Abstract
The immune microenvironment of primary tumors has been reported to be one of the factors influencing the prognosis of patients with cancer. The tumor-infiltrating regulatory T cell (Treg) count has previously been revealed to be positively correlated with intratumoral cyclooxygenase-2 (Cox-2) expression, and was also associated with poor survival among patients with non-small cell lung cancer (NSCLC). In addition, the urinary levels of a prostaglandin E2 (PGE2) metabolite (PGE-M) were used as a biomarker in clinical trials of the Cox-2 inhibitor celecoxib. In the current prospective study, the association of urinary PGE2 and PGE-M levels with intratumoral Cox-2 expression and Treg count was examined in patients with NSCLC. A total of 21 patients with NSCLC who underwent complete resection of the tumor at Kawasaki Medical School Hospital (Kurashiki, Japan) were enrolled. Urine specimens were obtained prior to surgery in order to examine urinary PGE2 and PGE-M levels. A significant positive association was observed between urinary PGE2 levels and the intratumoral Treg count (P=0.023), but not the intratumoral Cox-2 expression levels. No significant associations were identified between urinary PGE2 levels and any of the other clinicopathological characteristics examined, including age, sex, smoking history, histology, tumor size, nodal status and disease stage. However, no significant association was observed between urinary PGE-M levels and the intratumoral Treg count (P=0.069) or Cox-2 expression. In conclusion, urinary PGE2 levels were positively correlated with intratumoral Treg counts in patients with NSCLC in the current study. This indicates that urinary PGE2 may be an improved biomarker, compared with PGE-M, for the prediction of intratumoral Treg numbers.
Keywords: non-small cell lung cancer, regulatory T-cell, cyclooxygenase-2, urinary prostaglandin E2, urinary prostaglandin metabolites
Introduction
Lung cancer is a major cause of mortality in developed countries (1). Surgical resection is the prominent curative treatment option for this type of disease, particularly during the early stages of non-small cell lung cancer (NSCLC) (1). However, the 5-year survival rate for patients with NSCLC who undergo surgery remains ~70% (1,2). Several biomarkers have been reported as predictors of survival and recurrence in patients with NSCLC, including tumor-infiltrating regulatory T cells (Tregs) (3). A number of previous studies have demonstrated that the immune microenvironment of the primary tumor is a significant prognostic factor. Immunological biomarkers in the tumor microenvironment are useful prognostic predictors, in addition to promising targets for novel therapeutic approaches (4–9). In particular, a promising immunological biomarker may be Tregs; the potential mechanism underlying the induction of Tregs is the expression of cyclooxygenase-2 (Cox-2) in tumor cells (10). These findings may facilitate the development of individualized immunomodulatory therapies to deplete the tumor microenvironment from Tregs.
A major limitation to individualized immunomodulatory therapies is the requirement for adequate tumor specimens, which frequently necessitates an invasive procedure (11). Furthermore, in patients with recurrent disease, further tissue specimens are required; however, rebiopsies are difficult to perform in certain cases, including those with brain metastasis (11). Urinary prostaglandin E2 (PGE2) metabolite (PGE-M) is a major urinary metabolite of PGE2 and may be used as an index of systemic PGE2 production (11). Cox-2-derived PGE2 serves important roles in cancer progression. PGE2 is an unstable compound that is rapidly metabolized to stable PGE-M in vivo by the enzyme 15-hydroxyprostaglandin dehydrogenase (12). Furthermore, the direct quantification of PGE2 levels has been revealed to be an unreliable indicator of a biomarker of inflammation caused by infection or malignancy (12); therefore, several previous studies have used measurements of urinary PGE-M instead (13,14).
In the present study, urinary PGE2 levels were directly quantified using a highly sensitive PGE2 ELISA kit, to investigate whether urinary PGE2 levels were associated with the expression of Cox-2 protein or levels of Tregs in patients with NSCLC.
Patients and methods
Study population
Urinary and paraffin-embedded tumor samples were obtained from 21 consecutive patients with NCSLC who underwent surgical resection at Kawasaki Medical School Hospital (Kurashiki, Japan) between September 2014 and March 2015. None of the patients had received radiotherapy or chemotherapy prior to surgery. This prospective study was conducted with the approval of the Institutional Ethics Committee of Kawasaki Medical School, and informed consent for the use of urine and tumor specimens was obtained from all patients. The histological diagnosis of the tumors was based on the criteria of the World Health Organization, and the tumor-node-metastasis (TNM) stage was determined according to the criteria established in 2009 (15). Fluorodeoxyglucose (18FDG)-positron emission tomography-computed tomography scanning was used to calculate the maximal standardized uptake value (SUVmax). Scanning was performed 60 min following intravenous injection of 150–220 MBq of 18FDG. The regions of interest were placed three-dimensionally over the lung cancer nodules.
Patients were excluded from enrollment if they were taking, or had a history of regularly taking, aspirin or other nonsteroidal anti-inflammatory drugs (NSAID). Patients were also ineligible if they had concurrent severe or uncontrolled medical diseases, including active systemic infection, diabetes or renal failure.
Measurement of urinary PGE2 and PGE-M via ELISA
The urine samples were obtained prior to surgery and stored at −20°C following centrifugation at 500 × g for 5 min at room temperature. The urinary PGE2 level was determined using a Correlate-EIA™ PGE2 Enzyme Immunoassay kit (Assay Designs; Enzo Life Sciences, Inc., Farmingdale, NY, USA) according to the manufacturer's instructions. Plates were read at an absorbance wavelength of 450 nm (Varioskan® Flash Spectral Scanning Multimode Reader; Thermo Scientific, Inc., Waltham, MA, USA). The urinary PGE2 level was calculated in pg/ml, according to the protocol provided by the manufacturer of the assay kit. In addition, measurement of urinary PGE-M levels were performed at SRL, Inc. (Tokyo, Japan), and expressed in pg/ml.
Immunohistochemical (IHC) analysis
IHC analyses were performed using resected, paraffin-embedded lung cancer tissues. Following microtome sectioning, the tissue slides (4-µm-thick) were stained for Cox-2 and forkhead box P3 (Foxp3), a marker of Tregs, using an automated immunostainer (NexES Special Stainer; Ventana Medical Systems, Inc., Tucson, AZ, USA) according to the manufacturer's instructions. Slides were de-paraffinized using EZprep solution (Ventana Medical Systems, Inc.) for 30 min at 75°C. Epitope retrieval was accomplished on the automated stainer with cell conditioning 1 solution (Ventana Medical Systems, Inc.) for 60 min at 95°C. The antibodies were transferred with diluent to user-fillable dispensers for use on the automated stainer. Slides were developed using the Optiview DAB IHC detection kit (Ventana Medical Systems, Inc.). Briefly, the slides were treated with the inhibitor included was for 4 min, the multimer for 12 min, DAB/peroxide for 8 min and copper solution for 4 min at 37°C. Slides were subsequently counterstained with hematoxylin II (Ventana Medical Systems, Inc.) for 4 min at 37°C.
Primary antibodies directed against Cox-2 (dilution, 1:50; catalog no., CX-294; Dako; Agilent Technologies, Inc., Santa Clara, CA, USA) and Foxp3 (dilution, 1:100; catalog no., 22510; Abcam, Cambridge, UK) were used at 37°C according to the manufacturer's protocol. Secondary antibody (Discovery Universal Secondary Antibody, Ventana Medical Systems, Inc.; catalog no., 760-4205) was used at 37°C according to the manufacturer's protocol. The expression levels of each marker protein were examined and evaluated according to a previously reported original protocol (16,17). For Cox-2, the slides were scored according to the intensity of staining (0–3) and the percentage of positively stained cells (0, 0%; 1, 1–9%; 2, 10–49%; and 3, 50–100%). The IHC score (0–9) was calculated as the product of multiplying the intensity and percentage scores. Cox-2 expression was considered positive when the IHC score was ≥4 (16). To evaluate the immunostaining of the Tregs, digital high-power field (HPF) images of the tumor area were taken using a light microscope (Axiophot microscope; Carl Zeiss AG, Oberkochen, Germany), of which 10 were selected and the absolute number of Foxp3+ lymphocytes in these images determined (17). The number of immunostained Foxp3 cells was then determined as the mean count from the images and used to obtain the tumor-infiltrating Foxp3+ Treg count (Treg score; 0–24). IHC staining demonstrated high levels of Cox-2 expression (Fig. 1A) and Foxp3+ Tregs (Fig. 1B).
Figure 1.
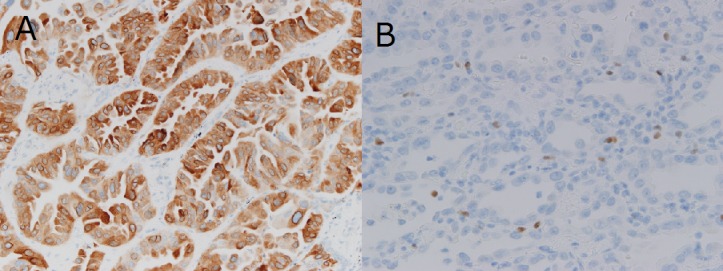
Immunohistochemical staining revealed high levels of (A) cyclooxygenase-2 expression and (B) tumor-infiltrating forkhead box P3+ regulatory T-cells. Magnification, ×200.
Statistical analysis
All statistical analyses were performed using SPSS software (version 17.0; SPSS, Inc., Chicago, IL, USA). The χ2 test and Fisher's exact test were used to examine the association between urinary PGE2 or PGE-M levels and various clinicopathological parameters of the patients. P<0.05 was considered to indicate a statistically significant difference.
Results
Patient clinicopathological characteristics
Clinicopathological characteristics of the patients are summarized in Table I. The patients ranged in age from 40–83 years old (mean, 69.1 years), and included 8 males and 13 females. Adenocarcinoma was detected in 18 patients (85.7%) and squamous cell carcinoma was observed in 3 patients (14.3%). Pathological lymph node N0 disease was detected in 17 patients (80.9%), and N1 or N2 disease in 4 patients (19.1%). Pathological stage I disease was observed in 15 patients (71.5%), and stage II or stage IIIA disease was detected in 6 patients (28.5%).
Table I.
Clinicopathological characteristics of the patients (n=21).
| Clinicopathological characteristic | No. of patients (%) |
|---|---|
| Age | |
| <70 | 11 (52.4) |
| ≥70 | 10 (47.6) |
| Sex | |
| Male | 8 (38.0) |
| Female | 13 (62.0) |
| Tumor histology | |
| Adenocarcinoma | 18 (85.7) |
| Squamous cell carcinoma | 3 (14.3) |
| Tumor stage | |
| T1 | 11 (52.2) |
| T2 | 9 (43.0) |
| T3 | 1 (4.8) |
| Pathological lymph node status | |
| N0 | 17 (80.9) |
| N1 | 1 (4.8) |
| N2 | 3 (14.3) |
| Pathological tumor stage | |
| IA | 10 (47.7) |
| IB | 5 (9.5) |
| II (A+B) | 2 (23.8) |
| IIIA | 4 (19.0) |
| Surgical procedure undergone | |
| Lobectomy | 20 (95.2) |
| Wedge resection | 1 (4.8) |
SD, standard deviation; T, tumor; N, node.
Association between clinicopathological characteristics and urinary PGE2/PGE-M levels
No significant correlation was observed between the urinary PGE2 and PGE-M levels (r=0.372; P=0.097; data not shown). However, urinary PGE2 levels (P=0.023), but not the urinary PGE-M levels (P=0.069), were significantly positively correlated with Treg score (Fig. 2 and Table II). The mean value of the urinary PGE2 level was 1467±478 pg/ml in the group with a Treg score <5 (n=13) and 1844±204 pg/ml in the group with a Treg score ≥5 (n=8) (Table II). No significant association was observed between the urinary PGE2 levels and the Cox-2 IHC score (P=0.986; Table II). In addition, no significant associations were identified between urinary PGE2 and any of the other clinicopathological characteristics examined, including age (P=0.863), sex (P=0.265), smoking history (P=0.465), histology (P=0.094), tumor size (P=0.524), nodal status (P=0.395), disease stage (P=0.680) and the SUVmax (P=0.308) (Table II).
Figure 2.
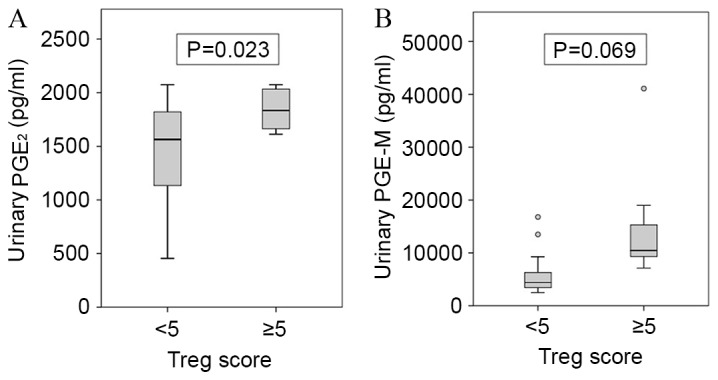
Association of (A) urinary PGE2 and (B) PGE-M levels with the tumoral Treg count. The results are presented as the mean ± standard deviation. Treg, regulatory T cell; PGE2, prostaglandin E2; PGE-M, PGE2 metabolite.
Table II.
Association of urinary PGE2 and PGE-M levels with the clinicopathological characteristics of patients with non-small cell lung cancer.
| Clinicopathological characteristic | No. of patients | Urinary PGE2 (pg/ml) | P-value | Urinary PGE-M (pg/ml) | P-value |
|---|---|---|---|---|---|
| Age | 0.863 | 0.177 | |||
| <70 | 11 | 1,594±384 | 6,818±3,225 | ||
| ≥70 | 10 | 1,629±501 | 12,349±11,651 | ||
| Sex | 0.265 | 0.423 | |||
| Female | 13 | 1,529±451 | 8,400±10,278 | ||
| Male | 8 | 1,744±392 | 11,161±5,076 | ||
| Smoking history | 0.465 | 0.785 | |||
| Never smoked | 14 | 1,561±450 | 9,157±10,274 | ||
| Smoker | 7 | 1,709±409 | 10,041±4,285 | ||
| Histology | 0.094 | 0.854 | |||
| Adenocarcinoma | 18 | 1,581± 77 | 9,334±9,095 | ||
| Squamous cell carcinoma | 3 | 1,788±392 | 10,160±6,247 | ||
| Tumor size | 0.524 | 0.741 | |||
| T1 | 11 | 1,672±295 | 10,052±10,920 | ||
| T2-3 | 10 | 1,543±557 | 8,792±5,628 | ||
| Pathological nodal status | 0.395 | 0.271 | |||
| N0 | 17 | 1,679±335 | 10,329±9,132 | ||
| N1+2 | 4 | 1,321±715 | 5,725±5,260 | ||
| Pathological stage | 0.680 | 0.332 | |||
| I | 15 | 1,645±342 | 10,354±9,757 | ||
| II+IIIA | 6 | 1,525±640 | 7,197±4,701 | ||
| SUVmax | 0.308 | 0.693 | |||
| <5 | 10 | 1,506±458 | 8,607±1,1652 | ||
| ≥5 | 11 | 1,705±406 | 10,220±5,004 | ||
| Cox-2 score | 0.986 | 0.657 | |||
| <4 | 14 | 1,609±416 | 9,924±10,312 | ||
| ≥4 | 7 | 1,613±499 | 8,509±3,963 | ||
| Treg score | 0.023 | 0.069 | |||
| <5 | 13 | 1,467±478 | 6,162±4,468 | ||
| ≥5 | 8 | 1,844±204 | 14,799±11,192 |
Treg, regulatory T cell; PGE2, prostaglandin E2; PGE-M, PGE2 metabolite; Cox-2, cyclooygenase-2; SUVmax, maximal standardized uptake value from fluorodeoxyglucose-positron emission tomography.
The mean urinary PGE-M level was 6,162±4,468 pg/ml in the group with a Treg score <5 (n=13), and 14,799±11,192 pg/ml in the group with a Treg score ≥5 (n=8) (Table II). No significant association was observed between the urinary PGE-M levels and the Cox-2 IHC scores (P=0.657) or any of the other clinicopathological characteristics examined (Table II).
Discussion
In 2010, it was demonstrated that the tumor-infiltrating Foxp3+ Treg count (Treg score) was positively correlated with intratumoral Cox-2 expression, and was also associated with recurrence-free survival, particularly in patients with lymph node-negative NSCLC (10). In the present study, the association of urinary PGE2 levels with the Cox-2 IHC score and Treg score were examined in 21 consecutive patients with NSCLC who underwent surgical tumor resection at Kawasaki Medical School Hospital. The results revealed a significant association between the urinary PGE2 levels and Treg score. In addition, to the best of our knowledge, the current study was the first to use the urinary PGE2 level, and not the PGE-M level, for the assessment of prognosis in patients with NSCLC.
Tregs were initially characterized as possessing a CD4+CD25+ phenotype and are considered to modulate the antitumor immune response (18). Tregs are able to suppress the activity of cytotoxic T cells through direct cell-to-cell contact or via the release of cytokines (19). The most specific Treg cell marker currently identified is the nuclear transcription factor Foxp3 (19,20). A high density of tumor-infiltrating Foxp3+ Tregs has been reported to be associated with a higher risk of recurrence and a poorer overall survival in patients with NSCLC (21). Sharma et al (22) demonstrated that tumor-derived Cox-2/PGE2 induces the expression of Foxp3 and increases Treg activity in lung cancer.
Cox-2-derived PGE2 has been demonstrated to be important in cancer progression (23). Previous studies have suggested that the majority of PGE2 formed in vivo is derived from Cox-2 (24,25). Urinary PGE-M levels in healthy patients or patients with lung cancer are suppressed significantly by nonselective Cox inhibitors, including aspirin, and by Cox-2-selective inhibitors (25). As the antitumor effects of NSAIDs depend on the inhibition of Cox-2 and subsequent reduction in the quantity of PGE2 produced, urinary PGE-M levels may serve as a valuable intermediate marker of the pharmacological activity of NSAIDs. A previous phase II clinical trial revealed that patients with NSCLC exhibiting complete and partial responses to adjuvant therapy with carboplatin, paclitaxel and celecoxib had significantly decreased urinary PGE-M levels (26). In another phase II clinical trial of combined treatment with celecoxib and docetaxel, patients with recurrent NSCLC with the greatest proportional decline in urinary PGE-M levels exhibited a longer survival time, compared with patients with no change or an increase in urinary PGE-M levels (27). These findings indicate that urinary PGE-M is a potential biomarker for predicting the efficacy of Cox-2 inhibitors in adjuvant therapies.
Depleting Tregs via targeting C-C motif chemokine receptor 4 (CCR4) may be a potential cancer immunotherapy, as CCR4 is highly expressed on the surface of type 2 helper T cells and Tregs (28). Mogamulizumab, a humanized anti-CCR4 monoclonal antibody, has been demonstrated to reduce the numbers of CCR4+ malignant T cells and Tregs in cutaneous T-cell lymphoma (28). However, to the best of our knowledge, no previous studies have examined whether urinary PGE2 may serve as a potential biomarker for predicting the efficacy of Treg-targeting therapy.
There were several limitations of the current study. Firstly, the sample size was small compared with previous studies. Secondly, urinary PGE2 levels were directly quantified in the current study, whilst previous studies have evaluated the urinary PGE-M levels in patients with cancer (11–14). To the best of our knowledge, this is the first study to utilize the direct quantification of urinary PGE2 levels. The direct quantification of PGE2 levels has been revealed to be an unreliable indicator, however, the optimal method for the assessment and use of this marker remains to be established (12).
In the current study, urinary PGE2 levels were not associated with tumor Cox-2 expression levels. Numerous single nucleotide polymorphisms (SNPs) in the Cox-2 gene have been identified, which may contribute to divergent Cox-2 expression levels and PGE2 activities in patients with cancer (29). Compared with patients with esophageal tumors harboring the Cox-2-1195G, carriers of the Cox2-1195AA variant exhibit significantly increased Cox-2 expression levels (29). In 2012 it was reported that Cox-2 SNPs contributed significantly to increased tumor infiltration by Tregs (30). The results from a previous study revealed that the AA genotype group exhibited a significantly higher Treg score compared with the GA/GG group, independent of the intratumoral Cox-2 expression levels (30). The results of the present study revealed that urinary PGE2 levels were positively correlated with tumor Treg expression, but not Cox-2 expression. This may be attributable to SNPs in the Cox-2 gene.
In conclusion, that present study demonstrated that urinary PGE2 levels were positively correlated with intratumoral Treg count in patients with NSCLC. In addition, urinary PGE2 levels may be an improved biomarker, relative to PGE-M, for the prediction of intratumoral Treg expression. Additional studies in larger patient populations are required to evaluate the efficacy of urinary PGE2 as a biomarker in this regard.
Acknowledgements
The authors would like to thank Mrs. Kiyomi Maitani (Department of General Thoracic Surgery, Kawasaki Medical School, Okayama, Japan) for providing technical assistance. This work was supported in part by a research project grant from Kawasaki Medical School (grant no. 26-64).
References
- 1.Asamura H, Goya T, Koshiishi Y, Sohara Y, Eguchi K, Mori M, Nakanishi Y, Tsuchiya R, Shimokata K, Inoue H, et al. A Japanese lung cancer registry study: Prognosis of 13,010 resected lung cancers. J Thorac Oncol. 2008;3:46–52. doi: 10.1097/JTO.0b013e31815e8577. [DOI] [PubMed] [Google Scholar]
- 2.Sawabata N, Miyaoka E, Asamura H, Nakanishi Y, Eguchi K, Mori M, Nomori H, Fujii Y, Okumura M, Yokoi K. Japanese Joint Committee for Lung Cancer Registration: Japanese lung cancer registry study of 11,663 surgical cases in 2004: Demographic and prognosis changes over decade. J Thorac Oncol. 2011;6:1229–1235. doi: 10.1097/JTO.0b013e318219aae2. [DOI] [PubMed] [Google Scholar]
- 3.Shimizu K, Okita R, Nakata M. Clinical significance of the tumor microenvironment in non-small cell lung cancer. Ann Transl Med. 2013;1:20. doi: 10.3978/j.issn.2305-5839.2013.06.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dieu-Nosjean MC, Antoine M, Danel C, Heudes D, Wislez M, Poulot V, Rabbe N, Laurans L, Tartour E, de Chaisemartin L, et al. Long-term survival for patients with non-small-cell lung cancer with intratumoral lymphoid structures. J Clin Oncol. 2008;26:4410–4417. doi: 10.1200/JCO.2007.15.0284. [DOI] [PubMed] [Google Scholar]
- 5.Al-Shibli K, Al-Saad S, Donnem T, Persson M, Bremnes RM, Busund LT. The prognostic value of intraepithelial and stromal innate immune system cells in non-small cell lung carcinoma. Histopathology. 2009;55:301–312. doi: 10.1111/j.1365-2559.2009.03379.x. [DOI] [PubMed] [Google Scholar]
- 6.Dai F, Liu L, Che G, Yu N, Pu Q, Zhang S, Ma J, Ma L, You Z. The number and microlocalization of tumor-associated immune cells are associated with patient's survival time in non-small cell lung cancer. BMC Cancer. 2010;10:220. doi: 10.1186/1471-2407-10-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Takanami I, Takeuchi K, Naruke M. Mast cell density is associated with angiogenesis and poor prognosis in pulmonary adenocarcinoma. Cancer. 2000;88:2686–2692. doi: 10.1002/1097-0142(20000615)88:12<2686::AID-CNCR6>3.3.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 8.Al-Shibli KI, Donnem T, Al-Saad S, Persson M, Bremnes RM, Busund LT. Prognostic effect of epithelial and stromal lymphocyte infiltration in non-small cell lung cancer. Clin Cancer Res. 2008;14:5220–5227. doi: 10.1158/1078-0432.CCR-08-0133. [DOI] [PubMed] [Google Scholar]
- 9.Wakabayashi O, Yamazaki K, Oizumi S, Hommura F, Kinoshita I, Ogura S, Dosaka-Akita H, Nishimura M. CD4+ T cells in cancer stroma, not CD8+ T cells in cancer cell nests, are associated with favorable prognosis in human non-small cell lung cancers. Cancer Sci. 2003;94:1003–1009. doi: 10.1111/j.1349-7006.2003.tb01392.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shimizu K, Nakata M, Hirami Y, Yukawa K, Maeda A, Tanemoto K. Tumor-infiltrating Foxp3+ regulatory T cells are correlated with cyclooxygenese-2 expression and are associated with recurrence in resected non-small cell lung cancer. J Thorac Oncol. 2010;5:585–590. doi: 10.1097/JTO.0b013e3181d60fd7. [DOI] [PubMed] [Google Scholar]
- 11.Ferretti A, Flanagan VP, Roman JM. Quantitative analysis of 11 alpha-hydroxy-9,15-dioxo-2,3,4,5,20-pentanor-19-carboxyprostanoic acid, the major urinary metabolite of E prostaglandins in man. Anal Biochem. 1983;128:351–358. doi: 10.1016/0003-2697(83)90385-8. [DOI] [PubMed] [Google Scholar]
- 12.Wang D, DuBois RN. Urinary PGE-M: A promising cancer biomarker. Cancer Prev Res (Phila) 2013;6:507–510. doi: 10.1158/1940-6207.CAPR-13-0153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cai Q, Gao YT, Chow WH, Shu XO, Yang G, Ji BT, Wen W, Rothman N, Li HL, Morrow JD, Zheng W. Prospective study of urinary prostaglandin E2 metabolite and colorectal cancer risk. J Clin Oncol. 2006;24:5010–5016. doi: 10.1200/JCO.2006.06.4931. [DOI] [PubMed] [Google Scholar]
- 14.Dong LM, Shu XO, Gao YT, Milne G, Ji BT, Yang G, Li HL, Rothman N, Zheng W, Chow WH, Abnet CC. Urinary prostaglandin E2 metabolite and gastric cancer risk in the Shanghai women's health study. Cancer Epidemiol Biomarkers Prev. 2009;18:3075–3078. doi: 10.1158/1055-9965.EPI-09-0680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goldstraw P, Crowley J, Chansky K, Giroux DJ, Groome PA, Rami-Porta R, Postmus PE, Rusch V, Sobin L. International Association for the Study of Lung Cancer International Staging Committee; Participating Institutions: The IASLC lung cancer staging project: Proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM Classification of malignant tumours. J Thorac Oncol. 2007;2:706–714. doi: 10.1097/JTO.0b013e31812f3c1a. [DOI] [PubMed] [Google Scholar]
- 16.Edelman MJ, Watson D, Wang X, Morrison C, Kratzke RA, Jewell S, Hodgson L, Mauer AM, Gajra A, Masters GA, et al. Eicosanoid modulation in advanced lung cancer: Cyclooxygenase-2 expression is a positive predictive factor for celecoxib+ chemotherapy -cancer and leukemia group B trial 30203. J Clin Oncol. 2008;26:848–855. doi: 10.1200/JCO.2007.13.8081. [DOI] [PubMed] [Google Scholar]
- 17.Perrone G, Ruffini PA, Catalano V, Spino C, Santini D, Muretto P, Spoto C, Zingaretti C, Sisti V, Alessandroni P, et al. Intratimoural FOXP3-positive regulatory T cells are associated with adverse prognosis in radically resected gastric cancer. Eur J Cancer. 2008;44:1875–1882. doi: 10.1016/j.ejca.2008.05.017. [DOI] [PubMed] [Google Scholar]
- 18.Curiel TJ. Tregs and rethinking cancer immunotherapy. J Clin Invest. 2007;117:1167–1174. doi: 10.1172/JCI31202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim JM, Rudensky A. The role of the transcription factor Foxp3 in the development of regulatory T cells. Immunol Rev. 2006;212:86–98. doi: 10.1111/j.0105-2896.2006.00426.x. [DOI] [PubMed] [Google Scholar]
- 20.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–1061. doi: 10.1126/science.1079490. [DOI] [PubMed] [Google Scholar]
- 21.Petersen RP, Campa MJ, Sperlazza J, Conlon D, Joshi MB, Harpole DH, Jr, Patz EF., Jr Tumor infiltrating Foxp3+ regulatory T-cells are associated with reccurence in pathologic stage l NSCLC patients. Cancer. 2006;107:2866–2872. doi: 10.1002/cncr.22282. [DOI] [PubMed] [Google Scholar]
- 22.Sharma S, Yang SC, Zhu L, Reckamp K, Gardner B, Baratelli F, Huang M, Batra RK, Dubinett SM. Tumor cyclooxygenase-2/prostaglandin E2-dependent promotion of FOXP3 expression and CD4+CD25+ T regulatory cell activities in lung cancer. Cancer Res. 2005;65:5211–5220. doi: 10.1158/0008-5472.CAN-05-0141. [DOI] [PubMed] [Google Scholar]
- 23.Wang D, Dubois RN. Eicosanoids and cancer. Nat Rev Cancer. 2010;10:181–193. doi: 10.1038/nrc2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morris PG, Zhou XK, Milne GL, Goldstein D, Hawks LC, Dang CT, Modi S, Fornier MN, Hudis CA, Dannenberg AJ. Increased levels of urinary PGE-M, a biomarker of inflammation, occur in association with obesity, aging and lung metastases in patients with breast cancer. Cancer Prev Res (Philla) 2013;6:428–436. doi: 10.1158/1940-6207.CAPR-12-0431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Murphey LJ, Williams MK, Sanchez SC, Byrne LM, Csiki I, Oates JA, Johnson DH, Morrow JD. Quantification of the major urinary metabolite of PGE2 by a liquid chromatographic/mass spectrometric assay: Determination of cyclooxygenase-specific PGE2 synthesis in healthy humans and those with lung cancer. Anal Biochem. 2004;334:266–275. doi: 10.1016/j.ab.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 26.Mutter R, Lu B, Carbone DP, Csiki I, Moretti L, Johnson DH, Morrow JD, Sandler AB, Shyr Y, Ye F, Choy H. A phase II study of celecoxib in combination with paclitaxel, carboplatin and radiotherapy for patients with inoperable stage IIIA/B non-small cell lung cancer. Clin Cancer Res. 2009;15:2158–2165. doi: 10.1158/1078-0432.CCR-08-0629. [DOI] [PubMed] [Google Scholar]
- 27.Csiki I, Morrow JD, Sandler A, Shyr Y, Oates J, Williams MK, Dang T, Carbone DP, Johnson DH. Targeting cyclooygenase-2 in recurrent non-small cell lung cancer: A phase II trial of celecoxib and docetaxel. Clin Cancer Res. 2005;11:6634–6640. doi: 10.1158/1078-0432.CCR-05-0436. [DOI] [PubMed] [Google Scholar]
- 28.Kurose K, Ohue Y, Sato E, Yamauchi A, Eikawa S, Isobe M, Nishio Y, Uenaka A, Oka M, Nakayama E. Increase in activated Treg in TIL in lung cancer and in vitro depletion of Treg by ADCC using an antihuman CCR4 mAb (KM2760) J Thorac Oncol. 2015;10:74–83. doi: 10.1097/JTO.0000000000000364. [DOI] [PubMed] [Google Scholar]
- 29.Zhang X, Miao X, Tan W, Ning B, Liu Z, Hong Y, Song W, Guo Y, Zhang X, Shen Y, et al. Identification of functional genetic variants in cyclooxygenase-2 and their association with risk of esophageal cancer. Gastroenterology. 2005;129:565–576. doi: 10.1053/j.gastro.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 30.Yukawa T, Shimizu K, Maeda A, Yasuda K, Saisho S, Okita R, Nakata M. Cyclooxygenaze-2 genetic variants influence intratumoral infiltration of FoxP3-positive regulatory T cells in non-small cell lung cancer. Oncol Rep. 2015;33:74–80. doi: 10.3892/or.2014.3561. [DOI] [PubMed] [Google Scholar]


