Abstract
Unfertilized vertebrate eggs are arrested in metaphase of meiosis II with high cyclin B/Cdc2 activity to prevent parthenogenesis. Until fertilization, exit from metaphase is blocked by an activity called cytostatic factor (CSF), which stabilizes cyclin B by inhibiting the anaphase-promoting complex (APC) ubiquitin ligase. The APC inhibitor early mitotic inhibitor 1 (Emi1) was recently found to be required for maintenance of CSF arrest. We show here that exogenous Emi1 is unstable in CSF-arrested Xenopus eggs and is destroyed by the SCFβTrCP ubiquitin ligase, suggesting that endogenous Emi1, an apparent 44-kDa protein, requires a stabilizing factor. However, anti-Emi1 antibodies crossreact with native Emi2/Erp1/FBXO43, a homolog of Emi1 and conserved APC inhibitor. Emi2 is stable in CSF-arrested eggs, is sufficient to prevent CSF release, and is rapidly degraded in a Polo-like kinase 1-dependent manner in response to calcium-mediated egg activation. These results identify Emi2 as a candidate CSF maintenance protein.
Keywords: cyclin B, meiosis, maturation-promoting factor, oocyte maturation
To prevent parthenogenesis, unfertilized eggs from many animals arrest in metaphase of meiosis II (MII). Sperm penetration triggers the release from metaphase arrest and the commencement of alternating cycles of DNA replication and cell division in the embryo. The regulatory basis for metaphase II arrest was first characterized in frog eggs >30 years ago and termed cytostatic factor (CSF) (1). CSF is operationally defined as an activity, rather than a single molecule, present in unfertilized eggs that blocks cleavage of dividing blastomeres upon injection (reviewed in ref. 2). Mos, an activator of the mitogen-activated protein kinase/Rsk pathway, is a key component of CSF that appears at the onset of meiosis I (MI) and activates CSF to block cleavage of blastomeres (3).
The anaphase-promoting complex (APC) is an E3 ubiquitin ligase that triggers M-phase exit by directing proteasome-dependent cyclin B destruction (4), resulting in the swift inactivation of the cyclin B/Cdc2 kinase, or maturation-promoting factor (MPF) (5, 6). A rise in intracellular calcium after fertilization induces metaphase II release by relieving the APC from repression. Early mitotic inhibitor 1 (Emi1), originally cloned from a Xenopus oocyte cDNA library, blocks the cleavage of injected blastomeres similar to CSF (7) and efficiently inhibits the APC in vitro (8). Recently, Emi1 was shown to be required for maintenance of CSF arrest in frog and mouse eggs. Immunodepletion of Emi1 from Xenopus CSF egg extract causes rapid cyclin B proteolysis and exit from metaphase arrest independent of calcium mobilization, and ablation of Emi1 by small interfering RNA in mouse oocytes induces parthenogenesis (9, 10). Recent work has shown that the Mos/mitogen-activated protein kinase/Rsk pathway establishes, but is not required to maintain, CSF arrest (11, 12). Therefore, CSF arrest is a complex process established by the mitogen-activated protein kinase pathway and maintained through inhibition of the APC.
Upon fertilization of Xenopus eggs, calcium signaling inactivates CSF arrest, which requires the Xenopus Polo-like kinase 1 (Plx1). The target of Plx1 in this pathway remains unknown (13). In human somatic cells, MPF and human Polo-like kinase 1 (Plk1) target Emi1 for degradation by the Skpl Cullin/F-box protein (SCF)βTrCP ubiquitin ligase (14–17). Specifically, Plk1 phosphorylates Emi1 on its DSGxxS sequence, creating a consensus degron recognized by βTrCP (17). Thus, Xenopus Emi1 (xEmi1) could be a Plx1 target downstream of calcium signaling. An apparent paradox is how Emi1 levels are sustained in the CSF-arrested egg amid high MPF and Plx1 activities. In line with this paradox, a recent report suggests that Emi1 is unstable and undetectable in Xenopus eggs (18). On the other hand, Emi1 appears to be present in mouse eggs (10). In this study, we want to clarify our understanding of Emi1 regulation in Xenopus eggs and find that Emi2, an Emi1 homolog, may contribute to CSF arrest.
Methods
Reagents. Sera from four rabbits immunized with maltose binding protein (MBP)-Emi1 fusion protein were affinity-purified by flowing over a column of GST-Emi1 immobilized on CNBr-Sepharose resin with acid elution. Other antibodies used were against β-catenin, cyclin B2, Plx1, Plk1 (Zymed), myc epitope, and actin (Santa Cruz Biotechnology). xEmi2 was PCR-cloned from an oocyte cDNA library, and a human Emi2 (hEmi2) clone was purchased from Invitrogen. pCS2-cDNA constructs were linearized and in vitro-transcribed to generate mRNA by using a mMessage Machine kit (Ambion, Austin, TX). pCS2-cDNA constructs were in vitro-translated (IVT) in rabbit reticulocyte lysate (TNT, Promega) and labeled with 35S-methionine. All Emi1 and Emi2 experiments used Xenopus sequences unless otherwise noted as hEmi1 and hEmi2 for human sequences. MBP-fusion proteins and GST-Plk1 were expressed in Escherichia coli and purified by batch binding bacterial protein lysate to affinity resin and elution with maltose or glutathione, then dialyzed into XB buffer (20 mM Hepes, pH 7.7/100 mM KCl). Point mutations were engineered with a QuikChange kit (Stratagene).
Handling of Xenopus Oocytes. Oocytes were obtained and processed for H1 kinase activity and immunoblot as described (19). Oocytes were injected with 30 ng of MBP-Emi1 fusion protein or 10 ng of various mRNA in total volumes not exceeding 50 nl. Maturation was induced by treating oocytes with 10 μg/ml progesterone. Eggs were activated with A23187 ionophore (Sigma).
Destruction and APC Ubiquitination Assays. Egg extract was prepared as described (20). Destruction assays and in vitro APC ubiquitination reactions were performed as described (8).
Immunodepletion and in Vitro Phosphorylation Assays. Plx1 immunodepletion, Plk1 in vitro kinase reactions, and βTrCP binding assays were performed as described (17).
Immunofluorescence Microscopy. Staining of Emi1 in a Xenopus cell line (XTC) and human cell lines was performed as described (7, 21).
Results
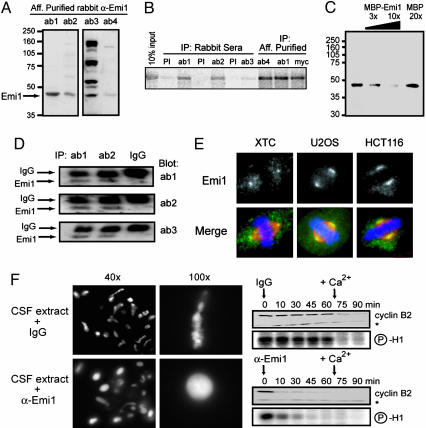
Characterization of Anti-Emi1 Antibodies. To examine Emi1 expression levels, high titer sera selected from the best four of six rabbits immunized with recombinant MBP-Emi1 fusion protein were purified against immobilized GST-Emi1 by affinity chromatography. These four affinity-purified antibodies (ab1–4) vary in affinity and specificity but each detects a band corresponding to the correct molecular mass of 44-kDa Emi1 in CSF extract (Fig. 1A). All four antibodies, using crude serum or affinity-purified antibodies, immunoprecipitate IVT myc-Emi1 (Fig. 1B).
Fig. 1.
Characterization of anti-Emi1 antibodies. (A) A 44-kDa protein is recognized by affinity-purified anti-Emi1 antibodies in CSF-arrested eggs. CSF extract was immunoblotted with affinity-purified antibodies from four rabbits immunized against Emi1. (B) Anti-Emi1 antibodies immunoprecipitate expressed Emi1. IVT myc-Emi1 was immunoprecipitated (IP) by reactive sera and affinity-purified antibodies, but not by preimmune (PI) sera. (C) Emi1 antibody recognition of the 44-kDa species is blocked with antigen. CSF extract was blotted with affinity-purified antibody that was unblocked or blocked with increasing purified MBP-Emi1 fusion protein up to 10-fold molar excess over antibody or blocked with 20-fold molar excess of MBP protein over antibody. (D) Each of the anti-Emi1 antibodies detects the same 44-kDa protein. Immunoprecipitates from CSF extract with two anti-Emi1 antibodies or control IgG were immunoblotted with three anti-Emi1 antibodies. (E) Anti-Emi1 antibody detects conserved Emi1 localization to the spindle poles. Metaphase chromosomes, spindles, and Emi1 were visualized in Xenopus somatic XTC cells, human U2OS cells, and human HCT116 cells by fluorescence microscopy. The merged images show DNA (blue), α-tubulin (red), and Emi1 (green). (Magnification: ×63.) (F) Addition of anti-Emi1 antibody to CSF extract induces chromatin decondensation, MPF inactivation, and cyclin B destruction without calcium addition. CSF extract was supplemented with CHX and sperm and treated with anti-Emi1 antibodies or control IgG. After 60 min, sperm chromatin was stained with Hoechst and visualized by epifluorescence microscopy. Similar extract was incubated with anti-Emi1 antibodies or IgG and incubated for 60 min before addition of calcium to trigger MII exit. Time points were processed for histone H1 kinase activity and immunoblot analysis. A nonspecific band (*) recognized by the anti-cyclin B2 antibody serves as a loading control.
We tested whether the 44-kDa band in CSF extract recognized by ab1, the most specific and highest-affinity antibody of the four in hand, is indeed Emi1. Preincubating ab1 with increasing MBP-Emi1 protein almost completely blocked detection of the 44-kDa band (Fig. 1C). Incubating ab1 with MBP at twice the blocking concentration of MBP-Emi1 did not block recognition of the 44-kDa species. Moreover, blotting ab1 and ab2 immunoprecipitates from egg extract with ab1–3 showed that all three antibodies recognize the same 44-kDa band (Fig. 1D). Using ab1, we estimate the concentration of 44-kDa Emi1 in CSF extract to be ≈50 nM (Fig. 7, which is published as supporting information on the PNAS web site), somewhat lower than our previous estimate of 300 nM (9). Additional validation demonstrated that these Emi1 antibodies detect overexpressed Emi1 and the endogenous 44-kDa protein in oocytes, embryos, and XTC cells (Fig. 7; see also Fig. 8, which is published as supporting information on the PNAS web site).
To validate the antibody ab1 further, we examined the subcellular localization of Emi1 in XTC cells by immunofluorescence microscopy. hEmi1 localizes specifically to the spindle poles in a variety of human cell lines (Fig. 1E and ref. 21). Importantly, this conserved and specific localization of Emi1 at the spindle poles is observed by ab1 staining in mitotic XTC cells in agreement with previous studies (7). Emi1 depletion in human cell lines by small interfering RNA abolishes the detection of Emi1 at spindle poles (data not shown). However, we could not validate ab1 in a similar fashion because we have found that XTC cells are refractory to small interfering RNA delivery.
To functionally validate the anti-Emi1 antibodies, we determined whether neutralizing Emi1 in CSF extract triggers calcium-independent metaphase release. Addition of ab1, but not control IgG, to CSF extract triggered rapid decline of cyclin B2 levels and Cdc2 activity and induced morphological decondensation of sperm nuclei (Fig. 1F). This effect is specific because MBP-Emi1 can block the effect of ab1 on meiotic progression (27). Taken together, the above immunological evidence and conserved localization of Emi1 suggest that these antibodies most likely detect Emi1 or a highly related protein in the oocyte, CSF-arrested egg, embryo, and XTC cells.
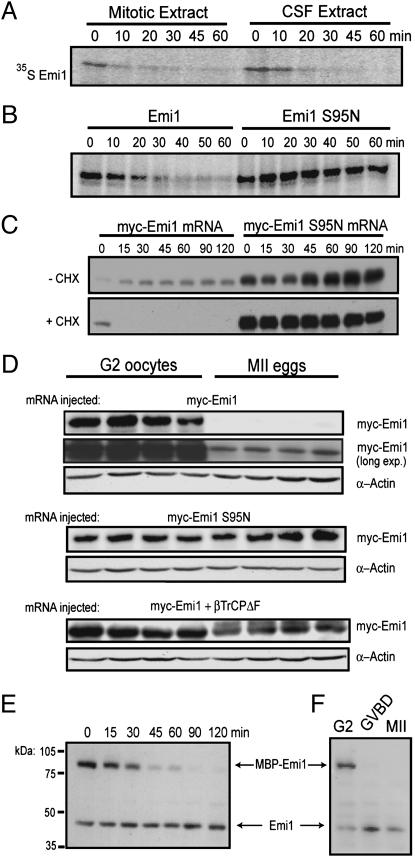
Exogenous Emi1 Is Destroyed in CSF-Arrested Eggs. In mitotic egg extract prepared by adding nondestructible Δ90 cyclin B to interphase extract, IVT Emi1 requires MPF for destruction (7). We suspected that although CSF extract contains high MPF activity IVT Emi1 would be refractory to destruction because Emi1 is required to maintain the CSF-arrested state. Instead, we found that radiolabeled IVT Emi1 is degraded with similar kinetics in mitotic and CSF extract (t1/2 ≈ 15 min; Fig. 2A). In contrast, IVT Emi1 is stable in interphase extract for >120 min (data not shown). hEmi1 is also destroyed in CSF extract (Fig. 9, which is published as supporting information on the PNAS web site). IVT Emi1 mutated in a critical serine residue (Ser-95) of the consensus βTrCP recognition degron is stable in CSF extract (Fig. 2B), indicating that CSF extract contains the factors required to ubiquitinate and destroy exogenous Emi1 through its DSGxxS sequence.
Fig. 2.
Exogenous Emi1 protein is destroyed through SCFβTrCP in CSF extract; nonetheless, Emi1 can accumulate by de novo translation. (A) IVT Emi1 is destroyed in mitotic extract and CSF extract. IVT, radiolabeled Emi1 was incubated in mitotic Δ90 cyclin B extract or CSF extract and processed for autoradiography at the indicated times. (B) Destruction of exogenous Emi1 in CSF extract is conserved and DSGxxS sequence-dependent. IVT [35S]Met Emi1 or Emi1 S95N was added to CSF extract and processed for autoradiography at the indicated times. (C) Accumulation of exogenous Emi1 protein translated in CSF egg extract. myc-Emi1 mRNA (WT or S95N mutant) was added to CSF extract with or without CHX and processed for immunoblot at the indicated times. (D) Exogenous Emi1 destruction in metaphase II eggs is mediated by the SCFβTrCP ligase. Stage VI oocytes were injected with myc-Emi1, myc-Emi1 S95N, or simultaneously with myc-Emi1 and βTrCPΔF mRNA. Injected oocytes were left at G2 arrest or matured by progesterone stimulation and processed for immunoblot. (E) Endogenous Emi1 is protected from destruction in CSF extract. Purified MBP-Emi1 protein was added to CSF extract supplemented with CHX and prepared for immunoblot at the indicated times after additions. (F) Endogenous Emi1 is stable in the maturing oocyte. Stage VI oocytes were injected with purified MBP-Emi1 protein and induced to mature by progesterone treatment. Emi1 was detected by immunoblotting lysates from immature oocytes, MI (GVBD) oocytes, and metaphase II eggs.
Emi1 levels in the egg may reflect the steady-state accumulation of unstable Emi1 protein. Therefore, we examined whether translation of myc-Emi1 mRNA in CSF would allow Emi1 to accumulate. Translated WT Emi1 accumulates to modest steady-state levels (Fig. 2C), whereas nondestructible Emi1 S95N mutant accumulates to increasingly high levels, suggesting that WT Emi1 is simultaneously translated and destroyed. To assess the stability of translated exogenous Emi1 directly, cycloheximide (CHX) was added to the CSF extract 2 h after transcript addition. WT Emi1 is rapidly destroyed (<15 min) in contrast to stable Emi1 S95N (Fig. 2C). Thus, newly synthesized Emi1 appears to be dynamically accumulated by a balance of translation and destruction, but could potentially accumulate to higher levels if a pool of the protein was sequestered from destruction.
G2 oocytes contain a stockpile of inactive MPF that is robustly activated at the onset of germinal vesicle breakdown (GVBD)/MI in response to hormonal stimulation (22). Thus, Emi1 may have important functions in G2-MI oocytes but could be destroyed after MPF activation after MI. We injected WT Emi1 or Emi1 S95N mRNA into G2 oocytes and induced maturation with progesterone. Both WT and mutant S95N ectopic Emi1 are stably expressed in the G2 oocyte, although Emi1 S95N accumulates at much higher steady-state levels than WT Emi1 in the CSF-arrested egg (Fig. 2D). Expression of dominant negative βTrCP (βTrCPΔF) missing the F-box domain enables exogenous WT Emi1 protein to accumulate to similar levels as Emi1 S95N in the MII egg (Fig. 2D). Thus, SCFβTrCP is active in the egg and directs exogenous Emi1 for proteolysis. The stability of both endogenous and exogenous Emi1 in the G2 oocyte would be consistent with a role in stabilizing APC substrates during G2-MI or possibly for the MI–MII transition, as seen in mouse (12).
Endogenous Emi1 Is Stabilized in CSF-Arrested Eggs. Next, we determined whether endogenous Emi1 is stable in MII eggs with characterized antibodies. CSF extract was treated with CHX, incubated with MBP-Emi1 protein (300 nM final concentration), and processed for immunoblotting at the indicated times postadditions. MBP-Emi1 is destroyed with similar kinetics as IVT Emi1 (Fig. 2E). Strikingly, levels of the 44-kDa protein detected by anti-Emi1 antibodies remain unchanged for up to 120 min in this destruction assay. Moreover, in CHX-treated CSF extract, the 44-kDa protein is extremely stable, with no apparent degradation in 48 h (Fig. 9B). Emi1 stability is also observed in vivo, as the endogenous 44-kDa band is present throughout oocyte maturation. On the other hand, recombinant MBP-Emi1 is destroyed at GVBD, when MPF first appears in MI (Fig. 2F). Differential stability of endogenous and exogenous forms of proteins is not uncommon. For example, IVT β-catenin is completely destroyed within 120 min in CSF extract by SCFβTrCP, yet endogenous β-catenin remains stable (Fig. 10, which is published as supporting information on the PNAS web site). If the 44-kDa band detected by anti-Emi1 is indeed Emi1, these results suggest that Emi1 is a stable protein in the egg and a mechanism exists to protect it from SCFβTrCP.
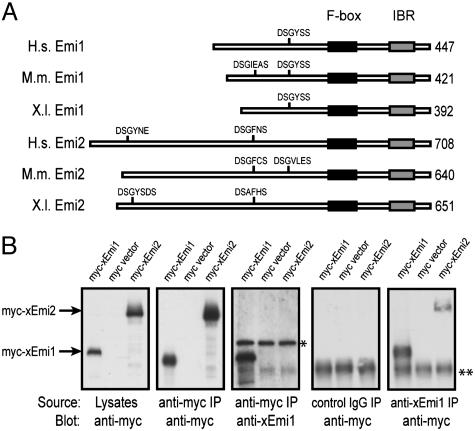
Emi2 Is a Homolog of Emi1 and Crossreacts with Anti-Emi1 Antibodies. Given that our anti-Emi1 antibodies dramatically inactivate CSF maintenance in egg extract, we considered the possibility that a previously unidentified Emi1 homolog could be immunologically crossreactive. A search of tblastn for xEmi1 identified a homologous ORF in Xenopus Fbx26. We originally identified Fbx26 from a Xenopus oocyte cDNA library as a highly abundant Skp1 interactor (384 of 444 clones isolated) in the same yeast two-hybrid screen that identified Emi1. However, frameshift errors in the Fbx26 cDNA sequence generated an incorrect ORF that masked its similarity to Emi1. The correct Fbx26 ORF is entered into the database as Emi2/Erp1 (GenBank accession no. AY928267), a 651-aa protein that is 25% identical to Emi1 that we refer to as Emi2. Human and mouse Emi2 orthologs are given the systematic name FBXO43 (23). In our following studies, we focus our attention on xEmi2 unless noted otherwise. An alignment of Emi1 and Emi2 orthologs is shown in Fig. 3A. Residues 434–651 of Emi2 are 35% identical to Emi1, sharing conserved F-box and IBR domains. Furthermore, Emi2 has conserved DSG-sequence degrons.
Fig. 3.
Emi2, a homolog of Emi1, is recognized by anti-Emi1 antibodies. (A) Emi2 is an Emi1-related protein conserved in vertebrate species. A schematic of Emi1 and Emi2 orthologs from human (H.s.), mouse (M.m.), and frog (X.l.) is shown. The conserved C-terminal F-box and zinc-binding “in-between-region” (IBR) domains are boxed. The identified βTrCP degrons in hEmi1 and xEmi1 and candidate degrons (DSG/A-X2-3-S/D/E) in Emi2 orthologs are shown. (B) Anti-xEmi1-specific antibodies can immunoprecipitate native xEmi2, but do not recognize denatured protein. HEK 293T cells were transfected with pCS2 myc-xEmi1 or pCS2 myc-xEmi2. Lysates were prepared after 48 h and either directly blotted with anti-myc antibodies or immunoprecipitated with anti-myc, anti-xEmi1 (Ab1), or control antibodies and then immunoblotted. The band indicated by * is an unknown anti-Emi1 crossreactive species. The band indicated by ** is IgG heavy chain.
To determine whether anti-Emi1 antibodies detect denatured Emi2 in addition to Emi1, anti-myc immunoprecipitates from 293T cells transfected with pCS2 myc-Emi2 or pCS2 myc-Emi1 were processed for immunoblotting with anti-Emi1 antibodies (Fig. 3B). Although anti-Emi1 antibodies recognize a robust Emi1 band, no signal was detected for Emi2. Equivalent amounts of myc-Emi1 and myc-Emi2 were immunoprecipitated because both were easily detected in a blot with anti-myc antibodies. We concluded that anti-Emi1 antibodies do not detect denatured Emi2 by immunoblot. On this basis, the 44-kDa band detected in egg extract is most likely not a form of Emi2.
Next, we asked whether anti-Emi1 antibodies crossreact with native Emi2. To test this idea, lysates from 293T cells transfected with myc-Emi1 or myc-Emi2 were immunoprecipitated with anti-Emi1 antibodies. Blotting the anti-Emi1 immunoprecipitates with anti-myc antibodies shows that anti-Emi1 antibodies recognize both native Emi1 and Emi2 (Fig. 3B). Additionally, anti-Emi1 antibodies immunoprecipitate an IVT C-terminal fragment of Emi2 (Fig. 11, which is published as supporting information on the PNAS web site). These results suggest that one feasible explanation for the ability of the anti-Emi1 antibodies to cause CSF release is through neutralization of Emi2.
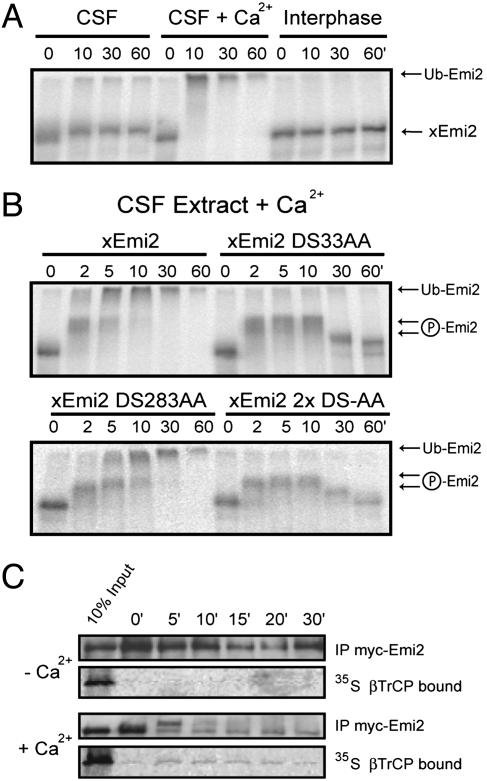
Emi2 Is Destroyed upon Egg Activation. The immunological crossreactivity of Emi1 and Emi2 prompted us to explore whether Emi2 exhibits properties consistent with a candidate CSF maintenance protein. One of Masui and Markert's (1) original postulates for the identity of CSF is that it is inactivated upon egg activation in response to calcium signaling. To test whether Emi2 fulfills this criterion, we incubated radiolabeled IVT myc-Emi2 in CSF extract in the absence or presence of calcium. The autoradiogram shows that Emi2 is stable in CSF extract but, upon calcium addition, becomes rapidly converted to an electrophoretically retarded form consistent with ubiquitination and is subsequently destroyed (Fig. 4A). Furthermore, Emi2 appears to be phosphorylated by an M-phase kinase, judging by its reduced electrophoretic mobility in CSF extract but not in interphase extract.
Fig. 4.
Emi2 is modified, apparently ubiquitinated by SCFβTrCP, and destroyed in CSF extract after calcium addition. (A) Emi2 is destroyed during CSF release. Full-length, radiolabeled IVT Emi2 was incubated in CSF extract with or without Ca2+ addition or in interphase extract for the indicated times. (B) Emi2 is destroyed through its conserved βTrCP recognition degron. Emi2 or mutants in one (DS33AA or DS283AA) or both (2xDS-AA) candidate degron sites were incubated in CSF extract and destruction was assayed at the indicated times after calcium addition. (C) Addition of calcium triggers Emi2 binding toβTrCP in CSF extract. IVT myc-Emi2 and radiolabeled IVT βTrCP were incubated in CSF extract with proteasome inhibitors, with or without calcium addition, for the indicated times. Anti-myc immunoprecipitates were analyzed for bound βTrCP.
Emi2 has two potential sequence degrons recognized by SCFβTrCP, one DS34GxxDS39 at the N terminus and a centrally located DS284AxxS288 sequence. We asked whether these two sequences contributed to Emi2 destruction upon CSF release by calcium. Whereas WT Emi2 is rapidly phosphorylated and ubiquitinated after calcium addition, mutating the N-terminal DSGxxDS degron (DS33AA) prevents Emi2 ubiquitination and destruction (Fig. 4B). An Emi2 mutant lacking the central DSAxxS sequence (DS283AA) is ubiquitinated and degraded with similar kinetics as WT Emi2 in response to calcium addition. Consistently, the stability of Emi2 mutant lacking both candidate degrons (2xDS-AA) is indistinguishable from the single DS33AA mutant, indicating that DS34GxxDS39 is the primary degron involved in Emi2 destruction during egg activation. Human IVT myc-Emi2 stability is regulated essentially the same way with one obvious exception: hEmi2 contains two destruction motifs that both contribute to calcium sensitivity (Fig. 12 A and B, which is published as supporting information on the PNAS web site). Because xEmi2 appears to use a potential βTrCP degron, we asked whether calcium triggers βTrCP binding to Emi2. Indeed, calcium addition to CSF extract promoted the binding of radiolabeled βTrCP to myc-Emi2 within 5 min (Fig. 4C), consistent with the kinetics of Emi2 ubiquitination. This finding suggests that Emi2 requires SCFβTrCP for ubiquitination.
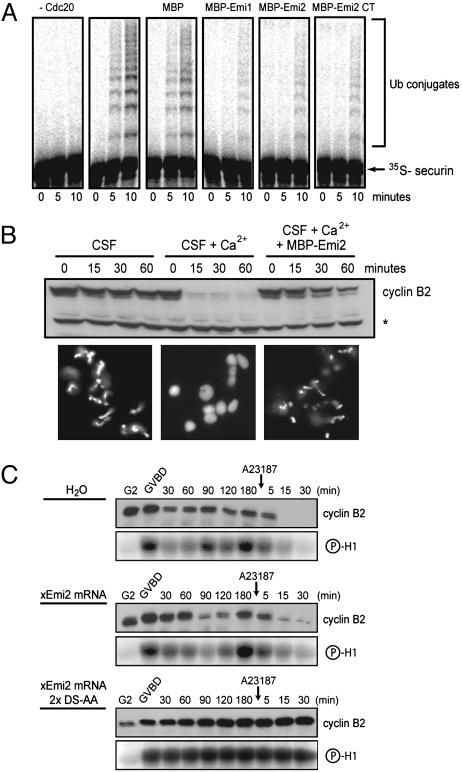
Emi2 Inhibits the APC and Is Sufficient to Prevent CSF Release. Because Emi2 is a homolog of Emi1, a well established APC inhibitor, we tested the likely activity of Emi2 as an APC inhibitor. Full-length Emi2 (MBP-Emi2) or a C-terminal fragment (MBP-Emi2 CT) blocks the ability of the APCCdc20 to polyubiquitinate radiolabeled securin substrate in vitro as effectively as Emi1 (Fig. 5A). Similar results were obtained with Cdh1 as the APC activator (Fig. 12C). These results indicate that the C terminus of Emi2, which bears the most identity with Emi1, is sufficient to inhibit the APC.
Fig. 5.
Emi2 inhibits the APCCdc20 complex and blocks exit from CSF arrest. (A) Emi2 is an APC inhibitor. Recombinant hEmi1, hEmi2, an hEmi2 C-terminal fragment (residues 541–708), or control (MBP) proteins (2 μM) were tested for their ability to inhibit the in vitro ubiquitination of human securin by the APCCdc20 complex. (B) Emi2 is sufficient to prevent CSF release. Addition of excess hEmi2 protein blocks the calcium-induced exit from MII in CSF extract. CSF extract was initiated to exit MII by calcium addition and assayed for cyclin B2 destruction (above) or the formation of interphase nuclei (below). Addition of MBP-hEmi2 to extract blocked MII exit. (C) CSF release requires Emi2 destruction. Injection of nondestructible xEmi2, but not WT, into maturing oocytes prevents MII exit. In vitro-transcribed xEmi2 (WT or nondestructable 2x DS-AA mutant) was injected into oocytes. Oocytes were matured with progesterone and harvested at GVBD and various times afterward. At 3 h post-GVBD, oocytes were released from MII arrest by addition of calcium ionophore A23187. Samples were immunoblotted for cyclin B2 and assayed for H1 kinase activity.
We determined whether Emi2 is sufficient to prevent calcium-induced CSF release. Addition of 2 μM MBP-Emi2 to CSF extract inhibited cyclin B degradation in response to calcium and prevented sperm nuclei decondensation (Fig. 5B). The slow partial decline in cyclin B levels is most likely the result of calcium-induced Emi2 destruction. The 2 μM concentration of MBP-Emi2 used here appears to saturate the destruction machinery and suffices to block CSF release in response to calcium. Finally, we examined whether Emi2 destruction is a prerequisite for CSF release in vivo. Calcium ionophore A23187 triggers the decline in cyclin B2 levels and H1 kinase activity in matured eggs injected at G2 with water or WT Emi2 mRNA, but not nondestructible Emi2 2xDS-AA mRNA (Fig. 5C). Together with the finding that Emi2 inhibits the APC in vitro, these results suggest that Emi2 is an APC inhibitor that must be destroyed for CSF release.
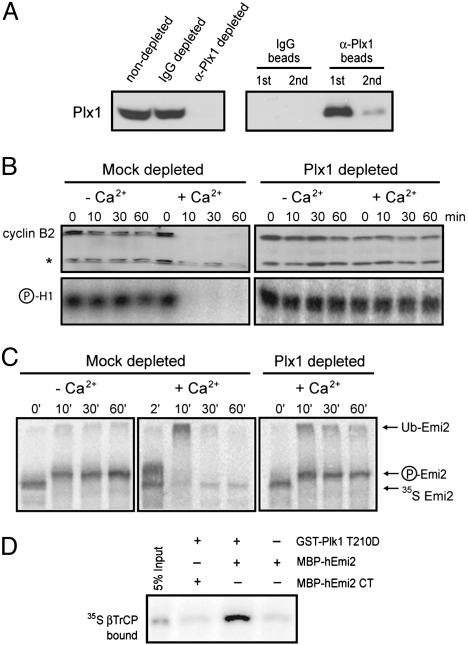
Emi2 Is a Plk1 Target During CSF Release. Another potential parallel between Emi1 and Emi2 is regulation by Plk1. Given that Plx1 is required for Emi1 proteolysis in mitotic egg extract (17), we supposed that Emi2 destruction during egg activation is a Plx1-dependent process. Two rounds of immunodepletion using anti-Plx1 antibodies effectively removed Plx1 from CSF extract (Fig. 6A). Consistent with prior work (13), calcium addition to Plx1-depleted, but not mock IgG-depleted, CSF extract failed to trigger cyclin B2 destruction and H1 kinase inactivation (Fig. 6B). Radiolabeled IVT myc-Emi2 is rapidly destroyed upon calcium addition to mock-depleted CSF extract, but remains stable in Plx1-depleted CSF treated with calcium (Fig. 6C). Finally, we determined whether Plk1 promotes Emi2 binding to βTrCP in vitro. Autoradiography of anti-MBP immunoprecipitates shows that constitutively active GST-Plk1 (T210D mutant) protein enhances the binding of radiolabeled βTrCP to full-length MBP-Emi2 fusion protein but not to the C terminus of Emi2 lacking βTrCP degrons (Fig. 6D). These results suggest that Plx1 is required for Emi2 destruction in response to calcium signaling by stimulating Emi2 and βTrCP binding.
Fig. 6.
Plx1 is required for the destruction of Emi2. (A) Depletion of Plx1 from egg extract. CSF extract was either mock-depleted with IgG beads or depleted with Plx1 antibodies for two rounds of depletion. Depleted extract or beads were immunoblotted for Plx1 protein. (B) Depletion of Plx1 prevents CSF release. CSF extract depleted as above was assayed for cyclin B2 destruction and H1 kinase inactivation with or without calcium addition. (C) Depletion of Plx1 stabilizes Emi2 in calcium-stimulated CSF extract. CSF extract depleted as above was incubated with IVT Emi2 with or without calcium addition, and samples were collected for autoradiography at the indicated times. (D) Plk1 stimulates the binding of hEmi2 to βTrCP.
Discussion
During our studies we learned that Ohsumi et al. (18) raised an antibody against Emi1 and failed to detect a 44-kDa band by immunoblot in the egg or developing embryo until gastrulation. Consistent with our results, Ohsumi et al. observed the destruction of exogenous Emi1 in CSF extract and maturing oocytes. However, they concluded from the inability to detect endogenous Emi1 and the observation that exogenous Emi1 is unstable in the egg that Emi1 does not and cannot possibly exist in Xenopus until gastrulation. However, no evidence is provided that the ≈44-kDa band, which Ohsumi et al. only see appearing at 10–12 h postfertilization in the embryo, is indeed Emi1. The species they see is induced at gastrulation, shortly after zygotic transcription is activated (24), but no specific validation or blocking experiment of the endogenous band shows this species is Emi1.
To shed light on this discrepancy in greater detail, we characterized four antibodies raised against Emi1 and present evidence that the 44-kDa species detected by these antibodies, presumably Emi1, is present in the egg. Importantly, another research group readily detects Emi1 at constant levels during oocyte maturation with an independently raised antibody (T. Lorca, personal communication). Ohsumi et al. propose that Emi1 cannot exist in the egg because exogenous Emi1 is destroyed. However, we show here that, although unstable, newly synthesized Emi1 can accumulate to detectable steady-state levels. During its synthesis, Emi1 could be sequestered by some cellular structure or stabilizing factor that would allow higher levels of accumulation. The instability of exogenous β-catenin in egg extract provides an example of an unstable protein that is sequestered in a stable complex (organized at the cell cortex). The simplest explanation for Ohsumi et al.'s data is that the antibody used in the study failed to detect endogenous Emi1 and these negative data by themselves are insufficient evidence to support the idea that the protein does not exist.
On the other hand, we remain cautiously skeptical that the 44-kDa species detected by our antibodies is unambiguously Emi1 for the simple reason that Xenopus laevis is not an organism allowing a direct gene knockout strategy to definitively settle this matter. Nonetheless, our functional evidence strongly suggests that Emi1 and/or an immunologically crossreactive protein is important in CSF arrest.
Bearing in mind that new synthesis of B-type cyclins was originally thought to be nonessential for Xenopus oocyte maturation (25) until the discovery of three additional cyclin B members a decade later (26), we considered the existence of unidentified Emi1 homologs highly plausible. In this study, we identify Emi2 as an Emi1 homolog that crossreacts with antibodies raised against full-length Emi1 in immunoprecipitation experiments but not in immunoblots. Thus, we conclude that the 44-kDa band detected by anti-Emi1 antibodies in eggs is unlikely to be a form of Emi2, but loss-of-function experiments using anti-Emi1 antibodies could have simultaneously or exclusively inhibited Emi2. This conclusion raises the possibility that the calcium-independent CSF release caused by Emi1 immunodepletion in our previous work (9) and antibody addition experiment (Fig. 1F) is a result of inactivating Emi2, which is a candidate CSF maintenance protein. In support for a role of Emi2 in CSF arrest, we show here that (i) Emi2 is an APC inhibitor sufficient to prevent CSF release, (ii) Emi2 destruction through its SCFβTrCP recognition sequence is a requirement for CSF release in response to calcium signaling, and (iii) Emi2 is targeted for destruction by Plk1 upon CSF release. These observations strongly suggest that Emi2 has a role in CSF arrest. It remains to be formally demonstrated that disabling either Emi1 or Emi2 function without perturbing the other causes loss of CSF maintenance.
Do the negative data from Ohsumi et al. (18) and the identification of Emi2 justify the dismissal of the role of Emi1 in maintaining CSF arrest? Recent work in mouse oocytes shows that Emi1 ablation by small interfering RNA causes spontaneous egg activation, providing plausible genetic evidence that Emi1 is indeed essential for CSF arrest (10). As was the case for B-type cyclins, without the luxury of a complete X. laevis genome sequence database, there may be additional members of the early mitotic inhibitor family of proteins awaiting identification. As our current knowledge stands, the production of Emi1–/–, Emi2 –/–, and double homozygous null mice will most constructively resolve the relative importance of these two homologs in CSF arrest.
Supplementary Material
Acknowledgments
We thank James Nelson (Stanford University, Stanford, CA) for β-catenin antibody, William Dunphy (California Institute of Technology, Pasadena) for Plx1 antibody, Tim Hunt (Imperial Cancer Research Fund, London) for cyclin B2 antibody, and Thierry Lorca for communicating unpublished results. This work was supported by Public Health Service Grants 5T32 CA09302-27 (to J.J.T.) and RO1 GM60439 and GM54811 (to P.K.J.).
Author contributions: J.J.T., D.V.H., K.H.B., A.V.L., M.K.S., and P.K.J. designed research; J.J.T., D.V.H., K.H.B., A.V.L., M.K.S., and J.R.A. performed research; D.V.H. contributed new reagents/analytic tools; J.J.T., D.V.H., K.H.B., and P.K.J. analyzed data; and J.J.T. and P.K.J. wrote the paper.
Abbreviations: APC, anaphase-promoting complex; CHX, cycloheximide; CSF, cytostatic factor; Emi, early mitotic inhibitor; hEmi, human Emi; xEmi, Xenopus Emi; GVBD, germinal vesicle breakdown; IVT, in vitro-translated; MI, meiosis I; MII, meiosis II; MBP, Maltose binding protein; MPF, mitosis-promoting factor; Plk1, human Polo-like kinase 1; Plx1, Xenopus Polo-like kinase 1; SCF, Skpl Cullin/F-box protein.
Data deposition: The sequence reported in this paper has been deposited in the GenBank database (accession no. AY928267).
References
- 1.Masui, Y. & Markert, C. L. (1971) J. Exp. Zool. 177, 129–145. [DOI] [PubMed] [Google Scholar]
- 2.Tunquist, B. J. & Maller, J. L. (2003) Genes Dev. 17, 683–710. [DOI] [PubMed] [Google Scholar]
- 3.Sagata, N., Watanabe, N., Vande Woude, G. F. & Ikawa, Y. (1989) Nature 342, 512–518. [DOI] [PubMed] [Google Scholar]
- 4.King, R. W., Peters, J. M., Tugendreich, S., Rolfe, M., Hieter, P. & Kirschner, M. W. (1995) Cell 81, 279–288. [DOI] [PubMed] [Google Scholar]
- 5.Gautier, J., Norbury, C., Lohka, M., Nurse, P. & Maller, J. (1988) Cell 54, 433–439. [DOI] [PubMed] [Google Scholar]
- 6.Gautier, J., Minshull, J., Lohka, M., Glotzer, M., Hunt, T. & Maller, J. L. (1990) Cell 60, 487–494. [DOI] [PubMed] [Google Scholar]
- 7.Reimann, J. D., Freed, E., Hsu, J. Y., Kramer, E. R., Peters, J. M. & Jackson, P. K. (2001) Cell 105, 645–655. [DOI] [PubMed] [Google Scholar]
- 8.Reimann, J. D., Gardner, B. E., Margottin-Goguet, F. & Jackson, P. K. (2001) Genes Dev. 15, 3278–3285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reimann, J. D. & Jackson, P. K. (2002) Nature 416, 850–854. [DOI] [PubMed] [Google Scholar]
- 10.Paronetto, M. P., Giorda, E., Carsetti, R., Rossi, P., Geremia, R. & Sette, C. (2004) EMBO J. 23, 4649–4659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bhatt, R. R. & Ferrell, J. E., Jr. (1999) Science 286, 1362–1365. [DOI] [PubMed] [Google Scholar]
- 12.Tunquist, B. J., Schwab, M. S., Chen, L. G. & Maller, J. L. (2002) Curr. Biol. 12, 1027–1033. [DOI] [PubMed] [Google Scholar]
- 13.Descombes, P. & Nigg, E. A. (1998) EMBO J. 17, 1328–1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Margottin-Goguet, F., Hsu, J. Y., Loktev, A., Hsieh, H. M., Reimann, J. D. & Jackson, P. K. (2003) Dev. Cell 4, 813–826. [DOI] [PubMed] [Google Scholar]
- 15.Guardavaccaro, D., Kudo, Y., Boulaire, J., Barchi, M., Busino, L., Donzelli, M., Margottin-Goguet, F., Jackson, P. K., Yamasaki, L. & Pagano, M. (2003) Dev. Cell 4, 799–812. [DOI] [PubMed] [Google Scholar]
- 16.Moshe, Y., Boulaire, J., Pagano, M. & Hershko, A. (2004) Proc. Natl. Acad. Sci. USA 101, 7937–7942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hansen, D. V., Loktev, A. V., Ban, K. H. & Jackson, P. K. (2004) Mol. Biol. Cell. 15, 5623–5634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ohsumi, K., Koyanagi, A., Yamamoto, T. M., Gotoh, T. & Kishimoto, T. (2004) Proc. Natl. Acad. Sci. USA 101, 12531–12536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Furuno, N., Nishizawa, M., Okazaki, K., Tanaka, H., Iwashita, J., Nakajo, N., Ogawa, Y. & Sagata, N. (1994) EMBO J. 13, 2399–2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Murray, A. W., Solomon, M. J. & Kirschner, M. W. (1989) Nature 339, 280–286. [DOI] [PubMed] [Google Scholar]
- 21.Hsu, J. Y., Reimann, J. D., Sorensen, C. S., Lukas, J. & Jackson, P. K. (2002) Nat. Cell Biol. 4, 358–366. [DOI] [PubMed] [Google Scholar]
- 22.Ferrell, J. E., Jr. (1999) BioEssays 21, 833–842. [DOI] [PubMed] [Google Scholar]
- 23.Jin, J., Cardozo, T., Lovering, R. C., Elledge, S. J., Pagano, M. & Harper, J. W. (2004) Genes Dev. 18, 2573–2580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Howe, J. A., Howell, M., Hunt, T. & Newport, J. W. (1995) Genes Dev. 9, 1164–1176. [DOI] [PubMed] [Google Scholar]
- 25.Minshull, J., Murray, A., Colman, A. & Hunt, T. (1991) J. Cell Biol. 114, 767–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hochegger, H., Klotzbucher, A., Kirk, J., Howell, M., le Guellec, K., Fletcher, K., Duncan, T., Sohail, M. & Hunt, T. (2001) Development (Cambridge, U.K.) 128, 3795–3807. [DOI] [PubMed] [Google Scholar]
- 27.Tung, J. J. & Jackson, P. K. (2005) Cell Cycle 4, 478–482. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.