Abstract
Emerging evidence has suggested that pancreatic adenocarcinoma is sustained by pancreatic cancer stem cells. The present study aimed to investigate the expression patterns of the pancreatic cancer stem cell surface markers cluster of differentiation CD44 and CD24 in a pancreatic adenocarcinoma cell line, and to investigate the possible mechanisms for their radiation resistance. Flow cytometry was used to analyze the expression patterns of CD44 and CD24 in the pancreatic adenocarcinoma PANC-1 cell line. In addition, a multi-target click model was used to fit cell survival curves and determine the sensitizer enhancement ratio. The apoptosis and cycle distribution of the four cell subsets was determined using flow cytometry, and the level of reactive oxygen species (ROS) was determined using the 2′,7′-dichlorofluorescin diacetate probe. The present results identified that the ratios of CD44+ and CD24+ in the sorted PANC-1 cell line were 92.0 and 4.7%, respectively. Prior to radiation, no statistically significant differences were observed among the four groups. Following treatment with 6 MV of X-rays, the rate of apoptosis was decreased in the CD44+CD24+ group compared with other subsets. The percentage of G0/G1 cells was highest in the CD44+CD24+ group compared with the three other groups, which exhibited increased radiosensitivity. In addition, the level of ROS in the CD44+CD24+ group was reduced compared with the other groups. In summary, the results of the present study indicated that CD44+CD24+ exhibited stem cell properties. The lower level of ROS and apoptosis in CD44+CD24+ cells may contribute to their resistance to radiation in pancreatic adenocarcinoma.
Keywords: pancreatic adenocarcinoma, PANC-1, stem cell, reactive oxygen species, radiosensitivity
Introduction
Pancreatic ductal adenocarcinoma (PDAC) is one of the most common malignant neoplasms of the pancreas and presents with poor prognosis (5-year survival rate, <5%) (1). Chemoradiation is the conventional option for patients with PDAC. However, due to the inherent chemoresistance and radioresistance of PDAC, such combined modality therapy consistently fails to improve outcomes (2).
Cancer stem cells (CSCs) are capable of unlimited self-renewal, and through asymmetric division, they give rise to further differentiated cells. CSCs are resistant to chemotherapy and radiation therapy, compared to differentiated cells; a number of previous studies revealed that tumor recurrence or metastasis following anticancer treatment could be attributed to CSCs (3–5). The existence of cancer stem cells was first shown in the context of acute myelogenous leukemia, and subsequently verified in breast and brain tumors. In 2007, Li et al (3) reported that the cluster of differentiation (CD)44+CD24+ epithelial-specific antigen+ pancreatic cancer cells exhibited the stem cell properties of self-renewal, the ability to produce differentiated progeny and increased expression of the developmental signaling molecule sonic hedgehog. These cells exhibited the following main characteristics: Tumorigenic capacity; specific molecular markers; and responsibility for the maintenance of tumor growth and resistant to chemo- or radiation therapy. Dou et al (6) used the cell-surface markers CD44+, CD24+ and CD133+ to identify cancer stem-like cells in murine melanoma B16F10 cells, and revealed that CD44+CD24+CD133+ cells exhibited biological properties of cancer stem-like cells and behaved similarly to CSCs. In addition, previous studies (7,8) identified that chemoradiation resistance in PDAC cells may be linked to pancreatic CSCs (PCSCs). Therefore, understanding the nascency and regulation of PCSCs may be critical for the identification of more effective treatments for patients with PDAC.
Reactive oxygen species (ROS) regulate a broad array of signal transduction pathways in multiple biological processes, including cell growth, differentiation, gene expression and apoptosis. ROS production contributes to tumor cell apoptosis following exposure to infrared and other stressors, including high glucose, angiotensin and tumor necrosis factor-α (9).
In the present study, PANC-1 cells were isolated and sorted into CD44+CD24+, CD44−CD24+, CD44+CD24− and CD44−CD24− using flow cytometry. The sensitizer enhancement ratio (SER) was then examined in the four subsets. At the same time, the effect of radiation on cell apoptosis, cycle distribution and the level of intracellular ROS was examined. In addition, it was also investigated whether ROS and cell cycle were able to affect radioresistance.
The results demonstrated that decreased levels of ROS and apoptosis in CD44+CD24+ cells may contribute to their resistance to radiation.
Materials and methods
Reagents
Dulbecco's modified Eagle's medium (DMEM), DMEM: Nutrient mixture F-12 (DMEM/F-12) and B-27 supplement were purchased from Gibco (Thermo Fisher Scientific, Inc., Waltham, MA, USA). Annexin V-fluorescein isothiocyanate (FITC) and propidium iodide (PI) were purchased from Nanjing KeyGen Biotech. Co. Ltd. (Nanjing, China). Fetal bovine serum (FBS) was purchased from Zhejiang Tianhang Biological Technology Co., Ltd. (Huzhou, China). Trypsin was purchased from Sigma-Aldrich (Merck KGaA, Darmstadt, Germany). Anti-human CD24 (cat. no. 173–820) and FITC-anti-human CD44 (cat. no. 193–040) were purchased from Ancell Corporation (Bayport, MN, USA). The 2′,7′-dichlorofluorescin diacetate (DCFH-DA) probe was purchased from Sigma-Aldrich (Merck KGaA). The Cell Lab Quanta SC flow cytometer was purchased from Beckman Coulter, Inc. (Brea, CA, USA). Medical linear accelerators were purchased from Siemens AG (Munich, Germany).
Cell culture
The human pancreatic cancer PANC-1 cell line was purchased from the Cell Bank of the Institute of Biochemistry and Cell Biology, Shanghai Institutes for Biological Sciences (Shanghai, China). Cells were cultured in DMEM supplemented with 10% FBS and penicillin/streptomycin at 37°C in a humidified 5% CO2 atmosphere.
Radiation
Cells were seeded onto 6-well tissue culture plates and incubated for 12 h as described previously, then treated with a single dose of radiation with 6 MV X-ray at room temperature. The initial dose rate was 300 cGy/min (SSD, 100 cm; gantry, 0°; and radiation field, 15×15 cm).
Flow cytometric analysis and fluorescence-activated cell sorting (FACS)
Cells in the exponential growth phase were dissociated by trypsin-EDTA solution (trypsin, 0.25%; EDTA, 0.02%) for 2–5 min at 37°C. Cells were then transferred to a 5-ml tube, washed twice with PBS and 2% heat-inactivated calf serum (Zhejiang Tianhang Biological Technology Co., Ltd., Huzhou, China), centrifuged for 5 min at 800 × g at 37°C, re-suspended in 100 µl (per 106 cells) of PBS, and counted by flow cytometry. The previously described anti-human CD24 or FITC anti-human CD44 antibodies were diluted to 1:100, added and incubated for 30 min at 4°C, and then washed twice with PBS. FACS was performed, and data were analyzed with the Cell Quest software (version 3.0; BD Biosciences, Franklin Lakes, NJ, USA). Using forward and side scatter profiles, debris and dead cells were gated out. Cells were routinely sorted twice and reanalyzed for purity. CD44+CD24+, CD44−CD24+, CD44+CD24− and CD44−CD24− were obtained. Cells were then cultured in DMEM/F-12 supplemented with FBS and penicillin/streptomycin/B-27 supplement at 37°C in a humidified 5% CO2 atmosphere.
Clonogenic assay
Cells in the exponential growth phase were seeded onto 6-well tissue culture plates (105 cells/well) with triplicate repeats for each cell group. Following 24 h, cells were treated with a single dose of radiation (0, 2, 4, 6 or 8 Gy) at room temperature, then incubated for 14 days without changing the culture medium. Cells were then fixed with methanol and stained with 0.05% crystal violet (Nanjing KeyGen Biotech Co., Ltd.), according to the manufacturer's protocol. The number of colonies with >50 cells were counted under a dissecting microscope (magnification, ×400). Cell survival was determined by a colony formation assay. The plating efficiency (PE) and survival fraction (SF) were calculated as follows: PE (%) = (number of colonies/inoculating cell number) × 100; SF = number of colonies counted [cells seeded × (PE/100)]. All experiments were repeated three times. According to the target model [S = 1 - (1 - eD/D0 N], in which S was the cell survival rate, D was the dose, D0 was the mean lethal dose and N was the extrapolation number), cell survival curves were drawn using GraphPad Prism version 6 software (GraphPad Software, Inc., La Jolla, CA, USA). The SER was calculated as SER = Dq (CD44+CD24+)/Dq (CD44+CD24−, CD44−CD24+, CD44−CD24−), where Dq was the quasi-threshold dose (Dq = D0 × lnN), as previously described (10).
Cell cycle and apoptosis analysis
The sorted cells were exposed to radiation dosages (2 Gy). The cells were collected following 48 h of radiation. For the detection of apoptotic cells, the cells were trypsinized and stained with acridine orange (Nanjing KeyGen Biotech Co., Ltd.), and the cells were observed and counted under a fluorescence microscope (magnification, ×400). The cells used for the apoptosis analysis were stained with PI and Annexin V. The cells used for cell cycle analysis were stained with PI subsequent to ethanol fixation. Each analysis was performed four times.
Measurement of intracellular ROS
The sorted cells were exposed to radiation dosages (2 Gy). The cells were collected following 48 h of radiation. The production of intracellular ROS was measured by performing flow cytometry using the oxidation-sensitive probe DCFH-DA. Briefly, 10 mM DCFH-DA stock solution (in methanol) was diluted 4,000-fold in cell culture medium without serum or other additives to yield a 2.5 mM working solution. Following the exposure of human umbilical endothelial cells to silica nanoparticles for 3 and 24 h, respectively, the cells in 6-well plates were washed twice with PBS and incubated in 2 ml working solution of DCFH-DA at 37°C in the dark for 30 min. The cells were then washed twice with cold PBS and re-suspended in the PBS for analysis of intracellular ROS by FACS. Experiments were repeated four times.
Statistical analysis
All analyses were performed using SPSS 16.0 (SPSS, Inc., Chicago, IL, USA). Data were expressed as the mean ± standard deviation. Statistically significant differences were determined by Student's t-test. P<0.05 was considered to indicate a statistically significant difference.
Results
Presence of CD44 and CD24 on the cell surface of pancreatic carcinoma cell lines
Flow cytometric analysis was used to determine the presence of CD44 and CD24 on the cell surface of the pancreatic adenocarcinoma PANC-1 cell line. A total of 92% of cells expressed the cell surface marker CD44, and 4.7% expressed CD24; CD44+CD24+, CD44+CD24−, CD44−CD24+ and CD44−CD24− were 0.6±0.2, 89.3±2.6, 4.1±1.3 and 6.0±1.7%, respectively. Typical samples are shown in Fig. 1.
Figure 1.
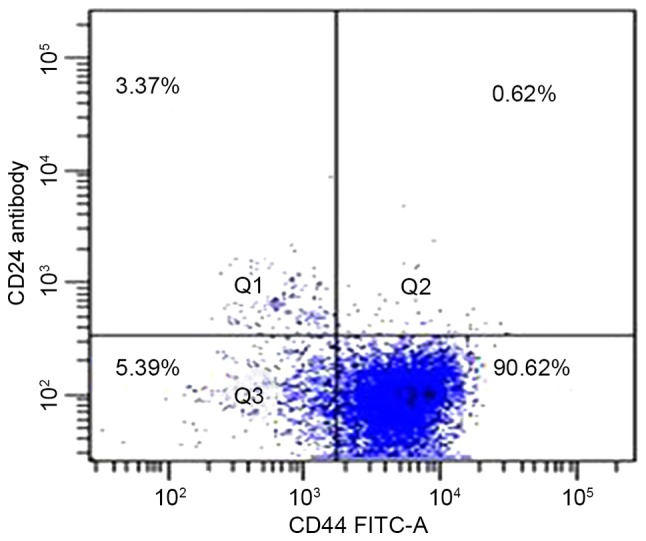
Expression levels of CD44 and CD24 on the cell surface of pancreatic carcinoma cell lines. A total of 92% of cells expressed the cell surface marker CD44, and 4.7% expressed CD24; while CD44+CD24+, CD44+CD24−, CD44−CD24+ and CD44−CD24− were 0.6±0.2, 89.3±2.6, 4.1±1.3 and 6.0±1.7%, respectively. CD, cluster of differentiation; FITC, fluorescein isothiocyanate.
Apoptosis of sorted cells
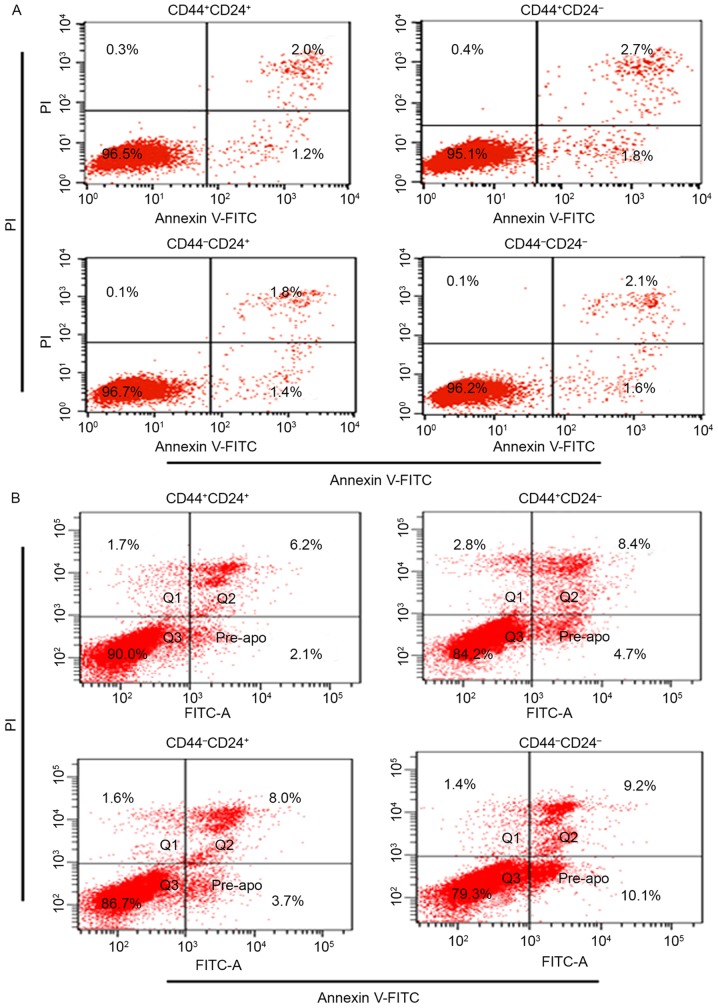
Prior to radiation, the percentage of apoptosis for CD44+CD24+ (3.3±0.6%), CD44+CD24− (4.0±0.8%), CD44−CD24+ (3.4±0.7%) and CD44−CD24− (3.5±0.8) was not significantly different (P>0.05; Fig. 2A).
Figure 2.
Apoptosis percentage for sorted cells. (A) Apoptosis percentage for sorted cells prior to radiation: CD44+CD24+ (3.3±0.6%); CD44+CD24− (4.0±0.8%); CD44−CD24+ (3.4±0.7%); and CD44−CD24− (3.5±0.8%). (B) Apoptosis percentage for sorted cells following 48 h of radiation: CD44+CD24+ (6.8±1.1%); CD44+CD24− (10.4±2.7%); CD44−CD24+ (13±3.1%); and CD44−CD24− (26.3±2.4%). Each analysis was performed four times. CD, cluster of differentiation; FITC, fluorescein isothiocyanate; PI, propidium iodide.
Following 48 h of radiation, the results revealed that radiation induced a lower percentage of apoptosis in CD44+CD24+ when compared with others (6.8±1.1 vs. 10.4±2.7%, P<0.01; 6.8±1.1 vs. 13±3.1%, P<0.01; 6.8±1.1 vs. 26.3±2.4%, P<0.01). The differences were significant (Fig. 2B).
Clonogenic survival rates
Sorted cells were exposed to various radiation dosages (0, 2, 4, 6 and 8 Gy) and their survival fractions were measured based on colony formation. The dose-response curves for the cell-killing effects of radiation are shown in Fig. 3. The survival fractions of CD44−CD24− were decreased exponentially as the dose of radiation increased. The SERs for CD44+CD24−, CD44−CD24+ and CD44−CD24− were 1.61, 1.81 and 1.94, respectively.
Figure 3.
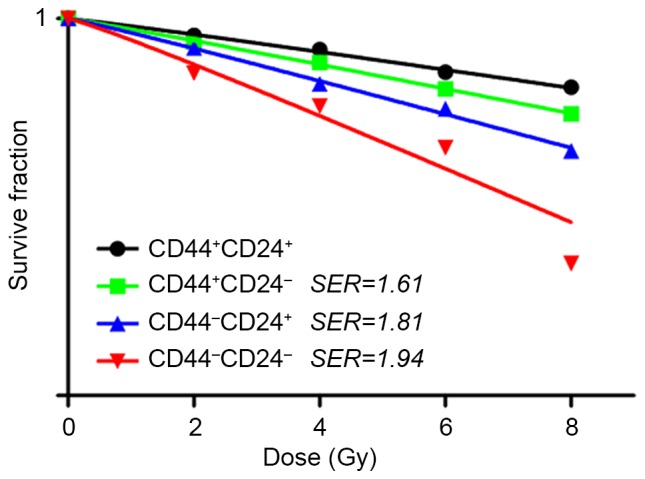
Cell survival curves. Sorted cells in the exponential growth phase were seeded onto 6-well tissue culture plates (105 cells/well) with triplicate repeats for each cell group. Following 24 h, cells were treated with a single dose of radiation (0, 2, 4, 6 or 8 Gy) at room temperature, and then incubated for 14 days without changing the culture medium. Their survival fractions were measured based on colony formation. CD, cluster of differentiation; SER, sensitizer enhancement ratio.
Cell cycle analysis
Prior to radiation, the percentage of G0/G1 was highest in CD44+CD24+ (63.8±1.7 vs. 58.2±2.2%, P<0.01; 63.8±1.7 vs. 53.4±2.7%, P<0.01; 63.8±1.7 vs. 50.1±3.4%, P<0.01; Table I).
Table I.
Cell cycle distribution for four types of cells.
| Cell cycle distribution | ||||||
|---|---|---|---|---|---|---|
| Sorted cells | G0/G1 phase | S phase | G2/M phase | |||
| Radiation | − | + | − | + | − | + |
| CD44+CD24+ | 63.8±1.7 | 67.2±3.4a | 20.2±3.3 | 18.9±2.1 | 16.0±1.6 | 13.9±2.5 |
| CD44+CD24− | 58.2±2.2b | 57.7±2.9 | 24.1±4.2 | 22.2±3.1b | 17.7±2.4b | 18.1±3.7 |
| CD44−CD24+ | 53.4±2.7 | 50.3±4.6 | 25.1±1.9a | 23.7±1.6b | 21.5±2.7 | 26.0±2.3a |
| CD44−CD24− | 50.1±3.4 | 42.8±2.7b | 25.8±2.5b | 27.7±1.9 | 24.1±3.8 | 29.5±4.1 |
Data are presented as the mean ± standard deviation.
P<0.01
P<0.05 compared with others. CD, cluster of differentiation.
Following 48 h of radiation, the percentage of G0/G1 was also highest in CD44+CD24+ (67.2±3.4 vs. 57.7±2.9%, P<0.01; 67.2±3.4 vs. 50.3±4.6%, P<0.01; 67.2±3.4 vs. 42.8±2.7%, P<0.01). The difference was significant (Table I).
Intracellular ROS
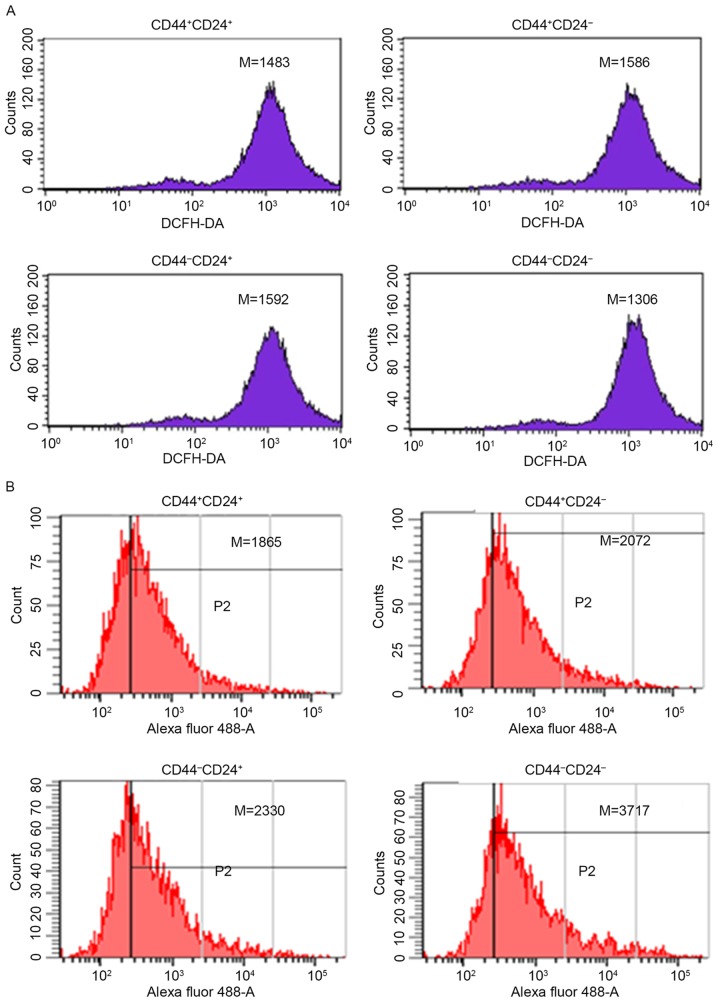
Prior to radiation, the level of intracellular ROS was lowest in CD44−CD24− (1,306±205 vs. 1,483±172, P<0.05; 1,306±196 vs. 1,586±201, P<0.05; 1,306±216 vs. 1,592±216, P<0.05; Fig. 4A).
Figure 4.
Level of intracellular ROS for sorted cells. (A) The level of intracellular ROS for sorted cells prior to radiation: CD44+CD24+ (1,483±172); CD44+CD24− (1,586±201); CD44−CD24+ (1,592±216); and CD44−CD24− (1,306±205). (B) The level of intracellular ROS for sorted cells following 48 h of radiation: CD44+CD24+ (1,865±315); CD44+CD24− (2,072±167); CD44−CD24+ (2,330±208); and CD44−CD24− (3,717±236). Experiments were repeated four times. ROS, reactive oxygen species; CD cluster of differentiation; M, median; DCFH-DA, 2′,7′-dichlorofluorescin diacetate.
Following 48 h of radiation, the level of intracellular ROS was lowest in CD44+CD24+ (1,865±315 vs. 2,072±167, P<0.01; 1,865±315 vs. 2,330±208, P<0.01; 1,865±315 vs. 3,717±236, P<0.01; Fig. 4B).
Discussion
Pancreatic cancer is a devastating disease with a median survival of only ~6 months (11,12). Pancreatic cancer is resistant to almost all conventional therapies. It is imperative to understand the unique logical characteristics of pancreatic cancer and the scientific mechanisms for its obstinate malignancy. The cancer stem cell hypothesis is important to improve understanding of the intrinsic biological characteristics of pancreatic cancer. This indicates that tumor progression is initiated and driven by a small subset of undifferentiated cells with the ability of self-renewal and differentiation into different integrated and heterogeneous tumor populations (13,14).
Emerging evidence (14–21) has indicated that pancreatic adenocarcinoma is hierarchically organized and sustained by a distinct subpopulation of CSCs, which are responsible for tumor growth, metastasis and resistance to therapy. In addition, elimination of these cells is possible and may subsequently improve the effect of clinical treatment. However, in previous studies, direct evidence for the existence of CSCs in human pancreatic cancer was not consistent (22,23). To isolate and identify pancreatic cancer stem cells more efficiently, new methods are required for additional studies.
In the present study, the PANC-1 cells were isolated and sorted into CD44+CD24+, CD44−CD24+, CD44+CD24− and CD44−CD24− by flow cytometry. The results revealed that 92% of cells expressed CD44 and 4.7% expressed CD24. The survival fractions of CD44−CD24− decreased exponentially as the dose of radiation increased. The SERs for CD44+CD24−, CD44−CD24+ and CD44−CD24− were 1.61, 1.81 and 1.94, respectively. Prior to radiation, no significant differences in apoptosis were observed among the four groups. However, the results indicated that radiation induced a significantly lower percentage of apoptosis in CD44+CD24+ when compared with others. Prior to or following radiation, the percentage of G0/G1 cells was significantly highest in CD44+CD24+, indicating that the relative stationary cell cycle is critical for radiation resistance.
In addition, the level of intracellular ROS was revealed to be lowest in CD44−CD24− prior to radiation. However, following radiation, the level of intracellular ROS was lowest in CD44+CD24+. In contrast to general cancer cells in which ROS levels are increased, CSCs exhibited reduced levels of ROS. The maintenance of low ROS levels is essential to preserve CSC self-renewal and stemness. Ishimoto et al (24) revealed that CD44+ gastrointestinal CSCs exhibited an enhanced capacity of glutathione synthesis and defense against ROS by activation of the cystine-glutamate exchange transporter. These properties contribute to the radioresistance of stem cells, since radiation exerts a cytotoxic effect through the generation of free radicals, and the critical mediator ROS is decreased in stem cells (25). CSCs are more radioresistant compared with non-CSCs, and this is partly attributable to the lower ROS levels and enhanced ROS defenses observed in CSCs (26).
In summary, the results from the present study have significant implications for the treatment of pancreatic cancer. The present study demonstrated that CD44+CD24+ pancreatic cancer cells possessed stem cell properties. These cells are more resistant to standard therapies used to treat pancreatic cancer, including radiotherapy. An improved understanding of pancreatic cancer stem cells may not only affect the ability to improve understanding of current therapeutics, but expression studies of pancreatic cancer stem cells may help in the identification of novel therapeutic targets.
Acknowledgements
The present study was supported by the Project of Lianyungang City Development Commission (grant no. SH1218).
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Gutt R, Liauw SL, Weichselbaum RR. The role of radiotherapy in locally advanced pancreatic carcinoma. Nat Rev Gastroenterol Hepatol. 2010;7:437–447. doi: 10.1038/nrgastro.2010.98. [DOI] [PubMed] [Google Scholar]
- 3.Li CW, Heidt DG, Dalerba P, Burant CF, Zhang L, Adsay V, Wicha M, Clarke MF, Simeone DM. Identification of pancreatic cancer stem cells. Cancer Res. 2007;67:1030–1037. doi: 10.1158/0008-5472.CAN-06-2030. [DOI] [PubMed] [Google Scholar]
- 4.Wang P, Zhang J, Zhang L, Zhu Z, Fan J, Chen L, Zhuang L, Luo J, Chen H, Liu L, et al. MicroRNA 23b regulates autophagy associated with radioresistance of pancreatic cancer cells. Gastroenterology. 2013;145:1133–1143.e12. doi: 10.1053/j.gastro.2013.07.048. [DOI] [PubMed] [Google Scholar]
- 5.Maugeri-Saccà M, Vigneri P, De Maria R. Cancer stem cells and chemosensitivity. Clin Cancer Res. 2011;17:4942–4947. doi: 10.1158/1078-0432.CCR-10-2538. [DOI] [PubMed] [Google Scholar]
- 6.Dou J, Pan M, Wen P, Li Y, Tang Q, Chu L, Zhao F, Jiang C, Hu W, Hu K, Gu N. Isolation and identification of cancer stem-like cells from murine melanoma cell lines. Cell Mol Immunol. 2007;4:467–472. [PubMed] [Google Scholar]
- 7.Du ZY, Qin RY, Wei CF, Wang M, Shi C, Tian R, Peng C. Pancreatic cancer cells resistant to chemoradiotherapy rich in ‘stem-cell-like’ tumor cells. Dig Dis Sci. 2011;56:741–750. doi: 10.1007/s10620-010-1340-0. [DOI] [PubMed] [Google Scholar]
- 8.Al-Assar O, Demiciorglu F, Lunardi S, Gaspar-Carvalho MM, McKenna WG, Muschel RM, Brunner TB. Contextual regulation of pancreatic cancer stem cell phenotype and radioresistance by pancreatic stellate cells. Radiother Oncol. 2014;111:243–251. doi: 10.1016/j.radonc.2014.03.014. [DOI] [PubMed] [Google Scholar]
- 9.Tothova Z, Kollipara R, Huntly BJ, Lee BH, Castrillon DH, Cullen DE, McDowell EP, Lazo-Kallanian S, Williams IR, Sears C, et al. FoxOs are critical mediators of hematopoietic stem cell resistance to physiologic oxidative stress. Cell. 2007;128:325–339. doi: 10.1016/j.cell.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 10.Xia S, Zhao Y, Yu S, Zhang M. Activated PI3K/Akt/COX-2 pathway induces resistance to radiation in human carvival cancer HeLa cells. Cancer Biother Radiopharm. 2010;25:317–323. doi: 10.1089/cbr.2009.0707. [DOI] [PubMed] [Google Scholar]
- 11.Philip PA, Mooney M, Jaffe D, Eckhardt G, Moore M, Meropol N, Emens L, O'Reilly E, Korc M, Ellis L, et al. Consensus report of the national cancer institute clinical trials planning meeting on pancreas cancer treatment. J Clin Oncol. 2009;27:5660–5669. doi: 10.1200/JCO.2009.21.9022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang J, Dhakal I, Yan H, Phillips M, Kesteloot H. SEER Cancer Registries: Trends in pancreatic cancer incidence in nine SEER cancer registries, 1973–2002. Ann Oncol. 2007;18:1268–1279. doi: 10.1093/annonc/mdm123. [DOI] [PubMed] [Google Scholar]
- 13.Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–111. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- 14.Gellmini S, Mangoni M, Serio M, Romagnani P, Lazzeri E. The critical role of SDF-1/CXCR4 axis in cancer and cancer stem cells metastasis. J Endocrinol Invest. 2008;31:809–819. doi: 10.1007/BF03349262. [DOI] [PubMed] [Google Scholar]
- 15.Dirks P. Cancer stem cells: Invitation to a second round. Nature. 2010;466:40–41. doi: 10.1038/466040a. [DOI] [PubMed] [Google Scholar]
- 16.Li YF, Xiao B, Tu SF, Wang YY, Zhang XL. Cultivation and identification of colon cancer stem cell-derived spheres from the Colo205 cell line. Braz J Med Biol Res. 2012;45:197–204. doi: 10.1590/S0100-879X2012007500015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nakanishi Y, Seno H, Fukuoka A, Ueo T, Yamaga Y, Maruno T, Nakanishi N, Kanda K, Komekado H, Kawada M, et al. Dclk1 distinguishes between tumor and normal stem cells in the intestine. Nat Genet. 2013;45:98–103. doi: 10.1038/ng.2481. [DOI] [PubMed] [Google Scholar]
- 18.Chen J, Li YJ, Yu TS, McKay RM, Burns DK, Kernie SG, Parada LF. A restricted cell population propagates glioblastoma growth after chemotherapy. Nature. 2012;488:522–526. doi: 10.1038/nature11287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Machado HL, Kittrell FS, Edwards D, White AN, Atkinson RL, Rosen JM, Medina D, Lewis MT. Separation by cell size enriches for mammary stem cell repopulation activity. Stem Cell Transl Med. 2013;2:199–203. doi: 10.5966/sctm.2012-0121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Driessens G, Beck B, Caauwe A, Simons BD, Blanpain C. Defining the mode of tumour growth by clonal analysis. Nature. 2012;488:527–530. doi: 10.1038/nature11344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hinohara K, Kobayashi S, Kanauchi H, Shimizu S, Nishioka K, Tsuji E, Tada K, Umezawa K, Mori M, Ogawa T, et al. ErbB receptor tyrosine kinase/NF-κB signaling controls mammosphere formation in human breast cancer; Proc Natl Acad Sci USA; 2012; pp. 6584–6589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang P, Wang CY, Gou SM, Wu HS, Liu T, Xiong JX. Isolation and biological analysis of tumor stem cells from pancreatic adenocarcinoma. World J Gastroentero. 2008;14:3903–3907. doi: 10.3748/wjg.14.3903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee HJ, You DD, Choi DW, Choi YS, Kim SJ, Won YS, Moon HJ. Significance of CD133 as a cancer stem cell markers focusing on the tumorigenicity of pancreatic cancer cell lines. J Korean Surg Soc. 2011;81:263–270. doi: 10.4174/jkss.2011.81.4.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ishimoto T, Nagano O, Yae T, Tamada M, Motohara T, Oshima H, Oshima M, Ikeda T, Asaba R, Yagi H, et al. CD44 variant regulates redox status in cancer cells by stabilizing the xCT subunit of system xc(−) and thereby promotes tumor growth. Cancer Cell. 2011;19:387–400. doi: 10.1016/j.ccr.2011.01.038. [DOI] [PubMed] [Google Scholar]
- 25.Schieber MS, Chandel NS. ROS links glucose metabolism to breast cancer stem cell and EMT phenotype. Cancer Cell. 2013;23:265–267. doi: 10.1016/j.ccr.2013.02.021. [DOI] [PubMed] [Google Scholar]
- 26.Rycaj K, Tang DG. Cancer stem cells and radioresistance. Int J Radiat Biol. 2014;90:615–621. doi: 10.3109/09553002.2014.892227. [DOI] [PMC free article] [PubMed] [Google Scholar]