Abstract
Itraconazole, a common anti-fungal agent, has demonstrated potential anticancer activity, including reversing chemoresistance mediated by P-glycoprotein, modulating the signal transduction pathways of Hedgehog, mechanistic target of rapamycin and Wnt/β-catenin in cancer cells, inhibiting angiogenesis and lymphangiogenesis, and possibly interfering with cancer-stromal cell interactions. Clinical trials have suggested the clinical benefits of itraconazole monotherapy for prostate cancer and basal cell carcinoma, as well as the survival advantage of combination chemotherapy for relapsed non-small cell lung, ovarian, triple negative breast, pancreatic and biliary tract cancer. As drug repurposing is cost-effective and timesaving, a review was conducted of preclinical and clinical data focusing on the anticancer activity of itraconazole, and discusses the future directions for repurposing itraconazole as an anticancer agent.
Keywords: itraconazole, repurposing, repositioning, anticancer, P-glycoprotein, Hedgehog, Wnt/β-catenin, mTOR, cancer-associated fibroblasts
1. Introduction
The development of anticancer drugs is a lengthy and expensive process (1). After a novel compound is identified or designed, preclinical and clinical data from phase I, II and III clinical trials are generated prior to approval. Drug repurposing represents the identification of the novel pharmacological effects of conventional drugs (2). As the pharmacokinetics, pharmacodynamics and safety in humans have already been established, expanding the application of a drug to additional diseases has advantages in terms of cost and time efficiency. Itraconazole is a common anti-fungal agent that was developed in the 1980s, which decreases ergosterol synthesis by inhibiting lanosterol 14α-demethylase (14DM), resulting in the destruction of the fungal membrane (3). However, the anti-fungal effect of itraconazole is unlikely to be associated with its anticancer activity. Preclinical and clinical data have proposed the use of itraconazole as a promising anticancer agent in monotherapy or in combination chemotherapy (3). This review focuses on the efficacy of itraconazole in cancer treatment and ongoing clinical trials.
2. Preclinical data
Certain chemotherapeutic drugs induce expression of the drug efflux protein P-glycoprotein (P-gp), also known as multi-drug resistance 1 or ATP-binding cassette (ABC) transporter B1 (ABCB1). In the 1990s, itraconazole was demonstrated to reverse chemoresistance in cancer cells overexpressing P-gp (Fig. 1; Table I) (4–6). In addition, the human breast cancer resistance protein is also inhibited by itraconazole (7).
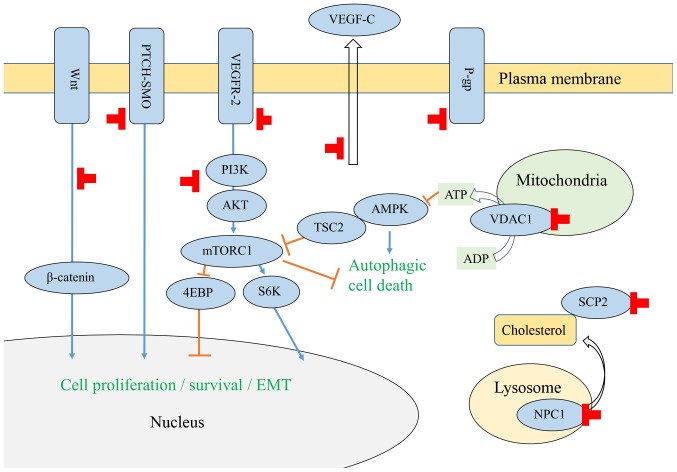
Figure 1.
Schematic representation of the anticancer activity of itraconazole. AKT, protein kinase B; AMPK, AMP-activated protein kinase; 4EBP, eukaryotic translation initiation factor 4E binding protein; EMT, epithelial-mesenchymal transition; mTORC1, mechanistic target of rapamycin complex 1; NPC1, Niemann-Pick C1 protein; P-gp, P-glycoprotein; PI3K, phosphoinositide 3-kinase; PTCH-SMO, transmembrane receptor protein patched-transmembrane protein Smoothened (SMO); S6K, ribosomal protein S6 kinase; TSC2, tuberous sclerosis complex 2; VDAC1, voltage-dependent anion-selective channel 1; VEGF, vascular endothelial growth factor; VEGFR-2, VEGF receptor 2.
Table I.
Potential anticancer activities of itraconazole.
| Modulation by itraconazole | Cell types (Refs.) |
|---|---|
| Signaling pathways | |
| mTOR | HUVEC (10,11), glioblastoma (12), rat glioma (12), EC (13), melanoma (14) |
| Hedgehog | Mouse fibroblast (9), mouse medulloblastoma (9), mesothelioma (16), breast cancer (17), BCC (38), melanoma (14) |
| Wnt/β-catenin | Melanoma (14) |
| AMPK | HUVEC (11) |
| Autophagy | Glioblastoma (12), EC (13), breast cancer (17) |
| Microenvironment | |
| Angiogenesis | HUVEC (8,10,11,25) |
| Lymphangiogenesis | Mouse lung cancer (18) |
| Cancer associated fibroblasts | Colon cancer (20) |
| Drug resistance | |
| P-glycoprotein (MDR1, ABCB1) | Pig kidney epithelial cells (5,6) |
| BCRP/ABCG2 | Breast cancer (7) |
| Transporter and pump of cholesterol | |
| SCP2 | Glioblastoma (12), rat glioma (12) |
| ABCA1 | EC (13) |
| NPC1 | HUVEC (25) |
mTOR, mechanistic target of rapamycin; AMPK, AMP-activated protein kinase; MDR1, multi-drug resistance 1; ABCB1, ATP-binding cassette transporter B1; BCRP, breast cancer resistance protein; ABCG2, ATP-binding cassette transporter G2; SCP2, sterol carrier protein 2; ABCA1, ATP-binding cassette protein A1; NPC1, Niemann-Pick C1 protein; P-gp, P-glycoprotein; HUVEC, human umbilical vein endothelial cell; EC, endometrial carcinoma; BCC, basal cell carcinoma.
A screen of US Food and Drug Administration (FDA)-approved drugs identified itraconazole as an anti-angiogenic agent in 2007 and as an inhibitor of Hedgehog signaling in 2010 (8,9). Itraconazole inhibits AKT (protein kinase B)/mechanistic target of rapamycin (mTOR) signaling in human umbilical vein endothelial cells (HUVECs), glioblastoma, endometrial carcinoma (EC) and melanoma cells (10–14). Inhibition of Hedgehog signaling was observed in basal cell carcinoma, medulloblastoma, pleural mesothelioma, breast cancer and melanoma cells (9,14–17), but not in EC cells (13). Inhibition of Wnt/β-catenin signaling was observed in basal cell and examined in melanoma cells (14). Itraconazole also induced autophagic cell death in medulloblastoma cells as well as in breast cancer cells (12,17), and suppressed lymphangiogenesis in lung carcinoma cells (18).
In HUVECs, itraconazole induced the accumulation of immature N-glycans on VEGFR2, which in turn inhibited autophosphorylation and downstream activation (19); itraconazole also exhibited synergistic effects with bevacizumab, a humanized monoclonal antibody against VEGF (20). Additionally, hypoglycosylation of the epidermal growth factor receptor was observed in renal cell carcinoma cells (19).
Itraconazole directly binds to the mitochondrial protein voltage-dependent anion channel 1 (VDAC1) and interferes with mitochondrial ATP production, leading to the activation of the AMP-activated protein kinase pathway and the subsequent inhibition of mTOR activity (11).
In 1909, White (21) observed that cholesterol accumulated in tumor cells. Since then, such changes in the lipid composition of cancer cells have been studied in association with drug resistance. In the 1970s, anti-fungal drugs were revealed to exert synergistic effects with certain chemotherapeutic drugs via altering the membrane lipid composition of cancer cells (22,23), and therapeutic strategies that target lipogenic enzymes have been investigated in preclinical and clinical studies (24). Aberrant activation of AKT is correlated with an increase in lipid raft formation, while the disruption of lipid rafts inhibits AKT activation (25). In HUVECs, itraconazole inhibited intracellular cholesterol trafficking to the plasma membrane by binding to Niemann-Pick C1 protein, resulting in cholesterol depletion (26). In glioblastoma cells, the redistribution of cholesterol was induced by the downregulation of sterol carrier protein (SCP2) (12), which is located in numerous organelles including mitochondria (27). In EC cells, the transcription of SCP2 was observed to be unaffected by itraconazole treatment. Among EC cells that were unaffected by itraconazole, the cholesterol efflux protein ABCA1 was downregulated (13).
The tumor microenvironment serves a key role in the cell proliferation, invasion and metastasis in cancer (28); however, the exact underlying mechanisms of cancer-stromal interactions are poorly understood. Cancer-associated fibroblasts (CAFs) are essential for tumor growth (29). Itraconazole inhibited the proliferation of CAFs established from human colon cancer cells, as well as the secretion of monocyte chemoattractant protein-1 (20). Monocyte/macrophage marker CD14 is a glycosylphosphatidylinositol-anchored glycoprotein present in cholesterol-rich lipid rafts, which contain a variety of signaling proteins and receptors (30,31). In mouse macrophages, itraconazole treatment altered the N-glycosylation of CD14, and increased CD14 transcription and protein expression (32).
3. Clinical data
In a randomized trial of leukemia, anti-fungal prophylactic treatment with itraconazole was proven to be effective and safe in patients receiving remission induction therapy, including daunorubicin (33). Based on preclinical data detailing the reversal of daunorubicin resistance by itraconazole (34), a sub-analysis of itraconazole anticancer activity was conducted in 27 patients with acute lymphoblastic leukemia (35), and itraconazole treatment was likely to be associated with improved disease-free survival (Table II). The results of the clinical trial (35), as well as preclinical data on itraconazole reversing the resistance of taxane-resistant cancer cells (5,6), supported the treatment of refractory solid tumors with taxane-based chemotherapy in combination with itraconazole. A prior retrospective study demonstrated that overall survival (OS) was prolonged in 19 patients with refractory ovarian cancer, who had been treated with taxane-based chemotherapy with itraconazole (36). Additional retrospective studies supported the survival advantage of itraconazole treatment in refractory malignancies including ovarian clear cell, triple-negative breast, pancreatic and biliary tract cancer, as compared with the previous reports (37–40). In pancreatic cancer, itraconazole treatment combined with chemotherapy was conducted in progressive disease during chemotherapy (39). A total of 38 patients received docetaxel (35 mg/m2), gemcitabine (1,000 mg/m2) and carboplatin (4 mg/min/ml) in combination with itraconazole (400 mg), following which a median OS of 11.4 months was observed. In addition, 28 patients with biliary tract cancer received itraconazole, and subsequently experienced a median OS of 12 months (40).
Table II.
Results of certain clinical trials.
| Cancer type | Phase | Eligibility | No. of previous regimens | No. patients treated with itraconazole | Combination chemotherapy | Results | (Refs.) |
|---|---|---|---|---|---|---|---|
| Leukemia | P2 RCT sub-analysis | ALL AML | 0 | 27 | Including daunorubicin | Tendency for longer DFS in itraconazole-treated ALL patients (P<0.06) | (35) |
| Ovarian | Retrospective comparative | Progression during previous chemotherapy | ≥1 | 19 | Including docetaxel | PFS HR=0.24 (P=0.002) OS HR=0.27 (P=0.006) in favor of the itraconazole arm | (36) |
| Ovarian | Retrospective | Recurrent clear cell carcinoma | ≥1 | 9 | Including docetaxel in 8 patients | Median OS 34.9 m 95% CI, 15.4.44.4 m | (37) |
| Breast | Retrospective | Triple negative | ≥2 | 13 | Including docetaxel | Median OS 20.4 m 95% CI, 13.1.41.4 m | (38) |
| Pancreatic | Retrospective | Relapse | ≥1 | 38 | Including docetaxel | Median OS 11.4 m 95% CI, 8.5.21.2 m | (39) |
| Biliary tract | Retrospective | Relapse | ≥1 | 28 | Including docetaxel | Median OS 12.0 m 95% CI, 9.1.24.6 m | (40) |
| NSCLC | P2 RCT | 2nd line | 1 | 15 | Pemetrexed | PFS HR=0.399 (P=0.089) OS HR=0.194 (P=0.012) in favor of itraconazole arm | (43) |
| Prostate | P2 RCT | Castration-resistant chemo.naive | 0 | 46 | None | PSA-PFS at 24 weeks, 11.8% vs. 48.0% in favor of the high dose arm | (49) |
| Basal cell carcinoma | P2 single arm | ≥1 tumor >4 mm in diameter | 0 | 29 | None | Decreased GLI1 and Ki67 among vismodegib-naive 8 patients | (51) |
No., number; m, months; P2, phase 2; RCT, randomized controlled clinical trial; DFS, disease-free survival; PFS, progression-free survival; OS, overall survival; CI, confidence interval; PSA, prostate-specific antigen; ALL, acute lymphoblastic leukemia; AML, acute myeloid leukemia; NSCLC, non-small cell lung cancer.
With the aim of enhancing the therapeutic efficacy of anticancer drugs, P-gp inhibitors were investigated in a clinical trial (41) that reported unsatisfactory outcomes. The phase III study was conducted in the ovarian cancer patients, in whom the paclitaxel dose was reduced from 175 mg/m2 in control patients to 80 mg/m2 with valspodar (5 mg/kg every 6 h for 12 doses) for patients undergoing the combination therpay (42). The addition of valspodar to standard chemotherapy regimens did not significantly improve progression-free survival (PFS) or OS, but increased the frequency of adverse events experienced. Therefore, the survival advantage conveyed by combination chemotherapy with itraconazole among patients with various types of cancer could not be explained by P-gp inhibition alone. Repurposing itraconazole for the targeting of angiogenesis has been examined since 2009. In a randomized phase II clinical trial of non-small cell lung cancer (43), 23 patients were enrolled in the second-line setting. Of these, 15 patients who were treated with pemetrexed (500 mg/m2, repeated every 21 days) and oral itraconazole (200 mg, daily) exhibited a prolonged OS time, as compared with the 8 patients who were treated with pemetrexed alone. A meta-analysis of randomized trials demonstrated that the VEGF inhibitor bevacizumab prolonged OS in colorectal, non-small cell lung and cervical cancer, but not in breast or ovarian cancer (44). Phase III trials of the VEGFR inhibitor ramucirumab reported prolonged OS in non-small cell lung, gastric and colorectal cancer, (45–48). Considering the results of the clinical trials using P-gp inhibitor or antiangiogenic agents (41,43–47), the clinical efficacy of itracoanzole treatment in various types of cancer (Table II) implicated the additional anticancer activities, which was demonstrated in preclinical studies (Table I).
In a randomized phase II clinical trial of metastatic castration-resistant prostate cancer (49), 46 chemotherapy-naïve patients were enrolled, of whom 29 received high-dose (600 mg/day) and 17 received low-dose (200 mg/day) itraconazole treatment. Prostate-specific antigen PFS rates at 24 weeks were 48.0 and 11.8% with median PFS of 11.9 and 35.9 weeks in the high- and low-dose arm, respectively. Plasma VEGF levels remained unchanged following itraconazole treatment in both arms, whereas the down-modulation of GLI1 was significantly correlated with the decline of PSA.
Basal cell carcinoma, the most common type of skin cancer, is associated with upregulated Hedgehog signaling, and two Hedgehog inhibitors, vismodegib and sonidegib, which target Smoothened have been approved by the FDA for treatment of basal cell carcinoma (50). In a recent study conducted on 29 patients with basal cell carcinoma (19 treated with itraconazole) (51), it was observed that the tumor area decreased by an average of 24% in 8 of the itraconazole-treated patients with accessible lesions. Among the vismodegib-naïve patients (n=8), the transcription of GLI1 and Ki-67 activity was significantly decreased after itraconazole treatment (51).
4. Future perspectives
Following exposure to cytotoxic agents, the residual tumors typically harbor cancer stem cells (CSCs) or develop stemness (52). The concept of CSCs was hypothesized to explain metastasis and recurrence following exposure to chemotherapy (53); CSCs are characterized by self-renewal, multi-differentiation and chemoresistance. Additional potential mechanisms underlying chemotherapy resistance may include dormant cell cycles, multidrug resistance transporters and protection by niche cells. The current focus is on the development of CSC-targeted therapy for preventing cancer relapse and improving survival rates (54). Aberrant signaling pathways, including AKT/mTOR, Hedgehog, and Wnt, have been reported in CSCs and multi-targeting therapies have been proposed (54). The first-in-class cancer stemness inhibitor napabucasin (BBI 608), which targets signal transducer and activator of transcription 3 (Stat3), Nanog, and Wnt/β-catenin pathways, has been reported to improve OS in patients with positive phospho-STAT3 recurrent colorectal cancer (55,56). Itraconazole may be a promising agent for targeting CSCs in relapsed disease of multiple types of cancer; therefore, further preclinical studies on CSCs and the surrounding stroma cells are warranted.
Ongoing clinical trials with itraconazole (as an anticancer agent) were identified from ClincalTrials.gov (https://clinicaltrials.gov/ct2/home) and UMIN-CTR Search Clinical Trials (http://www.umin.ac.jp/ctr/index.htm), as well as Google search (Table III). No ongoing clinical trials were registered at the EU Clinical Trial Register (https://www.clinicaltrialsregister.eu/ctr-search/search). Obtaining cancer tissues and blood from patients prior to and following itraconazole treatment is essential for exploring and characterizing novel targets in the tumor and the microenvironment, as well as for identifying biomarkers predictive of patient response for future enrichment clinical trials.
Table III.
Ongoing clinical trials.
| Type of cancer | Phase | Prior chemo | Primary endpoint | Treatment | Clinical trial identifier | Institution of principal investigator |
|---|---|---|---|---|---|---|
| Solid tumor | Window trial | – | Ki-67 index | Itra 400 mg BID | UMIN000018388 | Hyogo College of Medicine |
| NSCLC | P0 | Chemo-naïve | Tissue microvessel density | Itra 600 mg BID | NCT02357836 | University of Texas Southwestern Medical Center |
| Basal cell | P0 | – | GLI1 mRNA expression | Itra ointment | NCT02735356 | Stanford Cancer Institute |
| Esophageal cancer | P1 | – | Hh mRNA expression | Itra 300 mg BID | NCT02749513 | Dallas VA Medical Center |
| Glioblastoma | P1 | – | Toxicity | Multi-agent cocktail including Itra | NCT02770378 | University of Ulm School of Medicine |
| Prostate cancer | P2 | Chemo-naïve | Decline of PSA | Itra 300 mg BID | NCT01787331 | University of California |
| Gynecological cancer | P2 | ≥2nd line | PFS | DOC/Gem Itra 400 mg BID | UMIN000013951 | Hyogo College of Medicine |
| NSCLC | P2 | Chemo-naïve | Response rate | Nab-P/Carbo/Bev Itra 400 mg BID | UMIN000019049 | Meiwa Hospital |
| Gastric cancer | P2 | Chemo-naïve | Response rate operability | Nab-P/Ox/S-1 Itra 400 mg BID | UMIN000021340 | Meiwa Hospital |
| Pancreatic cancer | P2 | Chemo-naïve | Response rate operability | Nab-P/Ox/Gem Itra 400 mg BID | UMIN000029075 | Meiwa Hospital |
All trials are recruiting participants in March, 2017. NSCLC, non-small cell lung cancer; GLI1, Glioma-Associated Oncogene Homolog 1; Hh, hedgehog; PSA, prostate-specific antigen; PFS, progression-free survival; Itra, itraconazole; BID, twice a day; DOC, docetaxel; Gem, gemcitabine; nab-P, nanoparticle albumin-bound paclitaxel; Carbo, carboplatin; Bev, bevacizumab; Ox, oxaliplatin; S1, tegafur/gimeracil/oteracil; Tem, temozolomide.
References
- 1.Siddiqui M, Rajkumar SV. The high cost of cancer drugs and what we can do about it; Mayo Clin Proc; 2012; pp. 935–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pantziarka P, Bouche G, Meheus L, Sukhatme V, Sukhatme VP, Vikas P. The repurposing drugs in oncology (ReDO) project. Ecancermedicalscience. 2014;8:442. doi: 10.3332/ecancer.2014.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pantziarka P, Sukhatme V, Bouche G, Meheus L, Sukhatme VP. Repurposing drugs in oncology (ReDO)-itraconazole as an anti-cancer agent. Ecancermedicalscience. 2015;9:521. doi: 10.3332/ecancer.2015.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kurosawa M, Okabe M, Hara N, Kawamura K, Suzuki S, Sakurada K, Asaka M. Reversal effect of itraconazole on adriamycin and etoposide resistance in human leukemia cells. Ann Hematol. 1996;72:17–21. doi: 10.1007/BF00663011. [DOI] [PubMed] [Google Scholar]
- 5.Takara K, Tanigawara Y, Komada F, Nishiguchi K, Sakaeda T, Okumura K. Cellular pharmacokinetic aspects of reversal effect of itraconazole on P-glycoprotein-mediated resistance of anticancer drugs. Biol Pharm Bull. 1999;22:1355–1359. doi: 10.1248/bpb.22.1355. [DOI] [PubMed] [Google Scholar]
- 6.Shirakawa K, Takara K, Tanigawara Y, Aoyama N, Kasuga M, Komada F, Sakaeda T, Okumura K. Interaction of docetaxel (‘Taxotere’) with human P-glycoprotein. Jpn J Cancer Res. 1999;90:1380–1386. doi: 10.1111/j.1349-7006.1999.tb00723.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gupta A, Unadkat JD, Mao Q. Interactions of azole antifungal agents with the human breast cancer resistance protein (BCRP) J Pharm Sci. 2007;96:3226–3235. doi: 10.1002/jps.20963. [DOI] [PubMed] [Google Scholar]
- 8.Chong CR, Xu J, Lu J, Bhat S, Sullivan DJ, Jr, Liu JO. Inhibition of angiogenesis by the antifungal drug itraconazole. ACS Chem Biol. 2007;2:263–270. doi: 10.1021/cb600362d. [DOI] [PubMed] [Google Scholar]
- 9.Kim J, Tang JY, Gong R, Kim J, Lee JJ, Clemons KV, Chong CR, Chang KS, Fereshteh M, Gardner D, et al. Itraconazole, a commonly used antifungal that inhibits Hedgehog pathway activity and cancer growth. Cancer Cell. 2010;17:388–399. doi: 10.1016/j.ccr.2010.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu J, Dang Y, Ren YR, Liu JO. Cholesterol trafficking is required for mTOR activation in endothelial cells; Proc Natl Acad Sci USA; 2010; pp. 4764–4769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Head SA, Shi W, Zhao L, Gorshkov K, Pasunooti K, Chen Y, Deng Z, Li RJ, Shim JS, Tan W, et al. Antifungal drug itraconazole targets VDAC1 to modulate the AMPK/mTOR signaling axis in endothelial cells; Proc Natl Acad Sci USA; 2015; pp. E7276–E7285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu R, Li J, Zhang T, Zou L, Chen Y, Wang K, Lei Y, Yuan K, Li Y, Lan J, et al. Itraconazole suppresses the growth of glioblastoma through induction of autophagy: Involvement of abnormal cholesterol trafficking. Autophagy. 2014;10:1241–1255. doi: 10.4161/auto.28912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tsubamoto H, Inoue K, Sakata K, Ueda T, Takeyama R, Shibahara H, Sonoda T. Itraconazole inhibits Akt/mTOR signalling and proliferation in endometrial cancer cells. Anticancer Res. 2017;37:515–519. doi: 10.21873/anticanres.11343. [DOI] [PubMed] [Google Scholar]
- 14.Liang G, Liu M, Wang Q, Shen Y, Mei H, Li D, Liu W. Itraconazole exerts its anti-melanoma effect by suppressing Hedgehog, Wnt, and PI3K/mTOR signaling pathways. Oncotarget. 2017;8:28510–28525. doi: 10.18632/oncotarget.15324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim J, Aftab BT, Tang JY, Kim D, Lee AH, Rezaee M, Kim J, Chen B, King EM, Borodovsky A, et al. Itraconazole and arsenic trioxide inhibit Hedgehog pathway activation and tumor growth associated with acquired resistance to smoothened antagonists. Cancer Cell. 2013;23:23–34. doi: 10.1016/j.ccr.2012.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.You M, Varona-Santos J, Singh S, Robbins DJ, Savaraj N, Nguyen DM. Targeting of the Hedgehog signal transduction pathway suppresses survival of malignant pleural mesothelioma cells in vitro. J Thorac Cardiovasc Surg. 2014;147:508–516. doi: 10.1016/j.jtcvs.2013.08.035. [DOI] [PubMed] [Google Scholar]
- 17.Wang X, Wei S, Zhao Y, Shi C, Liu P, Zhang C, Lei Y, Zhang B, Bai B, Huang Y, Zhang H. Anti-proliferation of breast cancer cells with itraconazole: Hedgehog pathway inhibition induces apoptosis and autophagic cell death. Cancer Lett. 2017;385:128–136. doi: 10.1016/j.canlet.2016.10.034. [DOI] [PubMed] [Google Scholar]
- 18.Wang Y, Yao Y, Liu H, Ma X, Lv T, Yuan D, Xiao X, Yin J, Song Y. Itraconazole can inhibit malignant pleural effusion by suppressing lymphangiogenesis in mice. Trans Lung Cancer Res. 2015;4:27–35. doi: 10.3978/j.issn.2218-6751.2014.11.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nacev BA, Grassi P, Dell A, Haslam SM, Liu JO. The antifungal drug itraconazole inhibits vascular endothelial growth factor receptor 2 (VEGFR2) glycosylation, trafficking, and signaling in endothelial cells. J Biol Chem. 2011;286:44045–44056. doi: 10.1074/jbc.M111.278754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hara M, Nagasaki T, Shiga K, Takeyama H. Suppression of cancer-associated fibroblasts and endothelial cells by itraconazole in bevacizumab-resistant gastrointestinal cancer. Anticancer Res. 2016;36:169–177. [PubMed] [Google Scholar]
- 21.White CP. On the occurrence of crystals in tumours. J Pathol Bacteriol. 1909;13:3–10. doi: 10.1002/path.1700130103. [DOI] [Google Scholar]
- 22.Kuwano M, Kamiya T, Endo H, Komiyama S. Potentiation of 5-fluorouracil, chromomycin A3, and bleomycin by amphotericin B or polymyxin B in transformed fibroblastic cells. Antimicrob Agents Chemother. 1973;3:580–584. doi: 10.1128/AAC.3.5.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ikezaki K, Akiyama S, Miyazaki C, Kimura G, Kuwano M. Imidazole-resistant phenotype and virus transformation in cultured rat cells. Cancer Res. 1984;44:1791–1795. [PubMed] [Google Scholar]
- 24.Beloribi-Djefaflia S, Vasseur S, Guillaumond F. Lipid metabolic reprogramming in cancer cells. Oncogenesis. 2016;5:e189. doi: 10.1038/oncsis.2015.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Calay D, Vind-Kezunovic D, Frankart A, Lambert S, Poumay Y, Gniadecki R. Inhibition of Akt signaling by exclusion from lipid rafts in normal and transformed epidermal keratinocytes. J Invest Dermatol. 2010;130:1136–1145. doi: 10.1038/jid.2009.415. [DOI] [PubMed] [Google Scholar]
- 26.Head SA, Shi WQ, Yang EJ, Nacev BA, Hong SY, Pasunooti KK, Li RJ, Shim JS, Liu JO. Simultaneous targeting of NPC1 and VDAC1 by itraconazole leads to synergistic inhibition of mTOR signaling and angiogenesis. ACS Chem Biol. 2017;12:174–182. doi: 10.1021/acschembio.6b00849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gallegos AM, Atshaves BP, Storey SM, Starodub O, Petrescu AD, Huang H, McIntosh AL, Martin GG, Chao H, Kier AB, Schroeder F. Gene structure, intracellular localization, and functional roles of sterol carrier protein-2. Prog Lipid Res. 2001;40:498–563. doi: 10.1016/S0163-7827(01)00015-7. [DOI] [PubMed] [Google Scholar]
- 28.Plaks V, Kong N, Werb Z. The cancer stem cell niche: How essential is the niche in regulating stemness of tumor cells? Cell Stem Cell. 2915;16:225–238. doi: 10.1016/j.stem.2015.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kalluri R, Zeisberg M. Fibroblasts in cancer. Nat Rev Cancer. 2006;6:392–401. doi: 10.1038/nrc1877. [DOI] [PubMed] [Google Scholar]
- 30.Schmitz G, Orsó E. CD14 signalling in lipid rafts: New ligands and co-receptors. Curr Opin Lipidol. 2002;13:513–521. doi: 10.1097/00041433-200210000-00007. [DOI] [PubMed] [Google Scholar]
- 31.Martinez-Outschoorn UE, Sotgia F, Lisanti MP. Caveolae and signalling in cancer. Nat Rev Cancer. 2015;15:225–237. doi: 10.1038/nrc3915. [DOI] [PubMed] [Google Scholar]
- 32.Frey T, De Maio A. The antifungal agent itraconazole induces the accumulation of high mannose glycoproteins in macrophages. J Biol Chem. 2009;284:16882–16890. doi: 10.1074/jbc.M109.007609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vreugdenhil G, Van Dijke BJ, Donnelly JP, Novakova IR, Raemaekers JM, Hoogkamp-Korstanje MA, Koster M, de Pauw BE. Efficacy of itraconazole in the prevention of fungal infections among neutropenic patients with hematologic malignancies and intensive chemotherapy. A double blind, placebo controlled study. Leuk Lymphoma. 1993;11:353–358. doi: 10.3109/10428199309067926. [DOI] [PubMed] [Google Scholar]
- 34.Gupta S, Kim J, Gollapudi S. Reversal of daunorubicin resistance in P388/ADR cells by itraconazole. J Clin Invest. 1991;87:1467–1469. doi: 10.1172/JCI115154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vreugdenhil G, Raemaekers JM, van Dijke BJ, de Pauw BE. Itraconazole and multidrug resistance: Possible effects on remission rate and disease-free survival in acute leukemia. Ann Hematol. 1993;67:107–109. doi: 10.1007/BF01701730. [DOI] [PubMed] [Google Scholar]
- 36.Tsubamoto H, Sonoda T, Yamasaki M, Inoue K. Impact of combination chemotherapy with itraconazole on survival of patients with refractory ovarian cancer. Anticancer Res. 2014;34:2481–2487. [PubMed] [Google Scholar]
- 37.Tsubamoto H, Sonoda T, Yamasaki M, Inoue K. Impact of combination chemotherapy with itraconazole on survival for patients with recurrent or persistent ovarian clear cell carcinoma. Anticancer Res. 2014;34:2007–2014. [PubMed] [Google Scholar]
- 38.Tsubamoto H, Sonoda T, Inoue K. Impact of itraconazole on the survival of heavily pre-treated patients with triple-negative breast cancer. Anticancer Res. 2014;34:3839–3844. [PubMed] [Google Scholar]
- 39.Tsubamoto H, Sonoda T, Ikuta S, Tani S, Inoue K, Yamanaka N. Combination chemotherapy with itraconazole for treating metastatic pancreatic cancer in the second-line or additional setting. Anticancer Res. 2015;35:4191–4196. [PubMed] [Google Scholar]
- 40.Tsubamoto H, Sonoda T, Ikuta S, Tani S, Inoue K, Yamanaka N. Impact of itraconazole after first-line chemotherapy on survival of patients with metastatic biliary tract cancer. Anticancer Res. 2015;35:4923–4927. [PubMed] [Google Scholar]
- 41.Chung FS, Santiago JS, Jesus MF, Trinidad CV, See MF. Disrupting P-glycoprotein function in clinical settings: What can we learn from the fundamental aspects of this transporter? Am J Cancer Res. 2016;6:1583–1598. [PMC free article] [PubMed] [Google Scholar]
- 42.Lhommé C, Joly F, Walker JL, Lissoni AA, Nicoletto MO, Manikhas GM, Baekelandt MM, Gordon AN, Fracasso PM, Mietlowski WL, et al. Phase III study of valspodar (PSC 833) combined with paclitaxel and carboplatin compared with paclitaxel and carboplatin alone in patients with stage IV or suboptimally debulked stage III epithelial ovarian cancer or primary peritoneal cancer. J Clin Oncol. 2008;26:2674–2682. doi: 10.1200/JCO.2007.14.9807. [DOI] [PubMed] [Google Scholar]
- 43.Rudin CM, Brahmer JR, Juergens RA, Hann CL, Ettinger DS, Sebree R, Smith R, Aftab BT, Huang P, Liu JO. Phase 2 study of pemetrexed and itraconazole as second-line therapy for metastatic nonsquamous non-small-cell lung cancer. J Thorac Oncol. 2013;8:619–623. doi: 10.1097/JTO.0b013e31828c3950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Roviello G, Bachelot T, Hudis CA, Curigliano G, Reynolds AR, Petrioli R, Generali D. The role of bevacizumab in solid tumours: A literature based meta-analysis of randomised trials. Eur J Cancer. 2017;75:245–258. doi: 10.1016/j.ejca.2017.01.026. [DOI] [PubMed] [Google Scholar]
- 45.Garon EB, Ciuleanu TE, Arrieta O, Prabhash K, Syrigos KN, Goksel T, Park K, Gorbunova V, Kowalyszyn RD, Pikiel J, et al. Ramucirumab plus docetaxel versus placebo plus docetaxel for second-line treatment of stage IV non-small-cell lung cancer after disease progression on platinum-based therapy (REVEL): A multicentre, double-blind, randomised phase 3 trial. Lancet. 2014;384:665–673. doi: 10.1016/S0140-6736(14)60845-X. [DOI] [PubMed] [Google Scholar]
- 46.Fuchs CS, Tomasek J, Yong CJ, Dumitru F, Passalacqua R, Goswami C, Safran H, dos Santos LV, Aprile G, Ferry DR, et al. Ramucirumab monotherapy for previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (REGARD): An international, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet. 2014;383:31–39. doi: 10.1016/S0140-6736(13)61719-5. [DOI] [PubMed] [Google Scholar]
- 47.Wilke H, Muro K, Van Cutsem E, Oh SC, Bodoky G, Shimada Y, Hironaka S, Sugimoto N, Lipatov O, Kim TY, et al. Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): A double-blind, randomised phase 3 trial. Lancet Oncol. 2014;15:1224–1235. doi: 10.1016/S1470-2045(14)70420-6. [DOI] [PubMed] [Google Scholar]
- 48.Tabernero J, Yoshino T, Cohn AL, Obermannova R, Bodoky G, Garcia-Carbonero R, Ciuleanu TE, Portnoy DC, Van Cutsem E, Grothey A, et al. Ramucirumab versus placebo in combination with second-line FOLFIRI in patients with metastatic colorectal carcinoma that progressed during or after first-line therapy with bevacizumab, oxaliplatin, and a fluoropyrimidine (RAISE): A randomised, double-blind, multicentre, phase 3 study. Lancet Oncol. 2015;16:499–508. doi: 10.1016/S1470-2045(15)70127-0. [DOI] [PubMed] [Google Scholar]
- 49.Antonarakis ES, Heath EI, Smith DC, Rathkopf D, Blackford AL, Danila DC, King S, Frost A, Ajiboye AS, Zhao M, et al. Repurposing itraconazole as a treatment for advanced prostate cancer: A noncomparative randomized phase II trial in men with metastatic castration-resistant prostate cancer. Oncologist. 2013;18:163–173. doi: 10.1634/theoncologist.2012-314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.https://www.nccn.org/professionals/physician_gls/pdf/nmsc.pdf. [Apr 20;2017 ];NCCN: Clinical practice guidelines in oncology, basal cell skin cancer, version 1. 2017 doi: 10.6004/jnccn.2016.0065. [DOI] [PubMed] [Google Scholar]
- 51.Kim DJ, Kim J, Spaunhurst K, Montoya J, Khodosh R, Chandra K, Fu T, Gilliam A, Molgo M, Beachy PA, Tang JY. Open-label, exploratory phase II trial of oral itraconazole for the treatment of basal cell carcinoma. J Clin Oncol. 2014;32:745–751. doi: 10.1200/JCO.2013.49.9525. [DOI] [PubMed] [Google Scholar]
- 52.Zhou BB, Zhang H, Damelin M, Geles KG, Grindley JC, Dirks PB. Tumour-initiating cells: Challenges and opportunities for anticancer drug discovery. Nat Rev Drug Discov. 2009;8:806–823. doi: 10.1038/nrd2137. [DOI] [PubMed] [Google Scholar]
- 53.Zhao J. Cancer stem cells and chemoresistance: The smartest survives the raid. Pharmacol Ther. 2016;160:145–158. doi: 10.1016/j.pharmthera.2016.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ahmed M, Chaudhari K, Babaei-Jadidi R, Dekker LV, Nateri A Shams. Concise Review: Emerging drugs targeting epithelial cancer stem-like cells. Stem Cells. 2017;35:839–850. doi: 10.1002/stem.2579. [DOI] [PubMed] [Google Scholar]
- 55.Li Y, Rogoff HA, Keates S, Gao Y, Murikipudi S, Mikule K, Leggett D, Li W, Pardee AB, Li CJ. Suppression of cancer relapse and metastasis by inhibiting cancer stemness; Proc Natl Acad Sci USA; 2015; pp. 1839–1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jonker DJ, Nott L, Yoshino T, Gill S, Shapiro J, Ohtsu A, Zalcerg J, vickers MM, Wei A, Gao Y, et al. A randomized phase III study of napabucasin [BBI608] (NAPA) vs placebo (PBO) in patients (pts) with pretreated advanced colorectal cancer (ACRC): The CCTG/AGITG CO.23 trial. Ann Oncol. 2016;27(Suppl 6):S454O. doi: 10.1093/annonc/mdw370.03. [DOI] [Google Scholar]