Abstract
Primary small bowel adenocarcinoma (SBA) is a rare cancer for which effective treatment strategies have not yet been established. The results of previous retrospective studies suggest that chemotherapy contributes to a longer survival time in patients with SBA. However, there are few case reports about the efficacy of molecular targeted agent-containing chemotherapy for SBA. In the present study, the treatment and follow-up data of patients with SBA who received chemotherapy with or without molecular targeted agents were retrospectively analyzed. Each patient was treated in one of ten hospitals participating in the Osaka Gut Forum between April 2006 and March 2014. The following factors were evaluated: Age, sex, Eastern Cooperative Oncology Group performance status (PS), tumor location, tumor differentiation, chemotherapy regimen, resection of primary tumor, tumor biomarker expression, distant metastasis, best response under chemotherapy, time to disease progression, subsequent treatments, survival status and treatment toxicity. A total of 27 patients (17 males and 10 females; mean age, 63.4 years old; range, 36–83 years old) received chemotherapy due to non-curative tumor resection, unresectable tumor or post-operative recurrence. The median overall survival time was 14.8 months (range, 2–58 months). A univariate analysis revealed a PS of 0 (P=0.0228) and treatment with platinum-based chemotherapy (P=0.0048) were significant factors for an improved prognosis. An age-adjusted multivariate analysis also revealed that a platinum-based regimen was a significant positive prognostic factor (P=0.0373). Molecular targeted agents were administered to 8 patients, for whom it was their first- or second-line therapy. Among the 17 patients who received oxaliplatin-based chemotherapy as a first-line chemotherapy, a PS of 0 (P=0.0255) and treatment with bevacizumab (P=0.0121) were significant positive prognostic factors. Toxicities higher than Grade 3 occurred in 8/27 patients with SBA; however, serious side effects due to the molecular targeted agents were not experienced. The results of the present study indicate that chemotherapy containing molecular targeted agents is a well-tolerated and effective treatment option for SBA.
Keywords: small bowel adenocarcinoma, bevacizumab, molecular targeted therapy, chemotherapy, overall survival time, progression free survival
Introduction
Primary small bowel adenocarcinoma (SBA) is a rare cancer; 5,420 patients were diagnosed with SBA, accounting for 2.1% of all malignant gastrointestinal tumors, in the United States of America in 2005 (1,2). Early diagnosis of SBA is challenging due to the site of origin; for example, of 217 patients diagnosed with SBA at the University of Texas M. D. Anderson Cancer Center between 1978 and 998, 75 patients (35%) were diagnosed at stage IV (3). However, the use of capsule endoscopy and double balloon enteroscopy (DBE) has enabled the observation of the entire small intestine endoscopically (4–6). In addition, DBE can be used to sample mucosal tissues in order to diagnose SBA (5,6). The incidence of SBA diagnosis is increasing, partially due to the development of these novel methods for diagnosis (7,8). However, delayed diagnosis remains common despite technical advances in endoscopy.
Resection of the primary tumor and regional lymph nodes is the standard treatment for localized SBA. However, the curative resection rate of SBA is 40–60% (9–12). Chemotherapy is performed for unresectable or recurrent SBA. Raghav and Overman (13) reported that the median survival time (MST) of patients with SBA who received systemic chemotherapy was 13 months, which was longer than the MST for patients who received best supportive care (BSC) alone (4 months). The 5-year survival rates of SBA are 40–60% for resected tumors and 15–30% for unresectable tumors (9–12). Prospective evaluations of the efficacy of chemotherapy are rare due to the rarity of SBA. SBA is more similar to colorectal cancer than gastric cancer based on genome-wide DNA copy number aberrations (14); therefore, chemotherapy regimens used to treat colorectal cancer are typically selected for the treatment of SBA. Previous retrospective studies have demonstrated that chemotherapy, including oxaliplatin with fluorouracil and folinic acid (FOLFOX) chemotherapy, and oxaliplatin and capecitabine (CAPOX) chemotherapy, contributes to a longer survival time in patients with SBA (15–19). The overall survival (OS) time of patients who received chemotherapy was 15.1–22.2 months (15–19). Treatment strategies for colorectal cancer have drastically changed in the last 10 years due to the development of new molecular targeted therapies. Molecular targeted therapy has been suggested to be effective for the patients with SBA due to the similarities between SBA and colorectal cancer (20). Few studies have evaluated the efficacy of molecular targeted therapies for SBA (21–23) and there is an urgent need to explore effective strategies to treat SBA. In the present study, clinicopathological factors associated with the effective treatment of SBA, and the efficacy of chemotherapy, including molecular targeted agents for the treatment of SBA, were investigated.
Materials and methods
Patients
The treatment and follow-up data of 27 patients with recurrent, non-curatively resected or unresectable SBA who received chemotherapy between April 2006 and March 2014 in 10 hospitals participating in the Osaka Gut Forum (Table I) were retrospectively analyzed. SBA was defined as histologically diagnosed adenocarcinoma of the duodenum, jejunum or ileum excluding ampullary carcinoma. The present study was conducted in accordance with the Declaration of Helsinki. Ethical approval was obtained from the Institutional Review Boards of all the institutions where patients were recruited as listed in Table I; patients were able to opt out their data from inclusion in the retrospective study.
Table I.
Hospitals included in the present study and the number of patients with small bowel adenocarcinoma from each.
| Hospital | Number of patients |
|---|---|
| Osaka Universitya | 7 |
| Osaka Police Hospitalb | 5 |
| Osaka Rosai Hospitalc | 3 |
| Sumitomo Hospitalb | 3 |
| JCHO Osaka Hospitalb | 3 |
| Saiseikai Senri Hospitala | 2 |
| Kansai Rosai Hospitald | 1 |
| Osaka Medical Center for Cancer | 1 |
| and Cardiovascular Diseasesb | |
| Hyogo Prefectural Nishinomiya Hospitale | 1 |
| Higashiosaka City General Hospitalf | 1 |
Suita
Osaka
Sakai, Osaka
Amagasaki
Nishinomiya, Hyogo
Higashiosaka, Osaka, Japan.
Data collection
The following data were collected from medical records: Patient demographics [age, sex, Eastern Cooperative Oncology Group (ECOG) performance status (PS) (24)], primary tumor locations (duodenum, jejunum, ileum), indications for chemotherapy (post-operative recurrence, non-curative resection, unresectable), adjuvant chemotherapy, histological type (differentiated, undifferentiated), tumor biomarker expression [serum carcinoembryonic antigen (CEA) and carbohydrate antigen (CA) 19–9], metastatic sites (liver and lung), number of metastatic organs including lymph nodes, resection of the primary tumor, chemotherapy agent and radiation therapy. The clinical course of the disease was also investigated as follows: Best response under chemotherapy, time for disease progression, subsequent therapies and survival status. Best responses to chemotherapy were evaluated according to the Response Evaluation Criteria in Solid Tumors (version 1.1) (25). The National Cancer Institute Common Terminology Criteria (version 4.0) (26) were used to evaluate the toxicity of therapeutics. Progression free survival (PFS) was defined as the time from the initiation of chemotherapy until the confirmation of disease progression or mortality from any cause. OS was defined as the time from the initiation of chemotherapy until mortality. Surviving patients were censored on their last follow-up dates. The clinicopathological characteristics of the patients included in the present study are illustrated in Table II.
Table II.
Clinicopathological characteristics of patients with small bowel adenocarcinoma.
| Clinicopathological characteristic | Number of patients (%) |
|---|---|
| Age (<60/≥60) | 10 (37.0)/17 (63.0) |
| Sex (M/F) | 17 (63.0)/10 (37.0) |
| Location of primary tumor (duodenum/jejunum/ileum) | 8 (29.6)/11 (40.7)/8 (29.6) |
| Reasons for chemotherapy (non-curative resection/unresectable/post-operative recurrence) | 9 (33.3)/13 (48.2)/5 (18.5) |
| Adjuvant chemotherapy treatment (yes/no) | 4 (14.8)/23 (85.2) |
| Histological type (differentiated/undifferentiated) | 18 (66.7)/9 (33.3) |
| Performance status (0/1-2) | 19 (70.4)/8 (29.6) |
| CEA (ng/ml), (≤5/>5) | 13 (50.0)/13 (50.0) |
| CA19-9 (U/ml), (≤40/>40) | 15 (60)/10 (40) |
| Liver metastasis (present/absent) | 8 (29.6)/19 (70.4) |
| Lung metastasis (present/absent) | 2 (7.4)/25 (92.6) |
| Number of metastatic organs (1/2/3) | 16 (59.3)/9 (33.3)/2 (7.4) |
| Resection of primary tumor (yes/no), | 15 (55.6)/12 (44.4) |
M, male; F, female; CEA, carcinoembryonic antigen; CA19-9, carbohydrate antigen 19-9.
Chemotherapy regimens
The modified FOLFOX6 (mFOLFOX6) regimen consisted of the following: L-leucovorin (LV; 200 mg/m2), oxaliplatin (85 mg/m2) and bolus 5-fluorouracil (5-FU; 400 mg/m2), followed by infusion of 5-FU (2,400 mg/m2) for 46 h, every 2 weeks. The mFOLFOX6 with bevacizumab regimen included mFOLOFOX6, as described, with 5 mg/kg bevacizumab, every 2 weeks. mFOLFOX6 with cetuximab included an initial dose of 400 mg/m2 cetuximab and 250 mg/m2/week thereafter. The CAPOX regimen was as follows: Oxaliplatin (130 mg/m2) intravenously on day 1 and capecitabine (2,000 mg/m2/day) orally on days 1–14 every 3 weeks. The titanium silicate (TS)-1 ± cisplatin (CDDP) regimen was a follows: TS-1 (80 mg/m2/day) orally on days 1–21 plus CDDP (60 mg/m2) on day 8, every 5 weeks or TS-1 (80 mg/m2/day) orally on days 1–28 every 6 weeks. The 5-FU + LV regimen was as follows: Bolus 5-FU (600 mg/m2) plus LV (250 mg/m2) once a week for 6 weeks, every 8 weeks. The irinotecan (CPT-11) + CDDP regimen consisted of the following: CPT-11 (30 mg/m2) plus CDDP (60 mg/m2) intravenously on days 1 and 15 every 4 weeks. The FOLFILI regimen was as follows: LV (200 mg/m2), CPT-11 (180 mg/m2) and bolus 5-fluorouracil (5-FU; 400 mg/m2), followed by infusion of 5-FU (2,400 mg/m2) for 46 h, every 2 weeks.
Statistical analysis
The median and interquartile ranges are reported for continuous variable, and categorical variables are summarized as frequencies. Differences in the distribution of variables were evaluated using the Kruskal-Wallis test (reason for chemotherapy) or χ2 test (other data). PFS and OS were estimated using the Kaplan-Meier estimator method and compared using the log-rank test. Hazard ratios (HRs) and the corresponding 95% confidence intervals (CIs) for PFS and OS were estimated using multivariate Cox proportional hazards models following stepwise selection of the covariates. Other than the treatment groups, these covariates included the following: Age (<60/≥60 years old), sex (male/female), ECOG PS (continuous variable), primary tumor locations (duodenum/jejunum/ileum), indications for chemotherapy (post-operative recurrence/non-curative resection/unresectable), serum CEA level (≤5/>5 ng/ml), serum CA19-9 level (≤40/>40 ng/ml), liver metastasis (present/absent), lung metastasis (present/absent), number of metastatic organs (continuous variable), resection of primary tumor (present/absent), platinum-containing chemotherapy (yes/no), molecular targeted agents containing chemotherapy (yes/no), and combined-radiation therapy (yes/no). All reported P-values were two sided, and P<0.05 was considered to indicate a statistically significant difference. Statistical analyses were performed using JMP statistical software (version 11.1.1; SAS Institute, Inc., Cary, NC, USA).
Results
Patient clinicopathological characteristics
A total of 27 patients were included in the present study. Patient clinicopathological characteristics are illustrated in Table II. The median age was 63.4 years old (range, 36–83 years old). A total of 17 patients (63.0%) were ≥60 years old and 17 patients (63.0%) were male. The location of the primary tumor was in the duodenum in 8 patients (29.6%), in the jejunum in 11 patients (40.8%) and in the ileum in 8 patients (29.6%). Primary tumors were surgically resected in 15 patients (55.6%). Chemotherapy was introduced due to unresectable tumors in 13 patients (48.2%), non-curative resections in 9 patients (33.3%) and post-operative recurrence in 5 patients (18.5%). Among the patients with non-curative resections, 4 patients (14.9%) received adjuvant chemotherapy. The histological types of SBA were differentiated type in 18 patients (66.7%) and undifferentiated type in 9 patients (33.3%). The PS was 0 in 19 patients (70.4%), 1 in 7 patients (25.9%) and 2 in 1 patient (3.7%). The serum levels of CEA and CA19-9 were measured in 26 and 25 patients, respectively. Elevated levels of serum CEA and CA19-9 were observed in 50% (13/26) and 64% (16/25) of patients, respectively (data not shown). The number of metastatic organs was 1 in 16 patients (59.3%), 2 in 9 patients (33.3%) and 3 in 2 patients (7.4%) (Table II). A total of 8 patients (29.6%) had liver metastases and 2 (7.4%) had lung metastases (Table II).
Chemotherapy regimens and clinical efficacy of first-line chemotherapy
The following first-line chemotherapy regimens were used for patients in the present study: mFOLFOX6 in 14 patients, CAPOX in 4 patients, TS-1 ± CDDP in 7 patients, 5-FU + LV in 1 patient, CPT-11 + CDDP in 1 patient (Table III). A total of 4 patients received mFOLFOX6 with bevacizumab, 1 patient received CAPOX with bevacizumab, 1 patient received mFOLFOX6 with cetuximab and 8 patients received mFOLFOX6 without molecular targeted agents. The clinical efficacy of the first-line chemotherapy was evaluated; 2 patients exhibited a complete response (CR), 8 exhibited a partial response (PR), 12 exhibited stable disease (SD) and 5 exhibited progressive disease (PD). The response rate (RR) and disease control rate (DR) were 37.0 and 81.5%, respectively (Table III).
Table III.
Chemotherapy regimens and efficacy of the first-line chemotherapy treatment for small bowel adenocarcinoma.
| Regimen | Number of patients (%) |
|---|---|
| First-line chemotherapy | |
| mFOLFOX6 | 14 (51.9) |
| CAPOX | 4 (14.8) |
| TS-1 ± CDDP | 7 (25.9) |
| 5-FU + LV | 1 (3.7) |
| CPT-11 + CDDP | 1 (3.7) |
| Platinum-containing 1st line chemotherapy treatment (yes/no) | 24 (88.9)/3 (11.1) |
| Molecular-targeted agent-containing 1st line chemotherapy treatment (yes/no) | 6 (22.2)/21 (77.8) |
| Combined-radiation therapy treatment (yes/no) | 2 (7.4)/25 (92.6) |
| Efficacy of 1st line chemotherapy (CR/PR/SD/PD) | 2 (7.4)/8 (29.6)/12 (44.5)/5 (18.5) |
| Response rate (RR)/Disease control rate (DR) | 10 (37.0%)/22 (81.5%) |
| Molecular-targeted agent-containing 1st or 2nd line chemotherapy treatment (yes/no) | 8 (7, bevacizumab; 1, cetuximab,1) |
| (29.6)/19 (70.4) |
n=27. CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease; mFOLFOX6, oxaliplatin, leucovorin and 5-fluorouracil regimen; CAPOX, oxaliplatin and capecitabine regimen; 5-FU, 5-fluorouracil; CDDP, cisplatin; LV, leucovorin; CPT-11, irinotecan.
Salvage chemotherapy following first-line therapy
A total of 16 patients (59.2%) received second-line chemotherapy and 5 patients (18.5%) received third-line chemotherapy. Molecular targeted agents (bevacizumab or cetuximab) were administered as the second-line therapy in 5 patients, as the third-line therapy in 4 patients, as the fourth-line therapy in 4 patients and as the sixth-line therapy in 1 patient (data not shown).
PFS and OS
At the time of analysis, 17 patients (63.0%) had succumbed to their disease with a median follow-up of 21.3 months following initiation of first-line chemotherapy. Kaplan-Meier estimator curves for PFS and OS were calculated. The median PFS of first-line chemotherapy was 7.2 months (95% CI, 6.2–11.2 months; Fig. 1). The MST for all patients in the OS curve was 14.8 months (95% CI, 9.3–45.1 months; Fig. 2).
Figure 1.
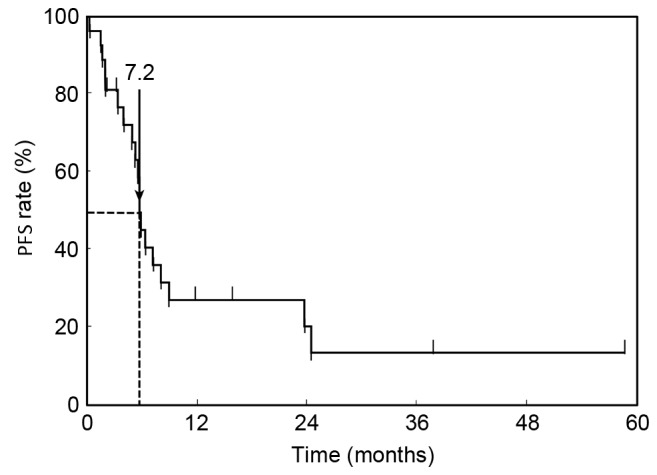
Cumulative PFS curve. The median PFS of first-line chemotherapy was 7.2 months (95% confidence interval, 6.2–11.2 months). PFS, progression-free survival.
Figure 2.
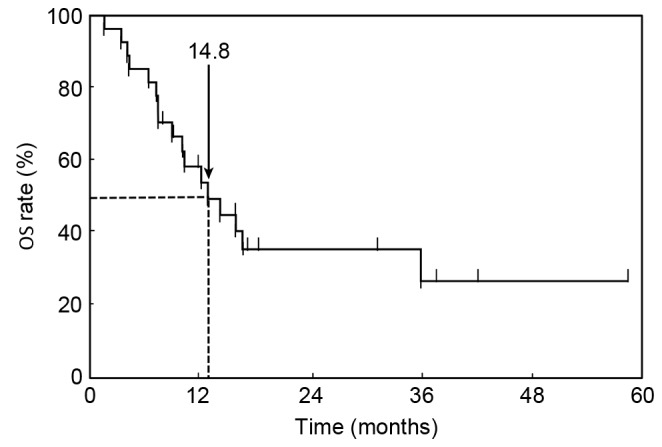
Cumulative OS curve. The median survival time for all patients was 14.8 months (95% confidence interval, 9.3 months-not yet reached). OS, overall survival.
Survival analysis and probability of receiving salvage chemotherapy
Univariate and multivariate analyses of the baseline clinicopathological characteristics associated with prognostic factors for survival were performed. The univariate analysis revealed that a PS of 0 and platinum-containing chemotherapy were significant prognostic factors for improved survival (P=0.0228 and P=0.0048, respectively; Table IV). Chemotherapy with molecular targeted agents was a borderline factor for improved prognosis (P=0.0827). Although SBA may exhibit different characteristics according to the location of the tumor, the survival time between patients with duodenal SBA and patients with non-duodenal SBA was not significantly different (data not shown). Multivariate analysis adjusted for age identified that a platinum-containing chemotherapy regimen was the most significant predictive factor for improved survival time (P=0.0373, Table V).
Table IV.
Univariate analysis of baseline and clinicopathological characteristics as prognostic factors for survival time in patients with small bowel adenocarcinoma.
| Clinicopathological characteristic | HR | 95% CI | P-value |
|---|---|---|---|
| Age (years) | N.S. | ||
| <60 | 1 | ||
| ≥60 | 0.85 | 0.32–2.36 | |
| Sex | N.S. | ||
| Male | 1 | ||
| Female | 1.01 | 0.36–2.66 | |
| Histological type | N.S. | ||
| Differentiated adenocarcinoma | 1 | ||
| Undifferentiated adenocarcinoma | 0.57 | 0.16–1.64 | |
| Performance status | 0.0228 | ||
| 0 | 1 | ||
| 1/2 | 3.40 | 1.20–9.25 | |
| Primary tumor location | N.S. | ||
| Duodenum | 1 | ||
| Non-duodenum (Jejunum, Ileum) | 1.16 | 0.40–4.17 | |
| Reason for chemotherapy | |||
| Post-operative recurrence | 1 | ||
| Non-curative resection | 2.69 | 0.72–17.5 | N.S. |
| Unresectable | 3.92 | 0.64–30.0 | N.S. |
| CEA (ng/ml) | N.S. | ||
| ≤5 | 1 | ||
| >5 | 0.78 | 0.28–2.05 | |
| CA19-9 (ng/ml) | N.S. | ||
| ≤40 | 1 | ||
| >40 | 0.72 | 0.22–1.95 | |
| Liver metastasis | N.S. | ||
| Absent | 1 | ||
| Present | 1.93 | 0.65–5.19 | |
| Lung metastasis | N.S. | ||
| Absent | 1 | ||
| Present | 0.92 | 0.05–4.60 | |
| Number of metastatic organs | |||
| 1 | 1 | ||
| 2 | 1.29 | 0.43–3.61 | N.S. |
| 3 | 2.48 | 0.11–10.4 | N.S. |
| Resection of primary tumor | N.S. | ||
| No | 1 | ||
| Yes | 0.79 | 0.29–2.22 | |
| Platinum-containing chemotherapy treatment | 0.0048 | ||
| No | 1 | ||
| Yes | 0.08 | 0.01–0.41 | |
| Molecular-targeted agent-containing chemotherapy treatment | 0.0827 | ||
| No | 1 | ||
| Yes | 0.38 | 0.10–1.12 | |
| Combined chemotherapy-radiotherapy treatment | N.S. | ||
| No | 1 | ||
| Yes | 1.60 | 0.25–5.88 |
CI, confidence interval; HR, hazard ratio; CEA, carcinoembryonic antigen; CA19-9, carbohydrate antigen 19-9; N.S., not significant.
Table V.
Multivariate analysis of clinicopathological characteristics as prognostic factors for survival time in small bowel adenocarcinoma.
| Clinicopathological characteristic | HR | 95% CI | P-value |
|---|---|---|---|
| Performance status | 0.2016 | ||
| 0 | 1 | ||
| 1/2 | 2.34 | 0.60–7.78 | |
| Platinum-containing chemotherapy treatment | 0.0373 | ||
| No | 1 | ||
| Yes | 0.14 | 0.02–0.88 |
CI, confidence interval; HR, hazard ratio.
Chemotherapy containing molecular target agents for SBA
A total of 8 patients were treated with chemotherapy regimens containing molecular targeted agents (Table III). Details of the regimens that these patients received are provided in Table VI. The clinical outcomes of these patients at the last follow-up period was CR in 1 patient, PR in 1 patient, SD in 1 patient, BSC in 2 patients and mortality in 3 patients (Table VI). The MST for the 8 patients treated with molecular targeted agents-containing regimens was 27.2 months (data not shown).
Table VI.
Details of the 8 patients with small bowel adenocarcinoma treated with molecular targeted agent-containing chemotherapy regimens.
| Chemotherapy regimens (treatment periods, [months]) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Case | Age (years) | Sex | PS | 1st-line | 2nd-line | 3rd-line | 4th-line | 5th-line | 6th-line | Result |
| 1 | 62 | M | 0 | CAPOX + Bev (26.7) | CR | |||||
| 2 | 63 | F | 0 | mFOLFOX6 + Bev (29.6) | CAPOX −23.2 | PR | ||||
| 3 | 57 | M | 0 | mFOLFOX6 + Bev (2.5) | FOLFILI + Bev (12.1) | UFT + Bev −5.6 | Regorafenib −0.2 | BSC | ||
| 4 | 81 | F | 0 | mFOLFOX6 + Bev (6.0) | CPT-11+ Pani −8.4 | Mortality | ||||
| 5 | 74 | M | 0 | mFOLFOX6 + Bev (2.0) | TS-1 (1.0) | Mortality | ||||
| 6 | 62 | F | 1 | TS-1+CDDP (7.7) | FOLFILI + Bev (9.2) | mFOLFOX6+ Bev (1.5) | mFOLFOX6 + Pani (1.7) | PTX (1.5) | SD | |
| 7 | 36 | F | 0 | mFOLFOX6 (3.0) | FOLFILI + Bev (10.0) | Capecitabine + Bev (5.0) | SOX + Bev −1 | SOX (6.0) | TS-1 + Bev (3.0) | BSC |
| 8 | 38 | M | 0 | mFOLFOX6 + Cet (6.8) | FOLFILI + Cet (3.0) | FOLFILI + Bev (2) | FOLFILI + Pani (2) | Mortality | ||
PS, performance status; CAPOX, oxaliplatin and capecitabine regimen; Bev, bevacizumab; CR, complete response; mFOLFOX6, oxaliplatin, leucovorin and 5-fluorouracil regimen; PR, partial response; FOLFILI, 5-fluorouracil, leucovorin and irinotecan regime; UFT, uracil with tegafur; BSC, best supportive care; CPT-11, irinotecan; Pani, panitumumab; TS-1, titanium silicate 1; CDDP, cisplatin; PTX, paclitaxel; SD, stable disease; SOX, TS-1/oxaliplatin; Cet, cetuximab.
The efficacy of molecular targeted agents in patients treated with oxaliplatin-based chemotherapy, including mFOLFOX6 and CAPOX as the first-line therapy was investigated. A total of 8 patients were treated with molecular targeted agents as the first or second line of treatment, including 7 patients with bevacizumab and 1 patient with cetuximab (Table III) in 18 patients with SBA that were treated with oxaliplatin-based chemotherapy (data not shown). Following the exclusion of the 1 patient treated with cetuximab, the prognostic factors for survival time in the 17 patients treated with oxaliplatin-based chemotherapy with or without bevacizumab were analyzed. A univariate analysis revealed that a PS of 0 and treatment with bevacizumab were significant prognostic factors for improved survival time (P=0.0256 and P=0.0121, respectively; Table VII).
Table VII.
Univariate analysis of baseline and clinical characteristics as prognostic factors for survival time in oxaliplatin-based chemotherapy.
| Clinicopathological characteristic | HR | 95% CI | P-value |
|---|---|---|---|
| Age | N.S. | ||
| <60 | 1 | ||
| ≥60 | 1.19 | 0.38–3.65 | |
| Sex | N.S. | ||
| Male | 1 | ||
| Female | 0.44 | 0.09–1.50 | |
| Histological type | N.S. | ||
| Differentiated adenocarcinoma | 1 | ||
| Undifferentiated adenocarcinoma | 0.81 | 0.26–3.08 | |
| Performance status | 0.0256 | ||
| 0 | 1 | ||
| 1/2 | 5.61 | 1.09–25.8 | |
| Primary tumor location | N.S. | ||
| Duodenum | 1 | ||
| Jejunum, Ileum | 1.09 | 0.28–7.20 | |
| CEA (ng/ml) | N.S. | ||
| ≤5 | 1 | ||
| >5 | 1.17 | 0.38–3.98 | |
| CA19-9 (ng/ml) | N.S. | ||
| ≤40 | 1 | ||
| >40 | 0.97 | 0.29–4.34 | |
| Liver metastasis | N.S. | ||
| Absent | 1 | ||
| Present | 1.07 | 0.29–3.24 | |
| Lung metastasis | N.S. | ||
| Absent | 1 | ||
| Present | 1.38 | 0.21–5.32 | |
| Resection of primary tumor | N.S. | ||
| No | 1 | ||
| Yes | 0.40 | 0.12–1.27 | |
| Bevacizumab treatment | 0.0121 | ||
| No | 1 | ||
| Yes | 0.16 | 0.02–0.69 |
HR, hazard ratio; CI, confidence interval; N.S., not significant; CEA, carcinoembryonic antigen; CA19-9, carbohydrate antigen 19-9.
Molecular targeted agent treatment toxicity
A total of 8 patients suffered from toxicities higher than Grade 3 due to chemotherapy, including 2 patients treated with bevacizumab (data not shown). Dose reduction (40 mg/m2/day) was required due to neutropenia in 1 patient treated with TS-1, in 1 patient treated with mFOLFOX6 (LV; 200 mg/m2, oxaliplatin 65 mg/m2 and bolus 5-FU; 200 mg/m2, infusion of 5-FU 2,000 mg/m2), and due to thrombocytopenia in 1 patient treated with CAPOX (capecitabine 1,500 mg/m2, oxaliplatin 100 mg/m2). Neurotoxicity was observed in 2 patients treated with mFOLFOX6 and 1 patient treated with mFOLFOX6 and bevacizumab. The 2 patients treated with mFOLFOX6 continued chemotherapy with either 5-FU + LV + CPT-11 (FOLFILI) or uracil + tegafur (UFT) with bevacizumab, and the 1 patient treated with mFOLFOX6 and bevacizumab continued chemotherapy with FOLFILI with bevacizumab due to oxaliplatin-induced neurotoxicity. Renal dysfunction was observed in 1 patient treated with TS-1; however, chemotherapy with TS-1 could be continued by reducing the dose to 60 mg/m2/day. Liver dysfunction was observed in 1 patient treated with mFOLFOX6 and bevacizumab; however, an alteration of the TS-1 dosage was effective (80 mg/m2/day). Although bevacizumab treatment was discontinued due to the toxicity of concomitant chemotherapies in 2 patients, no serious side effects in response to the molecular targeted agents, including bleeding, thrombosis, gastrointestinal perforation, allergic reactions and rash, occurred in any of the patients included in the present study. Therefore, chemotherapy with bevacizumab for SBA was considered well tolerated by patients.
Discussion
SBA is the most common histological subtype of carcinoma of the small bowel according to the National Cancer Database, accounting for 36.9% of all small bowel malignancies (27). The dominant immunophenotypic pattern of SBA is cytokeratin (CK) 20+/CK7− and this pattern is observed in 75–94% of colorectal cancer cases (28). Caudal-type homeobox transcription factor 2 (CDX2), which is highly expressed in colorectal cancer, is also expressed in the majority of cases of SBA, particularly in well-differentiated tumors (28). The molecular characteristics of SBA are more similar to those of colorectal adenocarcinoma compared with gastric adenocarcinoma, with low expression of receptor tyrosine-protein kinase erbB-2 and high frequencies of GTPase KRAS (KRAS) mutations (29). A genome-wide DNA copy number analysis demonstrated that the profiles of SBA overlapped more with colorectal adenocarcinoma compared with gastric adenocarcinoma (26). These results indicate that the characteristics of SBA resemble colorectal cancer. A previous study demonstrated that there was no significant difference in the immunophenotype determined by the expression of cytokeratin (CK) 7, CK20 and CDX2 between duodenal and non-duodenal SBA (28). Zaanan et al (15,30) reported that OS was not significantly different between duodenal and non-duodenal SBA. Consistent with previous studies, OS was not significantly different between duodenal and non-duodenal SBA in the present study.
Bevacizumab has been demonstrated to prolong the OS of patients with colorectal cancer from 15.6 to 20.3 months (20). Bevacizumab is a recombinant Immunolobulin G1 humanized monoclonal antibody that targets vascular endothelial growth factor (VEGF), which is a critical and highly pleiotropic factor that promotes new blood vessel formation in tumors. Bevacizumab inhibits tumor vessel hyperplasticity and facilitates the intra-tumoral transition of anticancer drugs by promoting tumor vessel permeability and decreasing intratumoral stromal pressure (31,32). Willett et al (33) demonstrated that a single infusion of bevacizumab decreases tumor perfusion, vascular volume, microvascular density, interstitial fluid pressure and the number of viable circulating endothelial and progenitor cells, but increases the fraction of vessels with pericyte coverage in patients with rectal carcinoma (33). These results indicate that the inhibition of VEGF has a direct and rapid antivascular effect in human tumors. In addition, bevacizumab has also been suggested to be an effective treatment for SBA, as the VEGF expression is detectable in 96% of SBA tumors (18).
Cetuximab is a monoclonal antibody directed against the epidermal growth factor receptor. Cetuximab has been demonstrated to improve first-line chemotherapy efficacy in colorectal cancer; however, the benefit was limited to patients with wild-type KRAS tumors (34). There are only a small number of case reports about the use of molecular targeted therapies, including bevacizumab and cetuximab, for SBA (17,19,21,35). Therefore, the results from the current multicenter retrospective cohort study may aid in determining whether molecular targeted agents for SBA are safe and effective. No serious side effects were observed as a result of molecular targeted agents in the current study. A univariate analysis of oxaliplatin-based chemotherapy revealed that bevacizumab-containing chemotherapy was the most significant positive prognostic factor. The current multicenter study demonstrated that molecular targeted agent-containing chemotherapy is safe and may improve survival times in patients with SBA.
The current study had a number of limitations, including a small sample size and the fact that the patients' disease status was heterogeneous, despite conducting a multi-center study, due to the rarity of SBA. In addition, there was no standardized treatment regimen; the physicians in each hospital selected the chemotherapy regimens for patients with SBA. The current study also included patients that had undergone surgical resection with varying degrees of success, which may have affected the outcome of chemotherapy. Despite these limitations, preliminary results from the present study indicate that molecular targeted agents are effective and safe for the treatment of patients with SBA. However, further studies are required to confirm these results in a larger cohort of patients with SBA.
References
- 1.Lowenfels AB. Why are small-bowel tumours so rare? Lancet. 1973;1:24–26. doi: 10.1016/S0140-6736(73)91228-2. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Murray T, Ward E, Samuels A, Tiwari RC, Ghafoor A, Feuer EJ, Thun MJ. Cancer statistics, 2005. CA Cancer J Clin. 2005;55:10–30. doi: 10.3322/canjclin.55.1.10. [DOI] [PubMed] [Google Scholar]
- 3.Dabaja BS, Suki D, Pro B, Bonnen M, Ajani J. Adenocarcinoma of the small bowel: Presentation, prognostic factors and outcome of 217 patients. Cancer. 2004;101:518–526. doi: 10.1002/cncr.20404. [DOI] [PubMed] [Google Scholar]
- 4.Mishkin DS, Chuttani R, Croffie J, Disario J, Liu J, Shah R, Somogyi L, Tierney W, Song LM, Petersen BT, Technology Assessment Committee, American Society for Gastrointestinal Endoscopy ASGE technology status evaluation report: Wireless capsule endoscopy. Gastrointest Endosc. 2006;63:539–545. doi: 10.1016/j.gie.2006.01.014. [DOI] [PubMed] [Google Scholar]
- 5.Yamamoto H, Sekine Y, Sato Y, Higashizawa T, Miyata T, Iino S, Ido K, Sugano K. Total enteroscopy with a nonsurgical steerable double-balloon method. Gastrointest Endosc. 2001;53:216–220. doi: 10.1067/mge.2001.112181. [DOI] [PubMed] [Google Scholar]
- 6.Yamamoto H, Yano T, Kita H, Sunada K, Ido K, Sugano K. New system of double-balloon enteroscopy for diagnosis and treatment of small intestinal disorders. Gastroenterology. 2003;125:1556–1557. doi: 10.1016/j.gastro.2003.03.004. [DOI] [PubMed] [Google Scholar]
- 7.Chow JS, Chen CC, Ahsan H, Neugut AI. A population-based study of the incidence of malignant small bowel tumours: SEER, 1973–1990. Int J Epidemiol. 1996;25:722–728. doi: 10.1093/ije/25.4.722. [DOI] [PubMed] [Google Scholar]
- 8.Hatzaras I, Palesty JA, Abir F, Sullivan P, Kozol RA, Dudrick SJ, Longo WE. Small-bowel tumors: Epidemiologic and clinical characteristics of 1260 cases from the connecticut tumor registry. Arch Surg. 2007;142:229–235. doi: 10.1001/archsurg.142.3.229. [DOI] [PubMed] [Google Scholar]
- 9.Ouriel K, Adams JT. Adenocarcinoma of the small intestine. Am J Surg. 1984;147:66–71. doi: 10.1016/0002-9610(84)90036-9. [DOI] [PubMed] [Google Scholar]
- 10.Barnes G, Jr, Romero L, Hess KR, Curley SA. Primary adenocarcinoma of the duodenum: Management and survival in 67 patients. Ann Surg Oncol. 1994;1:73–78. doi: 10.1007/BF02303544. [DOI] [PubMed] [Google Scholar]
- 11.Bauer RL, Palmer ML, Bauer AM, Nava HR, Douglass HO., Jr Adenocarcinoma of the small intestine: 21-year review of diagnosis, treatment, and prognosis. Ann Surg Oncol. 1994;1:183–188. doi: 10.1007/BF02303522. [DOI] [PubMed] [Google Scholar]
- 12.Rose DM, Hochwald SN, Klimstra DS, Brennan MF. Primary duodenal adenocarcinoma: A ten-year experience with 79 patients. J Am Coll Surg. 1996;183:89–96. [PubMed] [Google Scholar]
- 13.Raghav K, Overman MJ. Small bowel adenocarcinomas-existing evidence and evolving paradigms. Nat Rev Clin Oncol. 2013;10:534–544. doi: 10.1038/nrclinonc.2013.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haan JC, Buffart TE, Eijk PP, van de Wiel MA, van Wieringen WN, Howdle PD, Mulder CJ, van de Velde CJ, Quirke P, Nagtegaal ID, et al. Small bowel adenocarcinoma copy number profiles are more closely related to colorectal than to gastric cancers. Ann Oncol. 2012;23:367–374. doi: 10.1093/annonc/mdr122. [DOI] [PubMed] [Google Scholar]
- 15.Zaanan A, Costes L, Gauthier M, Malka D, Locher C, Mitry E, Tougeron D, Lecomte T, Gornet JM, Sobhani I, et al. Chemotherapy of advanced small-bowel adenocarcinoma: A multicenter AGEO study. Ann Oncol. 2010;21:1786–1793. doi: 10.1093/annonc/mdq038. [DOI] [PubMed] [Google Scholar]
- 16.Zhang L, Wang LY, Deng YM, Wang FH, Feng F, Chen YC, An X, Chen C, Xu RH, Li YH. Efficacy of the FOLFOX/CAPOX regimen for advanced small bowel adenocarcinoma: A three-center study from China. J BUON. 2011;16:689–696. [PubMed] [Google Scholar]
- 17.Tsushima T, Taguri M, Honma Y, Takahashi H, Ueda S, Nishina T, Kawai H, Kato S, Suenaga M, Tamura F, et al. Multicenter retrospective study of 132 patients with unresectable small bowel adenocarcinoma treated with chemotherapy. Oncologist. 2012;17:1163–1170. doi: 10.1634/theoncologist.2012-0079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Overman MJ, Varadhachary GR, Kopetz S, Adinin R, Lin E, Morris JS, Eng C, Abbruzzese JL, Wolff RA. Phase II study of capecitabine and oxaliplatin for advanced adenocarcinoma of the small bowel and ampulla of Vater. J Clin Oncol. 2009;27:2598–2603. doi: 10.1200/JCO.2008.19.7145. [DOI] [PubMed] [Google Scholar]
- 19.Xiang XJ, Liu YW, Zhang L, Qiu F, Yu F, Zhan ZY, Feng M, Yan J, Zhao JG, Xiong JP. A phase II study of modified FOLFOX as first-line chemotherapy in advanced small bowel adenocarcinoma. Anticancer Drugs. 2012;23:561–566. doi: 10.1097/CAD.0b013e328350dd0d. [DOI] [PubMed] [Google Scholar]
- 20.Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S, Holmgren E, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 21.Tsang H, Yau T, Khong PL, Epstein RJ. Bevacizumab-based therapy for advanced small bowel adenocarcinoma. Gut. 2008;57:1631–1632. doi: 10.1136/gut.2008.153866. [DOI] [PubMed] [Google Scholar]
- 22.Okubo K, Yoshioka S, Asukai K, Hata T, Nakanishi M, Maekawa T, Hama N, Kashiwazaki M, Taniguchi M, Tsujie M, et al. A case report of primary adenocarcinoma of small intestine. Gan To Kagaku Ryoho. 2010;37:2792–2794. (In Japanese) [PubMed] [Google Scholar]
- 23.Nagaraj G, Zarbalian Y, Flora K, Tan BR., Jr Complete response and prolonged disease-free survival in a patient with recurrent duodenal adenocarcinoma treated with bevacizumab plus FOLFOX6. J Gastrointest Oncol. 2014;5:E1–E6. doi: 10.3978/j.issn.2078-6891.2013.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET, Carbone PP. Toxicity and response criteria of the eastern cooperative oncology group. Am J Clin Oncol. 1982;5:649–655. doi: 10.1097/00000421-198212000-00014. [DOI] [PubMed] [Google Scholar]
- 25.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 26.National Institutes of health national cancer institute, corp-author. Common Terminology criteria for adverse events (CTCAE) Version 4.0. U.S. Department of Health and Human Services; 2009. [Google Scholar]
- 27.Bilimoria KY, Bentrem DJ, Wayne JD, Ko CY, Bennett CL, Talamonti MS. Small bowel cancer in the United States: Changes in epidemiology, treatment, and survival over the last 20 years. Ann Surg. 2009;249:63–71. doi: 10.1097/SLA.0b013e31818e4641. [DOI] [PubMed] [Google Scholar]
- 28.Overman MJ, Pozadzides J, Kopetz S, Wen S, Abbruzzese JL, Wolff RA, Wang H. Immunophenotype and molecular characterisation of adenocarcinoma of the small intestine. Br J Cancer. 2010;102:144–150. doi: 10.1038/sj.bjc.6605449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aparicio T, Svrcek M, Zaanan A, Beohou E, Laforest A, Afchain P, Mitry E, Taieb J, Di Fiore F, Gornet JM, et al. Small bowel adenocarcinoma phenotyping, a clinicobiological prognostic study. Br J Cancer. 2013;109:3057–3066. doi: 10.1038/bjc.2013.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zaanan A, Gauthier M, Malka D, Locher C, Gornet JM, Thirot-Bidault A, Tougeron D, Taïeb J, Bonnetain F, Aparicio T, Association des Gastro Entérologues Oncologues Second-line chemotherapy with fluorouracil, leucovorin, and irinotecan (FOLFIRI regimen) in patients with advanced small bowel adenocarcinoma after failure of first-line platinum-based chemotherapy: A multicenter AGEO study. Cancer. 2011;117:1422–1428. doi: 10.1002/cncr.25614. [DOI] [PubMed] [Google Scholar]
- 31.Dvorak HF. Vascular permeability factor/vascular endothelial growth factor: A critical cytokine in tumor angiogenesis and a potential target for diagnosis and therapy. J Clin Oncol. 2002;20:4368–4380. doi: 10.1200/JCO.2002.10.088. [DOI] [PubMed] [Google Scholar]
- 32.Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9:669–676. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- 33.Willett CG, Boucher Y, di Tomaso E, Duda DG, Munn LL, Tong RT, Chung DC, Sahani DV, Kalva SP, Kozin SV, et al. Direct evidence that the VEGF-specific antibody bevacizumab has antivascular effects in human rectal cancer. Nat Med. 2004;10:145–147. doi: 10.1038/nm0604-649c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van Cutsem E, Köhne CH, Hitre E, Zaluski J, Chien CR Chang, Makhson A, D'Haens G, Pintér T, Lim R, Bodoky G, et al. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med. 2009;360:1408–1417. doi: 10.1056/NEJMoa0805019. [DOI] [PubMed] [Google Scholar]
- 35.de Dosso S, Molinari F, Martin V, Frattini M, Saletti P. Molecular characterisation and cetuximab-based treatment in a patient with refractory small bowel adenocarcinoma. Gut. 2010;59:1587–1588. doi: 10.1136/gut.2009.196428. [DOI] [PubMed] [Google Scholar]


