactive in all sections. On the other hand, the sub-tegumentary cytons, the luminal aspect of the ductular system and the cyst fluid were variably labelled, suggesting distribution of the antigen in all these anatomical compartments. In the scolex, the surface of the spinal canal also revealed similar staining. At the host parasite interphase, where there was no host inflammatory reaction, the surrounding brain or the muscle failed to show any immunoreaction. On the other hand, in the presence of host inflammatory reaction of either acute or chronic type, the histiocytes and the adjoining stroma contained the immunolabel (Fig. 1C), suggesting sequestration of the parasite derived antigen. Degenerated cysticercal cysts, devoid of the glycocalyx showed only focal staining of the subtegumentary cytons and the underlying loose stroma of the bladder wall, but no surface immunostaining. Similarly, the cyst from the treated cases revealed marked reduction or lack of staining of the surface and subtegumetary cytons. The rabbit polyclonal antiserum showed identical topographic immunolabelling of the cysts on serial section, but of varying intensity confirming the specificity of CSF derived antibody.
-
(b)
Histochemical and Immunocytochemical labelling of cysts to look for metabolic parallel between the host and parasite : The bladder wall of the cyst showed intense staining for NADH (Fig. 2) and SDH, confirming the presence of mitochondrial enzyme reaction, similar to the porcine muscle and the vessel wall. The intensity of reaction was almost similar in the cyst and the adjoining porcine muscle. It was interesting to note the predominance of ‘type I muscle’ fibres close to the cyst, instead of usual checker board pattern of histochemical reaction for the mitchondrial enzymes in the skeletal muscles though this needs further investigation. The sucker muscle and the scolex part also revealed similar reaction.
Fig. 1A.

Cystical cyst showing absence of immunostaining of the surface (arrows), the subcuticular cells or the duct with control CSF. Immunoperoxidase method × 200
Fig. 1B.
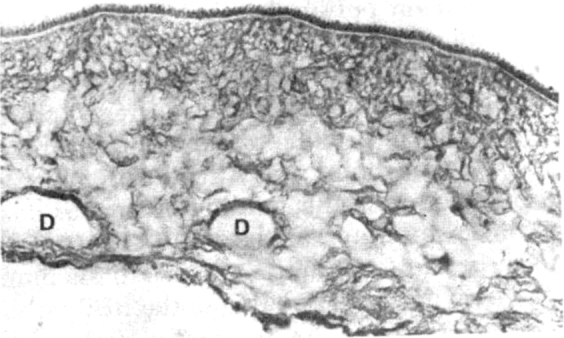
Strong immunolabelling of surface glycocalyx, and the walls of ducts (D) by the CSF from case of neurocysticercosis. Note absence of staining of the cuticle below the glycocalyx. Immunoperoxidase method × 360
Fig. 1C.
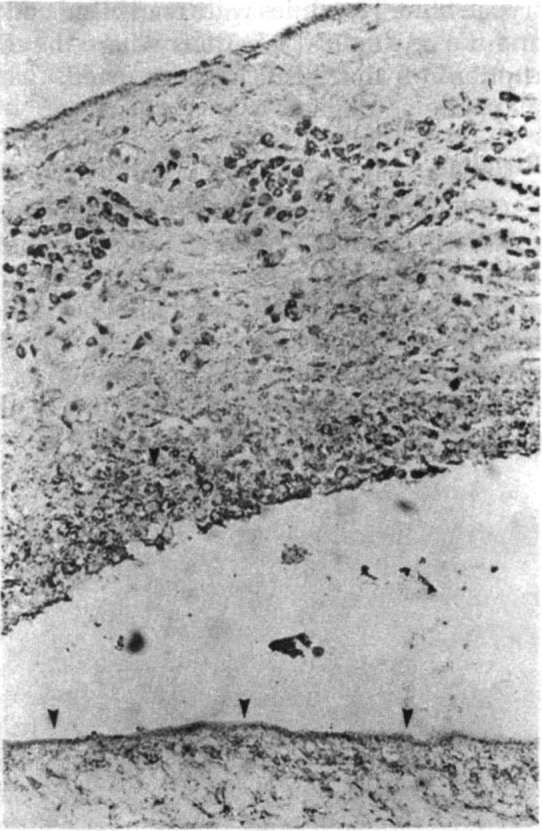
Reduced immunolabelling of the cyst wall following host reaction. Note strong staining of the histiocytes in the host tissue, suggesting sequestration of the antigen in the macrophage system. Immunoperoxidase method × 240
Fig. 2.

Strong histochemical reaction for NADH enzyme along the bladder wall and spiral canal in scolex (arrow head) of the cysticercal cyst, identical to host skeletal muscle (M) around. × 60
The cyst wall was found to be very rich in acetylcholine esterase (Fig. 3), the reaction pattern and intensity being similar to the motor end plates in the muscles (which are normally rich in acetylcholine esterase, and thus form a marker enzyme for the motor end plate). The bladder wall, the ductular system and the scolex were found to have rich neural innervation. Fine acetyl choline esterase positive (Fig. 4) argentophilic, nerve twigs were seen in the subcuticular area and traversing the cuticle to reach surface. This suggests rich sensory innervation of the surface and probable interaction with the host tissue. Similarly, the rostellar part of the scolex was found to have rich neural network, extending to the basal aspects of the hooklets and the suckers (Fig. 5). The acetylcholine esterase histochemical reaction could be abolished by physostigmine, confirming the specificity of the histochemical reaction and the neurotransmitter involved to be acetyl choline. Preliminary electron microscopic studies revealed synaptic complex in the cysticercal scolex sucker muscle, containing small, round, luscent synaptic vesicles, similar to the ones observed in the human and porcine motor end plates.
Fig. 3.
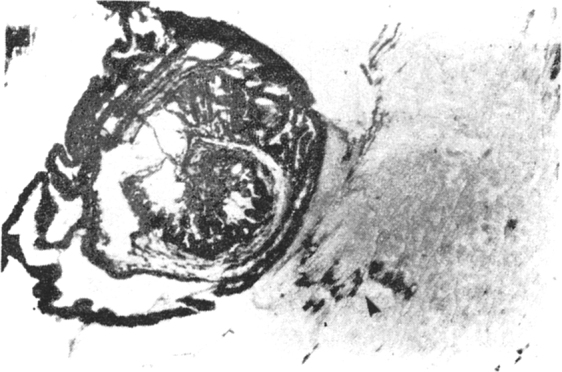
Rich acelyl choline esterase enzyme reaction along the bladder wall and scolex of the cysticercal cyst, similar to the motor end plates in the host skeletal muscle. × 60
Fig. 4.
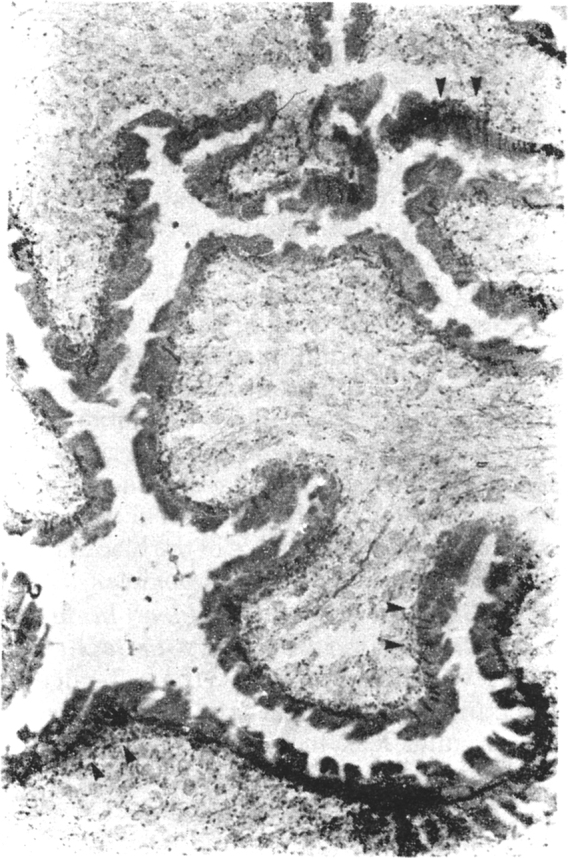
Acetyl choline esterase positive nerve twigs traversing the cuticle of the spiral canal, in Cysticercus cellulosae. Also note fine, horizontally oriented nerve fibres.
Histochemical reaction × 240
Fig. 5.
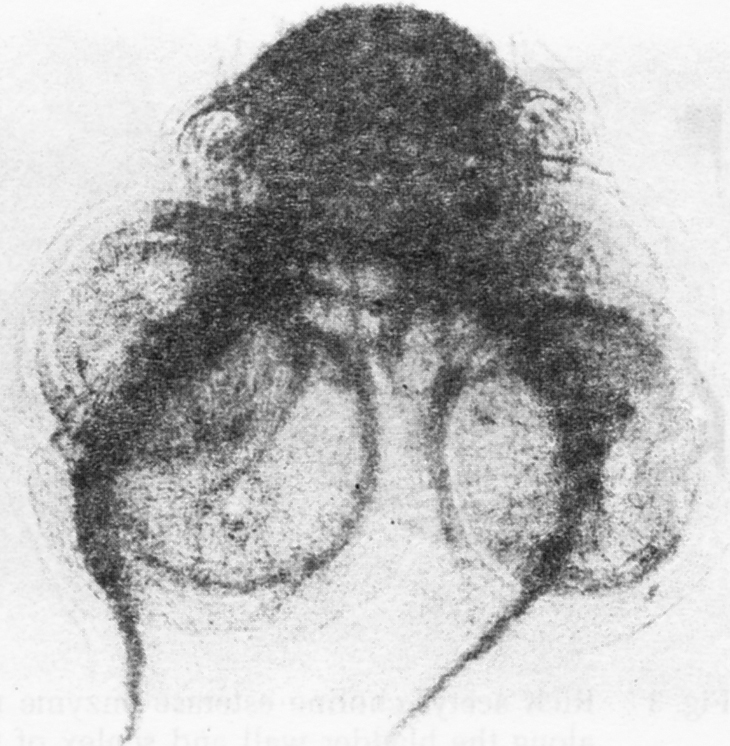
The nervous system of Cysticercus cellulosae, revealing the nerve cords, the transverse commissures, the innervation of the suckers and the rostellum, bearing the hooklets. Non specific esterase histochemical reaction, evaginated worm. × 60
The immunocytochemical staining with rabbit antiserum against porcine ACTH, revealed strong immunostaining of the surface of the bladder wall and cells around the ductular system (Fig. 6A), but not the subtegumentary cytons. In the spiral canal of the scolex, the subtegumentary zone showed staining along fine fibres. Similarly the base of the rostellum bearing the hooklets, was labelled, corresponding to the zone of neural labelling of the longitudinal lateral cords, the structures corresponding to the nerve cords, by the ACTH antiserum. This suggested colocalisation of ‘ACTH like’ substance in the neurons system of the cysticercal worm and its presence on the surface of the bladder wall and the rostellum with the hooklets.
Fig. 6A.
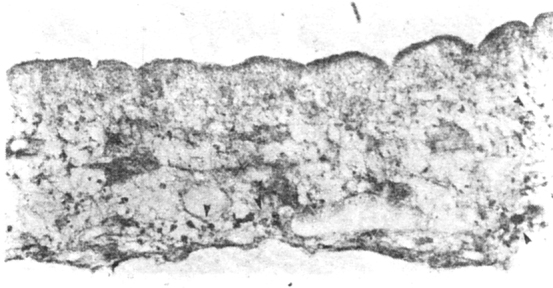
’ACTH like’ reaction product, along the surface of the bladder wall, and groups of cells close to duct. × 280
(c) Study of alteration in blood brain barrier (BBB) caused by the cyst extract : This study was carried out in 70 adult mice by injecting the cysticercal extract and observing for vascular leak in response to the foreign substance released by the parasite. The extravasation of HRP through medium sized and small arteries and arterioles was found almost immediately after the injection of the crude extract (Fig. 7A, B) and reached the maximum by 30 minutes and remained so upto 60 mins. With control saline or unrelated extract, there was no extravasation. The capillary network was intact, and no breach or leak was evident in them in the brain both, in the control and test groups. The breach of BBB does not exist. The dye was found seeping passively into the periventricular area. The arteriolar leak was evident in the striatum, thalamus the tegemental part of the mid-brain and pons. The HRP was found to spread around the vessel wall, for a distance of 80–120 µm into the neuropil (Fig. 8). When trypan blue dye was injected along with different doses of the crude extract, the striatum, thalamus, hippocampus and brain stem showed bluish staining, indicating opening of the BBB, in a dose dependent manner.
Fig. 7A.
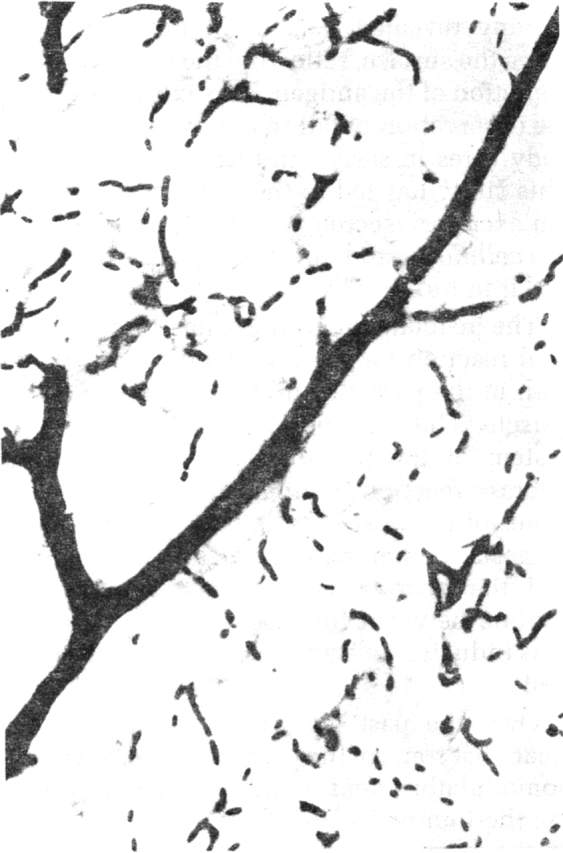
Control mouse brain showing normal arteriolar and capillary vasculature containing HRP along the lumen × 240
Fig. 7B.
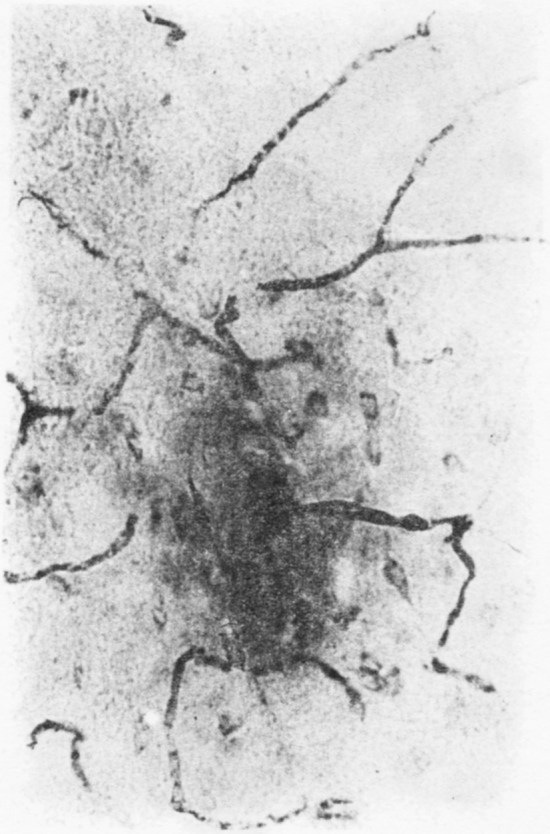
Arteriolar leak of HRP in mouse brain following IV injection of cysticercal cyst extract, indicating opening of blood brain barrier. Note labelling of adjacent neurons due to extra vascular leak of HRP into the interstitium × 240
Fig. 8.
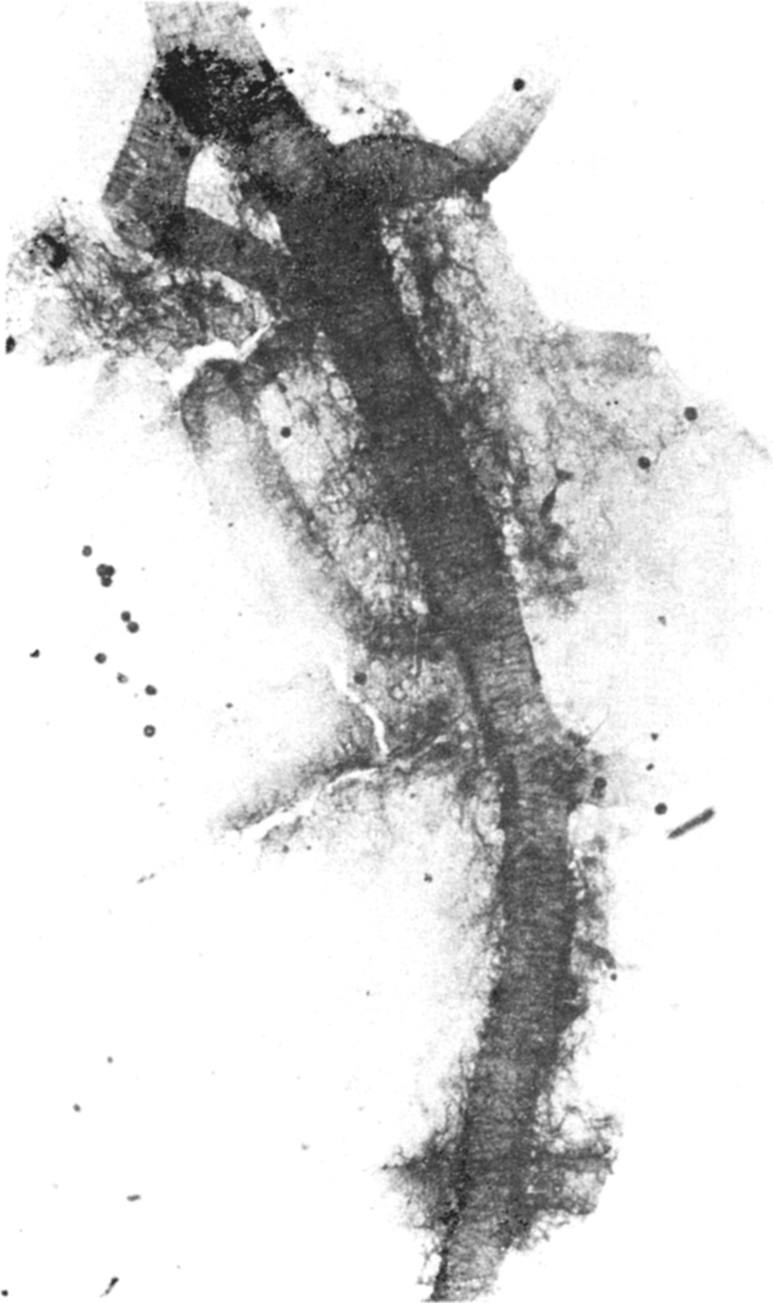
HRP leak from the small cerebral arteries and arterioles, due to opening of BBB by the cysticercal cyst extract. Note fine, horizontal neural innervation of the vasculature labelled by extravasating HRP × 200
Fig. 6B.
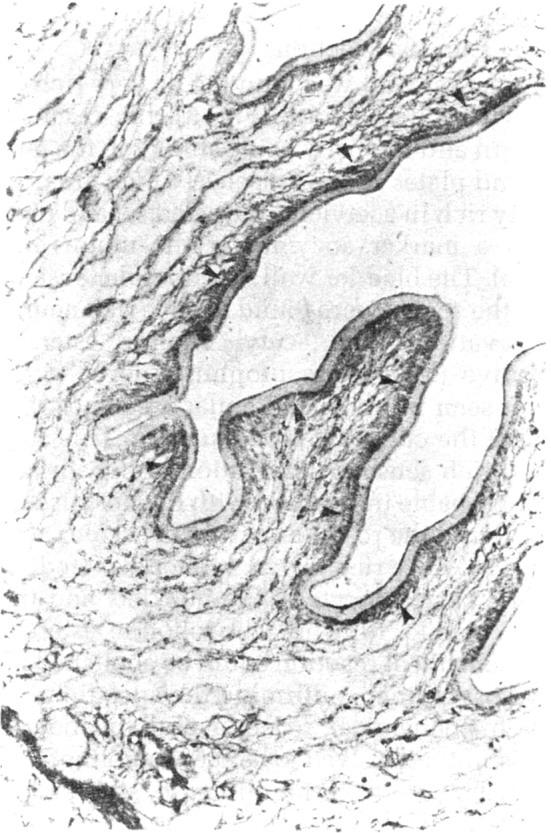
The subtegumentary zone of the spiral canal (arrows) corresponding to nerve fibres × 280
Discussion
This study has offered partial answers to some of the questions addressed.
To the question, which are structural locations of the antigen within the Cysticercus cellulosae that are recognised by the host immune system, immunocytochemistry, using CSF as the source of antibody, revealed maximal intensity of reaction and invariability of the response, along the external surface of the tegument, the glycocalyx. Following incubation in the in vitro maintained cysts with 35S methionine for variable periods of time, we observed maximum aminoacid incorporation, thus labelling of the surface glycocalyx and subtegumentary cytons in about 6 hours and the label persisted for 48 hours (unpublished data). This suggests a high metabolic activity and turnover of the glycocalyx and its likely “shedding” regularly into the CSF of patients of neurocysticercosis, thus sensitising the immune system, for a humoral response. The staining of the bladder wall stroma, ductular system and the bladder fluid indicates transcompartmental transport and accumulation of this surface glycoprotein into the cyst fluid, in a viable parasite. With the advent of host reaction, following the beginning of cyst degeneration, the antigen gets sequestrated in the granulomatous reaction and become an antigenic reservoir, as in nuerotuberculosis. With the depletion of this, the humoral response also wanes. Similarly, following praziquantel/albendezol therapy, it appears that, a large pool of antigen is released suddenly from the bladder, resulting in high antibody titres in serum and CSF. The cysticercal cysts, following antihelmenthic therapy revealed lack of immuno staining along the surface, reflecting the stripping and depletion of the antigen. This correlates with the observation of fall in anticysticercal antibody titres in serum and CSF on follow up. This study has led to the characterisation of the excretory/secretory antigens of Cysticercus cellulosae and development of a diagnostic kit in India.
The histochemical study, has shown identical reaction for the mitchondrial enzymes, both in the parasite and the porcine skeletal muscle. The bladder wall, and the nervous system of the parasite had acetyl choline esterase reaction identical to the motor end plates of the host muscle [18]. This strongly suggests, commonalities in the metabolism and the neurotransmitter nature of acetyl choline between the host and the parasite, thus inducing an acceptance of the cestode as ‘self’.
Over the past few years, it has become clear, that some of the parasites secrete immunomodulatory neuropeptides, thus influencing the immune and inflammatory response of the host, for its own benefit. Recently, Duvax-Miret et al [19] have observed that Schistosoma mansoni during its life cycle, secretes pro-opiomelanocortin (POMC) derived neuropeptides like ACTH, melanotrophin and β-endorphin. The ACTH, a 39 amino acid neuropeptide is converted to MSH (1–13 amino acid fragment) which is capable of blocking attack by phagocyte host attack. Similarly Trichinella pseudospirali is found to suppress inflamation in mice, by steroidogenesis [20]. The ACTH secreted is known to supress interferon produced by T-lymphocytes. Furthermore, the ACTH release can preferentially induce TH2 cells (subset of CD4 helper T cells) by faclitating production of cortisone and thus enhanced humoral response and suppressing the cell mediated response, detrimental to the parasite. The secretion of ACTH into the interphase between the parasite and host, is an elegant example of mimicry of the host neuromediators by the pathogens. The association of the ACTH secreting machinery within the nervous system of the parasite is noted in the present study. This phenomenon probably explains the presence of clinically asymptomatic cases, inspite of harbouring the cysticercal cysts and the variable immune and inflammatory response of the host to the presence of the parasite. Following praziquintel/albendazole therapy, probably this equilibrium on the worm is disturbed, triggering immune/inflammatory response detrimental both to the parasite and host. In schistosoma infested snails, the three neuropeptides, ACTH, β-endorphin and MSH have been found elevated in the haemolymph [21]. It will be interesting to study the levels of ACTH and cortisol levels in patients of neurocysticercosis, to further understand the pathobiology. The 4–9 amino acid fragments to ACTH are found to be neurotrophic [22]. Does this component cause aberrant sprouting of neuritis around the cyst and lead to seizure activity?
To the last question – does the oncosphere find entry into the brain by probably opening the blood brain barrier; the partial answer is that the cysts have some peptides capable of altering the BBB, at the arterial and arteriolar level. The physical egress of the oncosphere through these leaky blood vessels, into the brain parenchyma, is yet to be established. However, it is interesting to note that the crude extract has caused a leak in the BBB, at hippocampus, thalamus, stratum and brainstem. Cysts in human neurocysticercosis, are also commonly found in the cortical grey matter, the diencephalic nuclei, the brainstem and the cerebellum. Some of these clinically present as cases of benign intracranial hypertension/cycticercal encephalitis. Following the antihelminthic therapy also, some of the patients with multiple cysts manifest acute cerebral oedema, a clinical emergency. These cases respond well to steroids [2], similar to post traumatic vasogenic oedema, indicating a vascular pathophysiology in the case of neurocysticercosis. At this stage, the cell response in CSF is usually low. Could this be due to the sudden release of some peptides from the cyst fluid into the host-parasite interphase zone, altering the BBB? At this juncture, it is interesting to recall the writings of Aristotle in his treatise ‘History of Animals’: “Pigs whose meat is tender, have bladders which are like hail stones, in the region of the thigh, neck and loin – these are the regions they generally appear. If they are few in number, the meat is leaner. If they are many, the meat becomes soft and filled with serous fluid”. In clinical practice, the cysts in the muscle are usually not palpable, despite the bulk of the muscle and on biopsy the muscle may be normal or show minimal inflammation. Following treatment, initially, the muscles becomes tense and tender, similar to viral myostitis. The pathological phenomenon responsible for this could as well be marked interstitial oedema caused by the peptides in the cyst, acting on the systemic vasculature in the muscle.
Some of the phenomenon described are essentially related to the cestode parasite, but having a profound effect on the host. Some of the features appear to facilitate a successful parasitism and an aberration to this resulting in morbidity to both the partners. Can we view this as a symbiosis instead of parasitism? It is possible that the human host is deriving some, still unknown benefits, by accomodating the cyst. Can we use the neuropeptides in the vascular permeability, for therapeutic strategies? These are thoughts for the future.
REFERENCES
- 1.Srinivas HV, Shankar SK, Chandramukhi A. Neurocysticercosis. In: Sinha KK, Chandra P, editors. Progress in Clinical Neurosciences. Neurological Society of India; Ranchi (India): 1989. pp. 287–295. [Google Scholar]
- 2.Venkataraman S. Neurocysticercosis scene in India. In: Sinha KK, Chandra P, editors. Progress in clinical Neurosciences; Ranchi (India): 1989. pp. 297–314. [Google Scholar]
- 3.Tandon PN. Cerebral cysticercosis. Neurosurg Rev. 1983;6:119–127. doi: 10.1007/BF01742763. [DOI] [PubMed] [Google Scholar]
- 4.Sotelo J. Neurocysticercosis. In: Kennedy PGE, Johnson RT, editors. Infections of the Nervous System. Butterworths & Co; London: 1987. pp. 145–155. [Google Scholar]
- 5.Shankar SK, Vasudev RaoT, Mruthyunjayanna BP, Gourie-Devi M, Deshpande DH. Autopsy study of brains during an epidemic of Japanese encephalitis in Karnataka. Ind J Med Res. 1983;78:431–440. [PubMed] [Google Scholar]
- 6.Ravi V, Shankar SK. Japanese Encephalitis – The Indian Scene. In: Sinha KK, Chandra P, editors. Progress in clinical Neurosciences; Ranchi (India): 1989. pp. 69–84. [Google Scholar]
- 7.Yen-Fang Liu, Chin-Lung Teng, Liu-Kai Cerebral cysticercosis as a factor aggravating Japanese encephalitis. Chin Med J. 1957;75:101–107. [PubMed] [Google Scholar]
- 8.Pavri KM, Ghalasai GR, Dastur DK, Goverdhan MK, Lalitha VS. Dual infections of mice-visceral larva migrance and sublethal infection with Japanese encephalitis virus. Trans R Soc Trop Med Hyg. 1957:69–99. doi: 10.1016/0035-9203(75)90018-8. [DOI] [PubMed] [Google Scholar]
- 9.Nussenzweig R. Parasitic disease as a cause of immunosuppression. New Eng J Med. 1982;306:423–424. doi: 10.1056/NEJM198202183060711. [DOI] [PubMed] [Google Scholar]
- 10.Molinari JL, Meza R, Suarez B, Palaios S, Tato P, Retana A. Tenia solium: Immunity in hogs to the cysticercus. Exp Parasitol. 1983;55:340–357. doi: 10.1016/0014-4894(83)90031-0. [DOI] [PubMed] [Google Scholar]
- 11.Sotelo J, Guerrero V, Rubio F. Neurocysticercosis: A new classification based on active and inactive forms. A study of 758 cases. Arch Intern Med. 1985;145:442–445. [PubMed] [Google Scholar]
- 12.Flisser A. Neurocysticercosis in Mexico. Parasitol Today. 1988;4:131–137. doi: 10.1016/0169-4758(88)90187-1. [DOI] [PubMed] [Google Scholar]
- 13.Diwan AR, Coker-Vann M, Brown P. Enzyme linked immunosorbant assay (ELISA) for the detection of antibody to crysticerci of Tenia solium. Am J Trop Hyg. 1982;31:364–369. doi: 10.4269/ajtmh.1982.31.364. [DOI] [PubMed] [Google Scholar]
- 14.Espinoza B, Flisser A. Specific and cross reacting antigens of parasitic helminths. Arch Invest Med. 1987;17:299–312. [Google Scholar]
- 15.Bancroft JD, Cook CH. Manual of histological techniques. New York: Churchill Livingstone. 1984 [Google Scholar]
- 16.Karnovsky MJ, Roots L. A direct colouring' thiocholine method for cholinesterase. J Histochem Cytochem. 1964;12:219. doi: 10.1177/12.3.219. [DOI] [PubMed] [Google Scholar]
- 17.Reese TS, Karnovsky MJ. Fine structural localisation of blood brain barrier to exogenous peroxidase. J Cell Biol. 1967;34:207–217. doi: 10.1083/jcb.34.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vasantha S, Ravikumar BV, Roopashree SD, Sarala Das, Shankar SK. Neuroanatomy of Cysticercus cellulose (Cestoda) as revealed by acetylcholinesterase and non-specific esterase histochemistry. Parasitol Res. 1992;78:581–586. doi: 10.1007/BF00936456. [DOI] [PubMed] [Google Scholar]
- 19.Duvaux-Miret O, Stefano GB, Smith EM, Dissous C, Capron A. Immunosupppression in the definitive and intermediate host of human parasite Schistosoma mansoni by the release of neuroactive peptides. Proc Nat Acad Sci (USA) 1992;89:78–81. doi: 10.1073/pnas.89.2.778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stewart GL. Biological and immunological characterisation of Trichinella pseudospiralis. Parasitol Today. 1989;5:344–349. doi: 10.1016/0169-4758(89)90105-1. [DOI] [PubMed] [Google Scholar]
- 21.Pearce E, Appleton J. Production of Immunomodulatory neuropeptides by Schistosoma mansoni. Parasitol Today. 1992;8:353. doi: 10.1016/0169-4758(92)90161-t. [DOI] [PubMed] [Google Scholar]
- 22.Gispen WH. Therapeutic potential for melanocortin in peripheral nerve disease. Trends Pharmacol Sci. 1990;11:221–222. doi: 10.1016/0165-6147(90)90244-3. [DOI] [PubMed] [Google Scholar]


