Abstract
The efficacy of the current non-surgical treatments for advanced hepatocellular carcinoma (HCC) remains limited and novel treatments are required to improve patient outcomes. The majority of HCCs develop from chronically damaged tissue that contains a high degree of inflammation and fibrosis, which promotes tumor progression and resistance to therapy. Understanding the interaction between stromal components and cancer cells (and the signaling pathways involved in this interaction) could aid the identification of novel therapeutic targets. Numerous studies have demonstrated a marked association between high C-X-C chemokine receptor 4 (CXCR4) expression and the invasiveness, progression and metastasis of HCC. The present review will investigate the different roles of CXCR4 in the progression of HCC and discuss possible future treatments. Through the C-X-C chemokine ligand 12 (CXCL12)/CXCR4 signaling pathway, ephrin A1 activation enhances the migration of endothelial progenitor cells to HCC to enable the neovascularization of tumors. There is an association between nuclear CXCR4 expression and the lymph node metastasis of HCC to distant areas. CXCR4 enhances cell migration in vitro and cell homing in vivo. CXCR4 levels are concentrated at the border of a tumor and in perivascular areas, inducing invasive behavior. The binding of CXCL12 to CXCR4 activates intracellular signaling pathways and induces crosstalk with transforming growth factor-β signaling, which enhances the migration of cancer cells. The CXCL12/CXCR4 axis also activates expression of matrix metalloproteinase 10, which further stimulates migration. CXCR4 is likely to crosstalk with the sonic hedgehog signaling pathway, contributing to tumor invasiveness and supporting the cancer stem-cell population; as a result, CXCR4 can be regarded as a cancer stem-cell marker. CXCR4 influences interstitial fluid flow-induced invasion. CXCR4 expression and HCC cell migration are promoted by α-fetoprotein, which activates AKT/mechanistic target of rapamycin signaling. CXCR4 also has the potential to affect sorafenib treatment for HCC. Targeting the CXCL12/CXCR4 signaling pathway may, therefore, be a promising strategy in HCC treatment.
Keywords: hepatocellular carcinoma, C-X-C chemokine receptor 4, C-X-C chemokine ligand 12, progression
1. Introduction
Hepatocellular carcinoma (HCC) is one of the most common types of primary liver cancer and a major cause of cancer-associated mortality worldwide (1). The majority of HCCs develop from chronically damaged, inflamed and fibrotic tissue, which promotes tumor progression and resistance to therapy (2). For advanced HCC, the prolongation of survival with the present target therapy (sorafenib) remains limited (3). Understanding interactions between stromal components and cancer cells, and the involved signaling pathways could assist in the identification of new therapeutic targets (4).
Chemokines are small, pro-inflammatory chemoattractant cytokines that bind to specific G-protein-coupled receptors that span the plasma membranes of cells. They are key regulators of cell trafficking into and out of the tumor microenvironment (5). Over 50 different chemokines and 20 different chemokine receptors have been cloned to date (6), a number of of which regulate cell growth and survival. Chemokines have been implicated in various key steps of cancer development, including evasion of the immune system, angiogenesis, and cancer cell invasion and dissemination (7,8). The majority of chemokines can bind to various receptors and similarities between receptors afford them the ability to bind to a variety of chemokines (9). However, C-X-C chemokine receptor 4 (CXCR4) is one of the only receptors for C-X-C chemokine ligand 12 (CXCL12) (10). This suggests that the CXCL12/CXCR4 axis has an important biological role (11).
CXCR4 is expressed on cancer cells in >23 different malignancies (12). CXCL12/CXCR4 signaling affects the migration of tumor cells to metastasis sites; this has been reported in cancer of the breast, ovaries, prostate, lungs, pancreas, colon, thyroid glands and kidneys, and in neuroblastoma, rhabdomyosarcoma, leukemia and lymphoma (13).
Previous studies have observed that CXCR4 is expressed in HCC tissues, but not in healthy liver tissue (14,15). Other studies have demonstrated a significant association between high CXCR4 expression and the increased invasiveness, progression and metastasis of HCC (16,17). The CXCL12/CXCR4 axis contributes to the progression of HCC at various stages, using autocrine and paracrine mechanisms to support the growth of tumors, inducing angiogenesis and allowing the tumor to evade immune surveillance (18). However, certain mechanisms are unidentified and their clinical relevance remains controversial (19). To elucidate the contributory role of CXCR4 to the progression of HCC, the present review discusses the characteristics of CXCR4 activity in cancer, including in angiogenesis, migration and signaling pathways, and on stem cell markers and the stroma.
2. Ephrin A activation enhances the homing of endothelial progenitor cells towards HCC for neovascularization via CXCL12/CXCR4
Ephrin A1 may activate CXCL12/CXCR4 in HCC (20). The high expression of EphA1 in HCC cells may lead to a high CXCL12 concentration within the tumor microenvironment, which can then activate CXCL12/CXCR4 signaling and enhance the recruitment of endothelial progenitor cells (EPCs) to HCC. Wang et al (20) demonstrated that the upregulation of EphA1 expression in HCC cells promoted the chemotaxis of EPCs towards tumor cells and the tube formation of EPCs. In vivo experiments that blocked ephA1/CXCL12/CXCR4 signaling significantly reduced the growth of HCC xenografts (20). EphA1 increased CXCL12 expression in HCC cells via the phosphoinositide-3 kinase (PI3K), AKT and mechanistic target of rapamycin (mTOR) pathways (21). Inhibition of CXCL12 suppresses EphA1-induced chemotaxis and EPC tube formation (20). Targeting the EphA1/CXCL12 signaling pathway may prove to be a promising anti-angiogenic therapy for HCC.
3. Nuclear CXCR4 expression correlates with lymph node metastasis of HCC
Increased expression of nuclear CXCR4 increases the likelihood of lymph node metastasis, which is an indicator of poor outcomes in HCC (22,23). A previous study, however, found that cytoplasmic CXCR4 expression does not correlate with lymph node metastasis (24). Different sites of CXCR4 localization in HCC cells may therefore play different biologically relevant roles.
4. CXCL12 affects the homing of cluster of differentiation 34 (CD34)+ progenitors
CXCR4 is expressed in lymphocytes, myeloid cells, megakaryocytes and CD34+ cells (25). CXCR4 enables these cells to move across a gradient of CXCL12 concentrations. CXCR4 expression is downregulated by CXCL12 in vitro and in vivo, via a negative-feedback mechanism. Previous studies found that CXCL12 affects the mobilization of human hepatic progenitor cells in non-obese diabetic/severe compromised immunodeficient (NOD/SCID) mouse models (26,27). Kollet et al (26) found that CXCL12 increased the homing of CD34+ progenitors, whereas incubation with anti-CXCR4 antibodies abrogated this homing.
5. CXCR4 is concentrated at the tumor border and the perivascular space
Cells in the tumor border exhibit the highest levels of CXCR4 expression (28). In addition, the expression of CXCL12 usually concentrates in the perivascular space or in ductal cells, which may stimulate cell migration to these areas. Similarly, in a mouse model of HCC, the CXCR4+ cells were found to be mainly located at the tumor border or in the perivascular space, whereas CXCL12 expression localized to the infiltration areas, ductal and perivascular cells, and in the stroma (28). Notably, certain prior studies have reported that CXCR4+ cells can invade the stroma (12,13).
It is likely that CXCR4+ cells may attempt to migrate into the vasculature and infiltrate the peritumoral capsule. The CXCR4+ tumor cells that surround the vasculature exhibit the disorganized expression of E-cadherin, reflecting a less differentiated, more mesenchymal and migratory cell form (28).
The fact that CXCR4 concentrates at the perivascular areas and the tumor border suggests that it potentially contributes to tumor cell dissemination (28). In this way, CXCR4 and CXCL12 may behave as regulatory molecules, affecting the spread and progression of tumors.
6. Binding of CXCL12 to CXCR4 activates intracellular signaling
Following the binding of CXCL12, CXCR4 exerts its activity through a heterotrimeric G protein, which dissociates into activated α and βγ subunits. These subunits activate several intracellular signaling pathways, including those that activate chemotaxis, cellular migration, cell proliferation, gene transcription and cellular survival (29).
7. CXCL12/CXCR4 pathway crosstalks with transforming growth factor-β (TGF-β) to enhance cancer cell migration
Crosstalk has been observed between the TGF-β and CXCR4 pathways in liver tumors (28), suggesting a novel molecular mechanism that aids in explaining the tumorigenic effects of TGF-β and affords the possibility of a novel antitumor therapy. A previous study found that TGF-β upregulates CXCR4 expression in HCC cells to sensitize cells to CXCL12, increasing cell motility and survival (30). Patients with HCC who also have a history of cirrhosis have a significantly increased level of TGF-β and CXCR4 in comparison with those with non-cirrhotic livers (28). A high percentage (~76%) of HCC cells express a high level of CXCR4 coincident with the TGF-β pathway activation. These results demonstrate that TGF-β signaling affects CXCL12/CXCR4 signaling, resulting in increased cell migration and tumor function.
In a mouse model, diethylnitroamine-induced liver carcinogenesis led to a progressive increase in the expression of TGF-β1 and CXCR4, with maximal levels achieved at late disease stages (28).
Overactivation of the TGF-β pathway in HCC cells induces a mesenchymal-like phenotype and drives migratory behavior through activation of the CXCL12/CXCR4 axis (28), which contributes to HCC progression. CXCR4 localization to the migratory sites of tumors, with the corresponding over-activation of TGF-β signaling, could indicate a response in patients to drugs targeting the TGF-β pathway and provide a future prognosis (28).
8. CXCL12/CXCR4 axis induces matrix metalloproteinase 10 (MMP10) expression, stimulating cancer cell migration
MMP10 expression within HCC cells is induced by hypoxia and CXCR4 activation via the extracellular signal-regulated kinase 1/2 (ERK1/2) pathway, which transactivates the activator protein 1 responsive element in the MMP10 gene promoter (31). Human HCC cells that express MMP10 increase their CXCR4 expression and thus their migratory capacity. New reciprocal crosstalk between the CXCL12/CXCR4 axis and MMP10 contributes to the progression and metastasis in HCC. Conversely, the pharmacological inhibition of CXCR4 reduces the migration of HCC cells substantially (31).
9. CXCR4 performs crosstalk with the SHH signaling pathway to alter tumor invasiveness and cancer stem-cell behavior
The sonic hedgehog (SHH) signaling pathway can affect the behavior of cancer stem cells (32–34). Previous studies have demonstrated that the activation of the SHH pathway correlates with the increased invasiveness and progression of cancer (33–35). Singh et al (36) demonstrated that there was a strong association between CXCR4 and SHH signaling in human pancreatic cancer cells, which is not observed in the healthy pancreas. Similarly, a functional interaction between the SHH and CXCR4 signaling pathways was also observed in medulloblastoma (37). Hepatic cancer cells not only express cancer stem markers such as CD133, CD90 but also express CXCR4 (38). From the aforementioned findings, it can be concluded that CXCR4 is likely to crosstalk with the SHH signaling pathway in certain malignancies. Furthermore, CXCR4 also has a significant contributory role in the signaling of HCC cancer stem cells (12).
10. CXCR4 is a potential cancer stem-cell marker
It is possible that CD90+CXCR4+ HCC cells may form tumor spheres in cell culture (39). These cells developed into tumors following serial adoptive implantations in NOD/SCID mice. Furthermore, CD90+CXCR4+ HCC cells in the circulation were significantly more frequently detected compared with CD90−CXCR4−, CD90−CXCR4+ or CD90+CXCR4− HCC cells (39). Distant metastasis develops following the subcutaneous implantation of CD90+CXCR4+ HCC cells (39). CD90 is a cancer stem cell markers of HCC, whereas elevated CXCR4 expression was identified in HCC (12,40). Therefore, CD90+CXCR4+ HCC cells could be regarded as cancer stem cells of HCC. Selectively eliminating these cells may substantially improve upon current treatments to mitigate cancer metastasis (39).
Previous studies found that sphere-forming HCC cells expressed an increased level of MMP26 and CXCR4. Compared with MMP26−CXCR4−, MMP26−CXCR4+ and MMP26+CXCR4− HCC cells, MMP26+CXCR4+ HCC cells formed significantly more spheres in cell culture. These cells were also more frequently found in the circulation when mice underwent subcutaneous implantation with MMP26+CXCR4+ HCC cells (41). Thus, numerous studies have regarded MMP26+CXCR4+ cells as cancer stem cells. Such cells may express stem-cell markers, including octamer-binding transcription factor 3/4, CXCR4, CD133 and CD90 (42–44). The difference in importance between CD90+CXCR4+ HCC cells and MMP26+CXCR4+ cells requires elucidation.
11. CXCR4 influences interstitial fluid flow-induced invasion
A previous study demonstrated that autologous chemotaxis affects the invasion of HCC-derived cell lines, as induced by interstitial fluid flow, through the CXCR4/CXCL12 signaling axis (45). Another study revealed that mitogen-activated protein kinase kinase/ERK signaling was diverted from the CXCR4/CXCL12 axis (46). Autologous chemotaxis also influences interstitial fluid flow-induced invasion (45).
12. CXCR4 expression and HCC cell migration are promoted by α-fetoprotein (AFP)-activating AKT/mTOR signaling
The expression of certain metastasis-related genes is regulated by AFP (47). One study reported an association between levels of AFP and CXCR4 in HCC tissue. AFP depletion could reduce CXCR4 expression. AFP co-localizes and interacts with phosphatase and tensin homolog, thus inducing CXCR4 expression by activatory phosphorylation of AKT at Ser473 (47). In turn, phosphorylated mTOR (at Ser2448) can then enter the nucleus and bind the CXCR4 gene promoter. Thus, AFP enhances the migration of HCC cells via CXCR4 by activating the AKT/mTOR signaling pathway.
13. CXCL12/CXCR4 could affect sorafenib treatment in HCC
The hypoxia that is induced following sorafenib therapy increases the expression of CXCR4 and its ligand, CXCL12, in HCC (48). AMD3100 is an antagonist of CXCR4 that sensitizes HCC to sorafenib therapy by inhibiting CXCL12/CXCR4-induced HCC cell proliferation and the polarization of the tumor-promoting microenvironment. Inhibition of CXCR4 with AMD3100 prevents polarization towards the immunosuppressive microenvironment following sorafenib treatment, inhibits tumor growth, reduces lung metastasis and improves survival rates. CXCR4-targeted lipid-coated poly (lactic-co-glycolic acid) (PLGA) nanoparticles (NPs) with sorafenib and CXCR4 antagonist AM3100 can act in two ways, as tumor-targeting drug carriers for the delivery of sorafenib toward HCC and as inhibitors of CXCL12/CXCR4 in their own right (49). The NPs synergistically inhibited the growth and distant metastasis of HCC in mice, significantly improving overall survival when NPs were used to encapsulate anti-angiogenic drugs (49). The tumor stroma mediates the inhibition of HCC progression, which is induced by sorafenib-loaded CXCR4-targeted NPs. The study by Gao et al (49) indicated that NPs could act as multifunctional molecules, delivering anti-angiogenic drugs and directly targeting the tumor microenvironment, forming an efficient HCC therapy.
14. Targeting the CXCL12/CXCR4 signaling pathway may be a promising strategy
Chemokines and their cell-surface receptors in the microenvironment affect the progression of HCC in multiple ways, including through inflammation (which alters immune cells), angiogenesis and direct effects on HCC cells. The chemokine system has a key role in the orchestration of the immune response and serves as a core component of microenvironment maintenance in HCC. In affecting the progression of HCC, the CXCL12/CXCR4 signaling axis performs different functions in progenitor and stem cells, at the tumor border, in migration and on crosstalk between pathways. Therefore, targeting the CXCL12/CXCR4 signaling pathway may be a promising strategy for HCC treatment.
Figure 1.
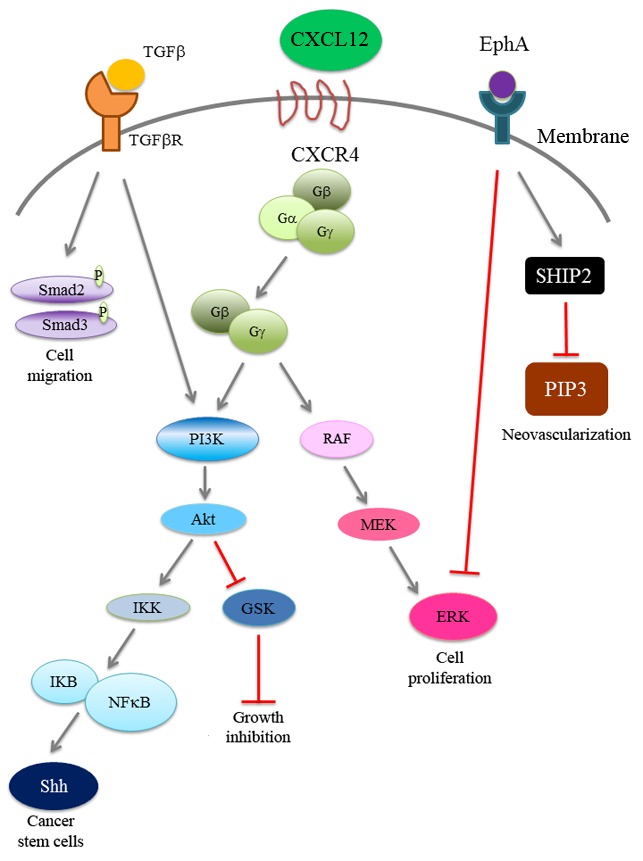
CXCL12/CXCR4 signaling and related pathways in HCC progression. CXCL12/CXCR4 signaling regulates the PI3K pathway and SHH expression, which correlates with tumor growth and cancer stem cell regeneration. In addition, CXCL12/CXCR4 signaling regulates the RAF/MEK/ERK signaling pathway for cell proliferation. The CXCL12/CXCR4 pathway crosstalks with TGF-β signaling to enhance cancer cell migration. Activated EphA1 promotes the homing of endothelial progenitor cells to HCC for tumor neovascularization. CXCL12, C-X-C chemokine ligand 12; CXCR4, C-X-C chemokine receptor 4; HCC, hepatocellular carcinoma; PI3K, phosphoinositide-3 kinase; TGFβR, transforming growth factor β receptor; MEK, mitogen-activated protein kinase kinase; ERK, extracellular-related kinase; EphA1, ephrin receptor A1.
Acknowledgements
The present review was supported by grants from Far Eastern Memorial Hospital (nos. FEMH-2016-C-004, FEMH 103-2314-B-418-007 and FEMH 104-2314-B-418-018). The authors thank research assistant Ms. Szu-Hua Wu and Ms. Ssu-Jung Lu from Far Eastern Memorial Hospital (New Taipei, Taiwan) for assistance with manuscript editing.
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Waller LP, Deshpande V, Pyrsopoulos N. Hepatocellular carcinoma: A comprehensive review. World J Hepatol. 2015;7:2648–2663. doi: 10.4254/wjh.v7.i26.2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 4.Hernandez-Gea V, Toffanin S, Friedman SL, Llovet JM. Role of the microenvironment in the pathogenesis and treatment of hepatocellular carcinoma. Gastroenterology. 2013;144:512–527. doi: 10.1053/j.gastro.2013.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gorbachev AV, Fairchild RL. Regulation of chemokine expression in the tumor microenvironment. Crit Rev Immunol. 2014;34:103–120. doi: 10.1615/CritRevImmunol.2014010062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Horuk R. Chemokine receptors. Cytokine Growth Factor Rev. 2001;12:313–335. doi: 10.1016/S1359-6101(01)00014-4. [DOI] [PubMed] [Google Scholar]
- 7.Singh S, Sadanandam A, Singh RK. Chemokines in tumor angiogenesis and metastasis. Cancer Metastasis Rev. 2007;26:453–467. doi: 10.1007/s10555-007-9068-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Singh R, Lillard JW, Jr, Singh S. Chemokines: key players in cancer progression and metastasis. Front Biosci (Schol Ed) 2011;3:1569–1582. doi: 10.2741/246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stone MJ, Hayward JA, Huang C, E Huma Z, Sanchez J. Mechanisms of Regulation of the Chemokine-Receptor Network. Int J Mol Sci. 2017;18(pii):E342. doi: 10.3390/ijms18020342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guo JC, Li J, Zhou L, Yang JY, Zhang ZG, Liang ZY, Zhou WX, You L, Zhang TP, Zhao YP. CXCL12-CXCR7 axis contributes to the invasive phenotype of pancreatic cancer. Oncotarget. 2016;7:62006–62018. doi: 10.18632/oncotarget.11330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kucia M, Reca R, Miekus K, Wanzeck J, Wojakowski W, Janowska-Wieczorek A, Ratajczak J, Ratajczak MZ. Trafficking of normal stem cells and metastasis of cancer stem cells involve similar mechanisms: Pivotal role of the SDF-1-CXCR4 axis. Stem Cells. 2005;23:879–894. doi: 10.1634/stemcells.2004-0342. [DOI] [PubMed] [Google Scholar]
- 12.Chatterjee S, Azad B Behnam, Nimmagadda S. The intricate role of CXCR4 in cancer. Adv Cancer Res. 2014;124:31–82. doi: 10.1016/B978-0-12-411638-2.00002-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sun X, Cheng G, Hao M, Zheng J, Zhou X, Zhang J, Taichman RS, Pienta KJ, Wang J. CXCL12/CXCR4/CXCR7 chemokine axis and cancer progression. Cancer Metastasis Rev. 2010;29:709–722. doi: 10.1007/s10555-010-9256-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shibuta K, Mori M, Shimoda K, Inoue H, Mitra P, Barnard GF. Regional expression of CXCL12/CXCR4 in liver and hepatocellular carcinoma and cell-cycle variation during in vitro differentiation. Jpn J Cancer Res. 2002;93:789–797. doi: 10.1111/j.1349-7006.2002.tb01321.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu H, Pan Z, Li A, Fu S, Lei Y, Sun H, Wu M, Zhou W. Roles of chemokine receptor 4 (CXCR4) and chemokine ligand 12 (CXCL12) in metastasis of hepatocellular carcinoma cells. Cell Mol Immunol. 2008;5:373–378. doi: 10.1038/cmi.2008.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xiang ZL, Zeng ZC, Tang ZY, Fan J, Zhuang PY, Liang Y, Tan YS, He J. Chemokine receptor CXCR4 expression in hepatocellular carcinoma patients increases the risk of bone metastases and poor survival. BMC Cancer. 2009;9:176. doi: 10.1186/1471-2407-9-176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhao S, Wang J, Qin C. Blockade of CXCL12/CXCR4 signaling inhibits intrahepatic cholangiocarcinoma progression and metastasis via inactivation of canonical Wnt pathway. J Exp Clin Cancer Res. 2014;33:103. doi: 10.1186/s13046-014-0103-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ghanem I, Riveiro ME, Paradis V, Faivre S, de Parga PM, Raymond E. Insights on the CXCL12-CXCR4 axis in hepatocellular carcinoma carcinogenesis. Am J Transl Res. 2014;6:340–352. [PMC free article] [PubMed] [Google Scholar]
- 19.Hu F, Miao L, Zhao Y, Xiao YY, Xu Q. A meta-analysis for C-X-C chemokine receptor type 4 as a prognostic marker and potential drug target in hepatocellular carcinoma. Drug Des Devel Ther. 2015;9:3625–3633. doi: 10.2147/DDDT.S86032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang Y, Yu H, Shan Y, Tao C, Wu F, Yu Z, Guo P, Huang J, Li J, Zhu Q, et al. EphA1 activation promotes the homing of endothelial progenitor cells to hepatocellular carcinoma for tumor neovascularization through the SDF-1/CXCR4 signaling pathway. J Exp Clin Cancer Res. 2016;35:65. doi: 10.1186/s13046-016-0339-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xi HQ, Wu XS, Wei B, Chen L. Eph receptors and ephrins as targets for cancer therapy. J Cell Mol Med. 2012;16:2894–2909. doi: 10.1111/j.1582-4934.2012.01612.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schimanski CC, Bahre R, Gockel I, Müller A, Frerichs K, Hörner V, Teufel A, Simiantonaki N, Biesterfeld S, Wehler T, et al. Dissemination of hepatocellular carcinoma is mediated via chemokine receptor CXCR4. Br J Cancer. 2006;95:210–217. doi: 10.1038/sj.bjc.6603251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xiang ZL, Zeng ZC, Tang ZY, Fan J, Sun HC, Wu WZ, Tan YS. Nuclear accumulation of CXCR4 and overexpressions of VEGF-C and CK19 are associated with a higher risk of lymph node metastasis in hepatocellular carcinoma. Zhonghua Zhong Liu Za Zhi. 2010;32:344–349. (In Chinese) [PubMed] [Google Scholar]
- 24.Kim SW, Kim HY, Song IC, Jin SA, Lee HJ, Yun HJ, Kim S, Jo DY. Cytoplasmic trapping of CXCR4 in hepatocellular carcinoma cell lines. Cancer Res Treat. 2008;40:53–61. doi: 10.4143/crt.2008.40.2.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ishii T, Nishihara M, Ma F, Ebihara Y, Tsuji K, Asano S, Nakahata T, Maekawa T. Expression of stromal cell-derived factor-1/pre-B cell growth-stimulating factor receptor, CXC chemokine receptor 4, on CD34+ human bone marrow cells is a phenotypic alteration for committed lymphoid progenitors. J Immunol. 1999;163:3612–3620. [PubMed] [Google Scholar]
- 26.Kollet O, Shivtiel S, Chen YQ, Suriawinata J, Thung SN, Dabeva MD, Kahn J, Spiegel A, Dar A, Samira S, et al. HGF, SDF-1, and MMP-9 are involved in stress-induced human CD34+ stem cell recruitment to the liver. J Clin Invest. 2003;112:160–169. doi: 10.1172/JCI17902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dar A, Kollet O, Lapidot T. Mutual, reciprocal SDF-1/CXCR4 interactions between hematopoietic and bone marrow stromal cells regulate human stem cell migration and development in NOD/SCID chimeric mice. Exp Hematol. 2006;34:967–975. doi: 10.1016/j.exphem.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 28.Bertran E, Crosas-Molist E, Sancho P, Caja L, Lopez-Luque J, Navarro E, Egea G, Lastra R, Serrano T, Ramos E, Fabregat I. Overactivation of the TGF-β pathway confers a mesenchymal-like phenotype and CXCR4-dependent migratory properties to liver tumor cells. Hepatology. 2013;58:2032–2044. doi: 10.1002/hep.26597. [DOI] [PubMed] [Google Scholar]
- 29.Vlahakis SR, Villasis-Keever A, Gomez T, Vanegas M, Vlahakis N, Paya CV. G protein-coupled chemokine receptors induce both survival and apoptotic signaling pathways. J Immunol. 2002;169:5546–5554. doi: 10.4049/jimmunol.169.10.5546. [DOI] [PubMed] [Google Scholar]
- 30.Bertran E, Caja L, Navarro E, Sancho P, Mainez J, Murillo MM, Vinyals A, Fabra A, Fabregat I. Role of CXCR4/SDF-1 alpha in the migratory phenotype of hepatoma cells that have undergone epithelial-mesenchymal transition in response to the transforming growth factor-beta. Cell Signal. 2009;21:1595–1606. doi: 10.1016/j.cellsig.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 31.García-Irigoyen O, Latasa MU, Carotti S, Uriarte I, Elizalde M, Urtasun R, Vespasiani-Gentilucci U, Morini S, Benito P, Ladero JM, et al. Matrix metalloproteinase 10 contributes to hepatocarcinogenesis in a novel crosstalk with the stromal derived factor 1/C-X-C chemokine receptor 4 axis. Hepatology. 2015;62:166–178. doi: 10.1002/hep.27798. [DOI] [PubMed] [Google Scholar]
- 32.Jeng KS, Sheen IS, Jeng WJ, Yu MC, Tsai HH, Chang FY, Su JC. Blockade of the sonic hedgehog pathway effectively inhibits the growth of hepatoma in mice: An in vivo study. Oncol Lett. 2012;4:1158–1162. doi: 10.3892/ol.2012.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jeng KS, Sheen IS, Jeng WJ, Yu MC, Hsiau HI, Chang FY, Tsai HH. Activation of the sonic hedgehog signaling pathway occurs in the CD133 positive cells of mouse liver cancer Hepa 1–6 cells. Onco Targets Ther. 2013;6:1047–1055. doi: 10.2147/OTT.S44828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jeng KS, Sheen IS, Jeng WJ, Lin CC, Lin CK, Su JC, Yu MC, Fang HY. High expression of patched homolog-1 messenger RNA and glioma-associated oncogene-1 messenger RNA of sonic hedgehog signaling pathway indicates a risk of postresection recurrence of hepatocellular carcinoma. Ann Surg Oncol. 2013;20:464–473. doi: 10.1245/s10434-012-2593-y. [DOI] [PubMed] [Google Scholar]
- 35.Jeng KS, Sheen IS, Jeng WJ, Yu MC, Hsiau HI, Chang FY. High expression of Sonic Hedgehog signaling pathway genes indicates a risk of recurrence of breast carcinoma. Onco Targets Ther. 2013;7:79–86. doi: 10.2147/OTT.S54702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Singh AP, Arora S, Bhardwaj A, Srivastava SK, Kadakia MP, Wang B, Grizzle WE, Owen LB, Singh S. CXCL12/CXCR4 protein signaling axis induces sonic hedgehog expression in pancreatic cancer cells via extracellular regulated kinase- and Akt kinase-mediated activation of nuclear factor κB: Implications for bidirectional tumor-stromal interactions. J Biol Chem. 2012;287:39115–39124. doi: 10.1074/jbc.M112.409581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sengupta R, Dubuc A, Ward S, Yang L, Northcott P, Woerner BM, Kroll K, Luo J, Taylor MD, Wechsler-Reya RJ, Rubin JB. CXCR4 activation defines a new subgroup of Sonic hedgehog-driven medulloblastoma. Cancer Res. 2012;72:122–132. doi: 10.1158/0008-5472.CAN-11-1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tomuleasa C, Soritau O, Rus-Ciuca D, Pop T, Todea D, Mosteanu O, Pintea B, Foris V, Susman S, Kacsó G, Irimie A. Isolation and characterization of hepatic cancer cells with stem-like properties from hepatocellular carcinoma. J Gastrointestin Liver Dis. 2010;19:61–67. [PubMed] [Google Scholar]
- 39.Zhu L, Zhang W, Wang J, Liu R. Evidence of CD90+CXCR4+ cells as circulating tumor stem cells in hepatocellular carcinoma. Tumour Biol. 2015;36:5353–5360. doi: 10.1007/s13277-015-3196-6. [DOI] [PubMed] [Google Scholar]
- 40.Sukowati CH, Anfuso B, Torre G, Francalanci P, Crocè LS, Tiribelli C. The expression of CD90/Thy-1 in hepatocellular carcinoma: An in vivo and in vitro study. PLoS One. 2013;8:e76830. doi: 10.1371/journal.pone.0076830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yu C, Wang Z, Xu X, Xiang W, Huang X. Circulating hepatocellular carcinoma cells are characterized by CXCR4 and MMP26. Cell Physiol Biochem. 2015;36:2393–2402. doi: 10.1159/000430201. [DOI] [PubMed] [Google Scholar]
- 42.Yang ZF, Ho DW, Ng MN, Lau CK, Yu WC, Ngai P, Chu PW, Lam CT, Poon RT, Fan ST. Significance of CD90+ cancer stem cells in human liver cancer. Cancer Cell. 2008;13:153–166. doi: 10.1016/j.ccr.2008.01.013. [DOI] [PubMed] [Google Scholar]
- 43.Yang ZF, Ngai P, Ho DW, Yu WC, Ng MN, Lau CK, Li ML, Tam KH, Lam CT, Poon RT, Fan ST. Identification of local and circulating cancer stem cells in human liver cancer. Hepatology. 2008;47:919–928. doi: 10.1002/hep.22082. [DOI] [PubMed] [Google Scholar]
- 44.Piao LS, Hur W, Kim TK, Hong SW, Kim SW, Choi JE, Sung PS, Song MJ, Lee BC, Hwang D, Yoon SK. CD133+ liver cancer stem cells modulate radioresistance in human hepatocellular carcinoma. Cancer Lett. 2012;315:129–137. doi: 10.1016/j.canlet.2011.10.012. [DOI] [PubMed] [Google Scholar]
- 45.Shah AD, Bouchard MJ, Shieh AC. Interstitial fluid flow increases hepatocellular carcinoma cell invasion through CXCR4/CXCL12 and MEK/ERK Signaling. PLoS One. 2015;10:e0142337. doi: 10.1371/journal.pone.0142337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Honma N, Genda T, Matsuda Y, Yamagiwa S, Takamura M, Ichida T, Aoyagi Y. MEK/ERK signaling is a critical mediator for integrin-induced cell scattering in highly metastatic hepatocellular carcinoma cells. Lab Invest. 2006;86:687–696. doi: 10.1038/labinvest.3700427. [DOI] [PubMed] [Google Scholar]
- 47.Zhu M, Guo J, Xia H, Li W, Lu Y, Dong X, Chen Y, Xie X, Fu S, Li M. Alpha-fetoprotein activates AKT/mTOR signaling to promote CXCR4 expression and migration of hepatoma cells. Oncoscience. 2015;2:59–70. doi: 10.18632/oncoscience.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen Y, Huang Y, Reiberger T, Duyverman AM, Huang P, Samuel R, Hiddingh L, Roberge S, Koppel C, Lauwers GY, et al. Differential effects of sorafenib on liver versus tumor fibrosis mediated by stromal-derived factor 1 alpha/C-X-C receptor type 4 axis and myeloid differentiation antigen-positive myeloid cell infiltration in mice. Hepatology. 2014;59:1435–1447. doi: 10.1002/hep.26790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gao DY, Lin TsT, Sung YC, Liu YC, Chiang WH, Chang CC, Liu JY, Chen Y. CXCR4-targeted lipid-coated PLGA nanoparticles deliver sorafenib and overcome acquired drug resistance in liver cancer. Biomaterials. 2015;67:194–203. doi: 10.1016/j.biomaterials.2015.07.035. [DOI] [PubMed] [Google Scholar]


