Abstract
In the last 15 years, it emerged that the practice of regular physical activity reduces the risks of many diseases (cardiovascular diseases, diabetes, etc.) and it is fundamental in weight control and energy consuming to contrast obesity. Different groups proposed many molecular mechanisms as responsible for the positive effects of physical activity in healthy life. However, many points remain to be clarified. In this mini-review we reported the latest observations on the effects of physical exercise on healthy skeletal and cardiac muscle focusing on muscle stem cells. The last ones represent the fundamental elements for muscle regeneration post injury, but also for healthy muscle homeostasis.
Interestingly, in both muscle tissues the morphological consequence of physical activity is a physiological hypertrophy that depends on different phenomena both in differentiated cells and stem cells. The signaling pathways for physical exercise effects present common elements in skeletal and cardiac muscle, like activation of specific transcription factors, proliferative pathways, and cytokines. More recently, post translational (miRNAs) or epigenetic (DNA methylation) modifications have been demonstrated. However, several points remain unresolved thus requiring new research on the effect of exercise on muscle stem cells.
Keywords: Stem cell, Physical exercise, Cardiac muscle, Skeletal muscle
1. Introduction
The World Health Organization (WHO) has recently published latest recommendations on physical activity for health, evidencing how physical inactivity is the fourth leading risk for global mortality after high blood pressure, tobacco use and high blood glucose [1].
On the other side, in the last 15 years, it has been shown that the practice of regular physical activity reduces the risks of cardiovascular diseases, diabetes, colon cancer, breast cancer and depression. Moreover, physical exercise has been demonstrated to be fundamental in weight control and energy expenditure to contrast the increase of obesity.
Pedersen and Saltin, in 2015 [2], elegantly reviewed the role of exercise as adjuvant therapy for 26 chronic diseases covering various aspects of the medical subjects (neurology, psychiatry, metabolism, cardiology, diseases of the locomotor apparatus). They also proposed the mechanisms that stand behind the positive effects of physical activity on these pathologies. However, the molecular players and pathways are still under debate. In fact physical exercise induces molecular adaptations like release of growth factors, cytokines and hormones that have therapeutic effects, improving both regeneration and function of many organs (i.e. skeletal muscles, bone, heart, lung, brain). The first effect of physical exercise is obviously found on cardiac and skeletal muscles where a crucial role in organ homeostasis is assumed by stem cells. In this mini-review we will describe the effect of physical exercise on skeletal and cardiac muscles focusing on the roles of physical exercise in activation of skeletal and cardiac muscle stem cells.
2. Exercise and training modalities
Before discussing the effect of physical exercise on muscle, in this paragraph, we summarize the different training modalities reported in this mini-review.
Aerobic (endurance) training is referred to exercises performed in aerobic conditions when glucose metabolism depends on oxygen. Generally, aerobic training induces adaptations of the cardiovascular and respiratory systems. In particular, aerobic training alters extrinsic modulation of the heart and improves intrinsic pump capacity [3] together with a variety of metabolic and morphological changes, comprising mitochondrial biogenesis and fast-to-slow skeletal muscle fiber-type conversion. It also increases muscle capillary density. Example of aerobic sports are cycling, marathon and triathlon. On the contrary, resistance exercise alludes to an exercise performed with weight or over-load in anaerobic condition. The last one induces muscle hypertrophy together with changes in muscle architecture and in the central nervous system [4]. Snijders et al. [5] showed that muscle fiber hypertrophy that occurs after prolonged resistance training in older men depends from Type II muscle fiber capillarization suggesting the importance of systemic released factors.
Resistance exercise decreases cardiac parasympathetic modulation suggesting an increased risk for cardiovascular dysfunction in young healthy adults. Resistance exercise training appears to have no effect on resting Heart Rate Value (HRV) in healthy young adults, while it may improve parasympathetic modulation in middle-aged adults with autonomic dysfunction [6].
Eccentric exercise is performed when the load placed on muscle is greater than the tension that can be created within the sarcomere determining the lengthening of the muscle. Eccentric contractions are present predominantly during participation in planned resistance exercise with loads like body-building; however, they also commonly occur during activities of daily living such as lifting and lowering heavy items or walking downstairs. While repeated shortening of muscle can increase endurance and fatigue resistance, exercise, that continually elongate muscles can increase sarcomere myofibrillar content, sarcomere number, and the muscle’s ability to generate force. These beneficial effects are now considered for designing therapeutic interventions to improve the rate and efficiency of skeletal muscle healing following injury.
Concentric exercise, instead, provokes shortening of the sarcomeres and transfer of force from muscle to the body’s lever system [4]. An example of concentric exercise is uphill walking. Generally, it is counter posed to downhill walking that is considered eccentric exercise.
Concerning the cardiovascular effect of the eccentric and concentric training Isner-Orobeti et al. [7] evidenced that, in case of a similar mechanical power, eccentric muscle work induces lower metabolic and cardiovascular responses than concentric muscle work. However, when both exercise modes are performed at a similar level of VO2max’, a greater cardiovascular stress is observed during eccentric muscle work. This observation induces the need of cautious interpretation of the heart rate values for training load management between eccentric and concentric muscle work.
3. Skeletal muscle and physical activity
Skeletal muscle has the fundamental role of maintaining skeletal architecture and locomotion [8] but also represents a huge site for glycogen synthesis [9] and amino-acid deposits [10]. Exercise induces molecular and cellular adaptations that lead to a general amelioration of physical performance.
Muscle homeostasis and metabolism depend on protein synthesis/degradation and activity of muscle stem cells. While total muscle protein synthesis and degradation seem to depend mainly on nutrition, physical exercise seems to act both on protein synthesis/degradation and on satellite cells. The role of protein metabolism is evident for muscle contraction speed. This parameter is very different among muscles where the structural fundamental component is myosin (protein) that is present both in slow and fast isoforms. Recent studies clarified that the important factor for this fast to slow protein switch is duration of training that should not be less than one year [11].
At level of the entire muscle, the principal effect of exercise is represented by hypertrophy that can be produced both by new satellite cell fusion or by increased protein synthesis. Probably, when an acute stimulus occurs (i.e. strong exercise with eccentric contractions) satellite cells are activated and contribute to hypertrophy increasing myonuclei number and fiber size, thus mimicking the regeneration pathway in response to damage. Differently, gradual exercise does not activate satellite cells and functional over-load induces a muscle growth response via protein synthesis alone [12].
Another important factor to consider in muscle homeostasis regulation is age. Metabolic and molecular regulation of responses to nutrition and physical exercise (both resistance and aerobic) in older and young people have been recently reviewed [13].
Cycling induces muscle hypertrophy as outcome of an increase in protein net balance both in older and young people but timing of this remains unknown [14].
Due to the slower hypertrophy rate of cycling training it is plausible that this practice requires a longer period to increase muscle mass compared to typical endurance training. Interestingly, while the effect on muscle hypertrophy is similar in older and young people, the strength gain is higher in older adults. Considering that muscle metabolism decreases with age leading to reduction of muscle mass, cycling would represent a plausible sport for older adults in presence of adequate medical advices. Instead, for young people, higher intensity intermittent cycling seems to be required to reach strength gain [15].
Concerning the link between physical exercise and age, it has to be mentioned that physical exercise opposes to age-induced sarcopenia. In fact, beside hypertrophy, the first effect of a training protocol is the fiber–type adaptation meaning a change from fast fiber to slow fiber associated with the release of reactive oxygen species. This causes an indirect protective role, stimulating production of anti-oxidant enzymes that are effective in muscle contractile dysfunction and fatigue. Furthermore, these phenomena are generated independently from the training intensity and have been observed both in fast fibers rich muscles (like tibialis anterior) and slow fibers rich muscles (like soleus) [16].
Although the molecular mechanisms that provoke this condition are still unknown some hypothesis have been formulated like: imbalance between protein synthesis and degradation, decrease in the size of type II fibers [17] and decrease in satellite cell content of type II fibers [18]. Since physical activity seems to act on all these levels, possible roles of physical activity to contrast aging sarcopenia have emerged, but not completely clarified. Sarcopenia has also been related to mitochondrial dysfunction [19], because it has been observed that mitochondrial DNA damage led to muscle wasting.
Importance of mitochondria and Calcium related proteins in sarcopenia has been evidenced also in Zampieri et al. 2016 [20] who showed that electrical stimulated-dependent effects on skeletal muscle size and force are associated with mitochondrial proteins involved in Calcium homeostasis (i.e. Mitochondrial Calcium Uniporter, MCU).
The effect of exercise on muscle hypertrophy has been demonstrated in myostatin null mice (MSTN-/-) with reduced muscle function. These mutant mice develop an increased muscle mass in the adulthood [21]. In this paper authors demonstrated that endurance exercise like running or swimming reduced muscle fiber size towards wild type values after exercise, increased muscle oxidative properties, capillary density and, most importantly, improved muscle force of myostatin null mice. Thus, endurance exercise can improve impaired skeletal muscle function of myostatin negative mice confirming the positive role of physical activity on skeletal muscle function.
Notably, also aerobic exercise has revealed an anabolic potential comparable to resistance exercise by altering protein metabolism and inducing skeletal muscle hypertrophy [22].
Possible roles for miRNAs (also called miRs) as intermediates for the effect of resistance exercise training have been proposed from different groups [23-26]. MicroRNAs are small non-coding RNAs (about 22 nucleotides) that negatively modulate gene expression at the post-transcriptional level by blocking translation or inducing degradation of mRNAs. Interestingly, all groups observed a down-regulation of mature isoforms of the miRs. In particular, McCarthy et al (2007) [24] showed a decrease of miR-1 e miR133a in mice. Davidsen et al. 2011 [25] observed, in humans, a down-regulation of miR-26a, miR-29a e miR-378 concomitantly to an up-regulation of miR-451. Finally, Mueller et al. [26] found, in elderly people, a decrease of miR-1 in parallel with an up-regulation of IGF-1.
On the contrary a single bout of resistance exercise followed by cycling determined a significant increase in miR-23a-3p (~90%), miR-23b-3p (~39%), miR-133b (~80%), miR-181-5p (~50%), and miR-378-5p (~41%) at 4 hours post-exercise [27].
A recent review by Masi et al. (2016) [28] reports the gene expression regulations by exercise-related miRs. Possible miRs targets have been suggested as well: 1) signaling pathways regulated by Calcium, AMP and PKD; 2) class IIa histone deacetylases; 3) muscle specific transcription factors (i.e. MyoD, Myogenin); 4) mitochondrial targets (mitochondrial transcription factor A (mtTFA) and Forkhead box J-3 (FoxJ-3)/MEF-2c); 5) MAPKs; 6) Runx1, Sox9, Pax3; 7) VEGF and IGF-1.
Collectively, these results evidence an emerging position for miRNAs in muscle metabolism regulation by physical activity. However further studies to clarify the roles of micro-RNAs in skeletal muscle training adaptation will be necessary.
Effects of physical exercise are also exerted through epigenetic alterations of DNA such as DNA de-methylations. Barrès et al. [29] showed that acute exercise induced transient DNA demethylation in the promoter region of Peroxisome proliferator activated receptor gamma (Pparg), Peroxisome proliferator activated receptor gamma coactivator-1alpha (Ppargc -1α), Pyruvate dehydrogenase kinase 4 (Pdk4), and Peroxisome Proliferator activated receptor delta (Ppard). These modifications corresponded to an up-regulation of these genes and reduced the risks of developing metabolic diseases.
Moreover, several authors have shown that, during perinatal development, mechanical stimuli might alter the epigenetic program, which has consequences for gene transcription and functional outcomes of the fetus [30]. Therefore, it is possible that physical training during pregnancy is associated with epigenetic regulation of critical genes in early life.
Finally, Gehlert et al. [31] recently showed that resistance exercise modulated nuclear and sarcoplasmic levels of SUMO-1 protein thus regulating muscle protein degradation and modifying muscle protein metabolism.
4. Skeletal muscle stem cells
Physical activity represents a continuous prod both for the entire muscle and satellite cells. Stem cells have been operatively defined as cells that are capable to self-regeneration giving origin to a cell that differentiates in a specific type and a cell identical to the mother. Satellite cells represent the principal reservoir of stem cells in skeletal muscle. Other skeletal muscle stem cells are mesenchymal-like stem cells, mesoangioblasts/pericytes, side population cells (SP cells), Skeletal Muscle Induced Pluripotent Stem Cells.
5. Satellite cells
In 1961 Alexander Mauro described, for the first time, satellite cells [32]. They are characterized by an exiguous cytoplasm, almost assuming the shape of the nucleus and localized in the peripheral region of the skeletal muscle fibers. Unlike other stem cells, satellite cells reside in a quiescent state and move to an activated state in response to damage or to stimuli coming from their microenvironment or from systemic circulation. In this chapter we shall analyze the effects of physical exercise on human and rodent satellite cells.
Moving from a quiescent to an activated state implies gene expression profile changes [33]. In particular, quiescent satellite cells are characterized by the homogeneous expression of PAX7 and, partially, of Myf5 transcription factors. Activated cells engage differentiation or proliferation pathways. Proliferating cells (also called myogenic precursor cells) express MyoD and Myf5. Entrance in the differentiated state determines downregulation of PAX7 and upregulation of Myogenin that, together with MyoD, will turn on muscle specific contractile genes like MEF2. Notably, the activated state of satellite cells is reversible permitting the return to a quiescent condition that is fundamental to maintain the stem cell pool.
One important effect of physical exercise regards the quantity of satellite cells. In fact, some groups observed an increase of satellite cell number from 9 hours to 24 hours post resistance exercise [34-37], determining muscle hypertrophy [34]. The activation process of satellite cells takes place within 24 hours from exercise. Also, a significant increase both in total and activated satellite cells was observed after 11 weeks of resistance training [38]. In rats a significant increase in satellite cell total content was observed 72 hours after eccentric exercise (running) [39]. Oishi et al. [40] showed an increase of satellite cell number and activity in rats after 4 weeks of running.
At molecular level, increased mRNA expression of PAX7, DLK1, NCAM, MYF5, MYOD and MYOGENIN were observed after 12 weeks of resistance training [41].
Finally, Macaluso et al. [42] postulated that physically active people, who has higher maximal oxygen volume (VO2max), has higher number of satellite cells. However, without injury, physical activity does not seem to stimulate fusion, but only proliferation of satellite cells. Instead, Frese et al. [43] reported a significant increase of fused myonuclei in human muscle biopsies of cyclists after resistance training. McKenzie et al. [44] proposed that satellite cell proliferation and myonuclear fusion occur under carbohydrate supplementation conditions, while protein supplementation stimulate muscle recovery and increased MyHC I and II fiber size minimizing the satellite cell involvement.
Recently, PGC-1a have been proposed to have an indirect role on sensitivity of satellite cells to exercise. In particular,PGC-1 null satellite cells have a higher propensity for activation and proliferation [45].
Another important component of satellite cell activation is Ca2+ ion that, through Calcineurin, regulates satellite cell transcription factors like MyoD, and MEF2 [46, 47]. Ca2+ is also important for cell cycle stage regulation in satellite cells via Calpains [47].
In conclusion, increase in the number and activity of satellite cells was found independently from the type of exercise suggesting that all the mechanical stimuli induce similar mechanisms on satellite cells.
Resistance type exercise is the best non-pharmacological intervention to reduce skeletal muscle loss associated to age or disease like cancer [48]. However, so far, the molecular mechanisms that stand behind this effect are still not completely understood. Thus further investigations on the related signaling pathways are warranted.
Quiescent and activated satellite cells differ, also, in metabolic status since the former have fewer mitochondria and a glycolytic metabolism compared to activated cells; moreover quiescent cells repair DNA damage more efficiently than their progeny [49].
Relation between muscle mitochondrial health and exercise has been evidenced by Taivassalo et al. [50] which examined the mitochondrial genotype in mature myofibers of patients with mitochondrial disease, following either an eccentric or concentric Resistance Training intervention and found a significant increase in the amount of wild type mitochondrial DNA together with a dramatic decrease in the proportion of COX-negative muscle fibers. This process depends on activated satellite cells that fuse to form new fibers [51]. The incorporation of satellite cell derived mitochondria explains the increase in wild type mitochondrial DNA (gene shifting). This was supported by the studies demonstrating that 12 weeks of resistance training induced an increase in muscle strength, myofiber damage and regeneration, NCAM-positive and COX-positive satellite cells and oxidative capacity in adults with mitochondrial DNA deletions [52, 53]. According to these authors mitochondrial DNA mutations, eventually occurred in satellite cells, are lost during muscle stem cell activation and proliferation. In general, this evidence supports the hypothesis that resistance training acts as a mitochondrial DNA shifting activating satellite cells. Further studies are necessary to quantify the extent of this phenomenon and the intensity of the exercise that is an important determinant of mitochondrial function.
Interestingly, Murach et al. [54] showed that resistance training induced coordinated gene expression pattern to promote adaptation of muscle to training.
Finally, a single bout of resistance exercise 24h after 16 weeks of resistance training activated satellite cells (increased number of Pax7/MyoD positive nuclei) and enhanced capillary density in both type I and type II fibers suggesting that satellite cell response to resistance exercise is accompanied by increased muscle fiber capillarization [55].
Endurance exercise induces inflammatory-myogenic processes during skeletal muscle recovery [56]. Thus, effect of physical exercise is exerted also by the production of many inflammatory cytokines.
Cytokines represent a large family of proteins that mediate intercellular communications both in the local microenvironment and in systemic circulation. mRNA levels of Interleukin 1b (IL-1b), Interleukin 6 (IL-6), Interleukin 7 (IL-7), Interleukin 8 (IL-8), Interleukin 10 (IL-10) were up-regulated following resistance and eccentric exercises, but only IL-6 protein was found to be up-regulated in response to exercise [57]. It is mainly localized in type II fibers [58]. Interestingly, also other cytokines like VEGF and MCP-1 were up-regulated in muscle stem cells in response to exercise. VEGF was found in the pericytes that surround the capillaries of skeletal muscle [59] while MCP-1 is secreted by macrophages and satellite cells [60]. Unfortunately, the mechanisms that regulate skeletal muscle and satellite cell function in response to these cytokines are still not understood [61], thus more research on this topic is needed. For an extensive review regarding a role for inflammation in the regenerative response to exercise, please refer to Saclier et al. [62]. Together with cytokines secretion, we have to remember that another important consequence of exercise is angiogenesis. In particular, a balance between pro- and anti-angiogenic factors (not all clarified) has to be maintained for vascular homeostasis. This topic is thoroughly reviewed by Olfert et al. [63].
Finally, the possible role of miRNAs in the regulation of satellite cells by physical exercise was investigated. It was demonstrated by two independent groups (Polesskaya et al., 2013; Hashemi Gheinani et al., 2015) [64, 65] that a particular miRNA (miR-199a-5p) inhibits the expression of several components of the Wnt signaling pathway that, in turn, regulates satellite cell maintenance and differentiation. Over-expression of these components of Wnt pathway during muscle regeneration resulted in the enhancement of the reparative process, generating more fibers of bigger caliber, independently from an effect on new myoblasts proliferation or differentiation.
6. Non-satellite cells
Beside satellite cells, resident stromal cells have been identified both in human and mouse skeletal muscle. These cells express myogenic potential but represent a heterogeneous population with different progenitors that directly or indirectly contribute to repair and remodel skeletal muscle after injury or disease. A recent review on the role of satellite and non-satellite cells for skeletal muscle therapy came from Rudnicki’s group [66]. Briefly, Side Population cells (SP), Mesoangioblasts/Pericytes, Bone Marrow Hematopoietic Stem Cells, Bone Marrow Mesenchymal Stem Cells, Adipose tissue-derived mesenchymal stem cells (ADMSC), Embryonic Stem cells (ES) and Induced-Pluripotent Stem cells (iPS) are under attentive consideration in muscle cell therapy.
We here shall analyze only the non-satellite stem cells that were shown to be involved in training adaptation processes. In particular, although limited, it has been demonstrated on the following cells: human skeletal muscle pericytes [67, 68] and human Mesenchymal Sca1+CD45− cells[69].
Muscle Pericytes are located on the basal membrane of vessels and are characterized by the expression of alkaline phosphatase (ALP), nerve/glial antigen 2 (NG-2) proteoglycan, CD146 [68,70]. Recently, Kostallari and colleagues [71] demonstrated that pericytes promoted satellite cell maintenance and post-natal myogenesis, thus playing a fundamental role in sustaining muscle stem cell niche.
After 3 hours from a single bout of eccentric exercises pericyte cell number remained unaltered, but the cells showed a significant increase in NF-kB activity [72] which is found to be involved in proliferation of endothelial cells in vitro and secretion of inflammatory molecules like MCP-1 and IL-8 [73]. In accordance, De Lisio et al. [67] found that, after 24 hours or 12 weeks from eccentric exercise, the quantity of pericytes in human muscle was unaltered. Instead, after 12 weeks of concentric exercise, NG2+ ALP+ cell number diminished [68].
Mesenchymal stem cells (MSCs) are a multipotent cell population located in a number of tissues of all the body and able to differentiate along the osteogenic, adipogenic and chondrogenic lineages [74]. They are positive for the cell surface markers CD105, CD73, CD90 and negative for CD45, CD34, CD14, or CD11b, CD79 or CD19, and HLA-DR.
In murine skeletal muscle a mesenchymal population with multi-lineage potential and labelled by Sca1 was found increased 24h post exercise but it did not give rise to new fibers suggesting that these cells might have a different role post-exercise [75]. In humans, PDGFRα positive mesenchymal cells increased following both concentric and eccentric training as well as differentiating satellite cells [68]. However, new fibers derived from mesenchymal stem cell were not found confirming that role of mesenchymal stem cells could be the regulation of satellite cell pool expansion through the release of paracrine factors. In fact the prevailing hypothesis is that mesenchymal stem cells are responsible for transfer the signal turned on following exercise. Further studies will be necessary to reveal the starting molecules and the mediators secreted by mesenchymal stem cells in this process.
7. Cardiac muscle cells and physical activity
The American Heart Association recommends that people perform moderately intense exercise for at least 30 minutes 2-3 days a week. In fact, exercise has several effects that benefit the heart and blood circulation. These positive effects include the decrease of fat levels helping weight loss programs, the reduction of inflammation and the maintenance of blood vessels flexible and open. In fact, sedentary people have a 35% greater risk, with respect to physically active people, of developing high blood pressure. Together with physical activity healthy eating is another successful mean of reaching and maintaining heart-healthy levels of fitness, weight and heart physiology [76].
Different authors suggested that the effects of exercise on cardiac tissue and vessels are dependent on frequency, intensity and duration of the exercise itself [77-79].
The principal effects of physical exercise in the heart are cardiac hypertrophy and an increased angiogenesis [80].
Cardiac hypertrophy following sustained physical exercise is due to an increase in left ventricular wall thickness and in the size of cardiomyocytes that are responsible for generating contractile force of the heart [81]. This is defined as physiological hypertrophy and is characterized by typical or even higher cardiac function. These characteristics are grouped under the definition of “athlete’s heart.” In contrast, pathological cardiac hypertrophy occurs in response to a series of stimuli, such as myocardial infarction, valve disease and dilated cardiomyopathy. Recently, it emerged that there is a “grey zone” between physiological and pathological hypertrophy that should be studied to distinguish these two hypertrophy types [82].
Physiological hypertrophy has a protective effect against some cardiovascular diseases such as ischemic injury. It is characterized by reduced cardiac apoptosis and by the down-regulation of cardiac fetal genes like α-MHC and sarcoplasmic reticulum Ca2+ ATPase (SERCA2a) [83-86]. Moreover, a complex network of cardiac transcription factors, like GATA4, GATA6, Csx/Nkx2.5, MEF2 is activated by physical exercise and induces physiological hypertrophy [87, 88].
Positive effects of physical exercise have been reported also on aging heart. Two different studies on rats by Kwak et al. showed that exercise training significantly attenuated age-induced increases of apoptosis in ventricles, as indicated by lower DNA fragmentation, TUNEL-positive staining, and caspase-3 cleavage, when compared with ventricles from the age-matched sedentary group [89, 90].
In the heart, the reported signaling molecules of physical exercise are Insulin Growth Factor 1 (IGF-1) and Neuregulin 1 (NRG-1) [91-94]. IGF increases cardiac telomerase activity and stimulates Phosphatidylinositol-3 kinase (PI3K)-RAC-alpha serine/threonine-protein kinase (AKT1) pathway; while NRG-1 activates cardiac transcription factors.
Recently, it has been reported by numerous studies that miRNAs play significant roles in cardiac pathophysiology, cardiovascular development and cardiac regeneration [95-97]. An emerging role for miRNAs in translation of exercise effect in heart has been recently reviewed in Fernandes et al. [80]. In particular, aerobic exercise reduces cardiac fibrosis through activation of miR-29, increases angiogenesis by miR-26 and, finally, regulates renin-angiotensin system through the miRNAs-27a/b and -143. Moreover, many miRNAs are involved in cardiac cell growth and survival and they are differently regulated by aerobic sports. For example swimming activated miRNA-1, -21, -27a/b, -29a/c, -30e, -99b, -100, -124, -126, -133a/b, -143, -144, -145, -208a, and -222, while running increased levels of miRNA-1, -26, -27a, -133, -143, -150, and -222.
Role of miRs in cardiac hypertrophy has been reviewed by Xu et al. [98] that also suggested possible targets like Fatty acid elongation pathway, Arrhythmogenic Right Ventricular Cardiomyopathy (ARVC) and ECM-receptor pathway.
Another miRNA, miR-214, is decreased upon resistance training, leading to increased left ventricular myocyte width and volume in rats [99]. Finally, circulating miRNAs may also play a relevant role in the physiological exercise response. These miRNAs, like miR-106a, -30b, -146, -338 and -21, are secreted or released from cells into the blood and play important roles in intercellular communication. Circulating miRNAs may be especially intriguing in the exercise response because they could potentially help drive similar effects, such as cellular proliferation, across multiple organ systems [100, 101].
8. Heart stem cells and physical activity
Although the principal player of cardiac hypertrophy is the enlargement of differentiated cardiomyocytes, Leite et al. [102] recently demonstrated that also c-kit+ cardiac stem cells (CSC) participate to cardiac hypertrophy generated by physical exercise. c-Kit+ CSC have been first reviewed 10 years ago [103]. Now the scientific community is convinced that the heart is an organ able to regenerate, although with a very low rate (1% per year at the age of 25) [104] and cardiac stem cells represent the principal players of this phenomenon.
Regarding the relationship between heart stem cells and physical exercise the first effect is the increase of cardiac stem cell number, through IGF-1-Akt signaling that induce cardiac stem cell proliferation [105].
Another important consequence of regular physical training is the increase of vascular nitric oxide (NO) concentration. NO is responsible for vasodilation, which results in the lowering of peripheral resistance and increase of perfusion [106]. In particular, it has been demonstrated by many authors that the shear stress generated with physical exercise increases intracellular level of the endothelial nitric oxide synthase (eNOS), the main source of NO. The up-regulation of this enzyme results as a complex pattern of intracellular regulation both in cardiac stem cells and endothelial progenitors [107], thus influencing both cardiac hypertrophy and increasing angiogenesis.
Endothelial progenitors are crucial for the increased capillary density observed both in heart and skeletal muscle following physical exercise and recently reviewed in Wilson et al. 2016 and Schuler et al. 2013 [77, 106]. Together with endothelial progenitors, also CD34+ circulating angiogenic cells contribute to vascular structures and their function is regulated by endurance exercise [108]. Other stem cells like bone marrow progenitors or induced pluripotent stem cells are under high consideration for cardiovascular repair [109], but their role in cardiac hypertrophy induced by physical exercise has not been clarified yet.
9. Conclusions
Physical activity is one of the most important factor to maintain general health population. Thus, long-term compliances need to be proposed to make it really effective on a large scale.
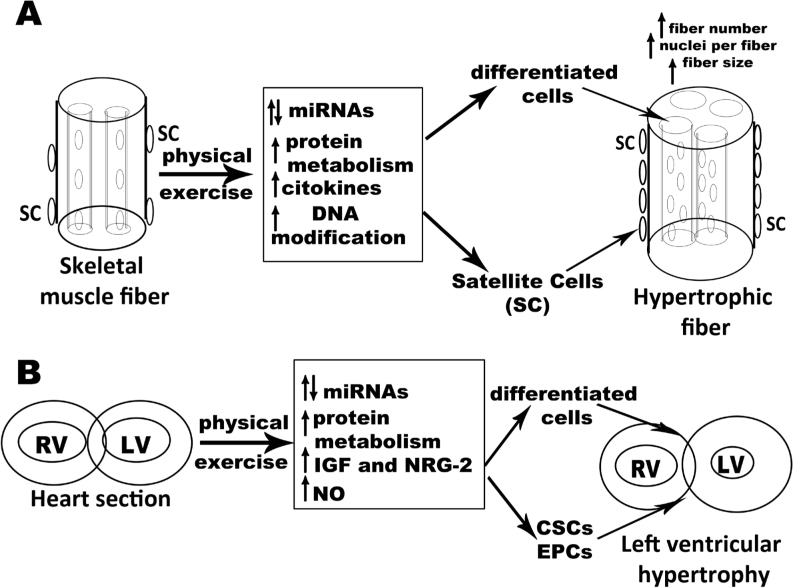
Benefits of physical exercise have been evidenced in many anatomical systems (musculo-skeletal, cardiovascular, digestive, etc.). In this mini-review, we analyzed the effect of physical exercises on skeletal and cardiac muscles focusing on muscle stem cell activation by physical activity. Interestingly, the morphological consequence of physical activity both in skeletal muscle and heart is hypertrophy (Figure 1A-B). In particular, in skeletal muscle activated satellite cells fuse with the existing fibers leading to an increase of fiber size. In the heart, physical exercise increases the width of cardiomyocytes and, in parallel, activates cardiac stem cells. While for skeletal muscle the effect of physical exercise is becoming more and more clear, in the myocardium definitive data are still lacking. This is probably due to the different accessibility between skeletal and cardiac muscle. In particular, while many assays are possible in skeletal muscle by a single biopsy, this is not possible in the human heart.
Figure 1.
Schematic representation of the effects of physical activity on skeletal muscle and heart.
Panel A: muscle is shown like a cylinder with inside poly-nucleated fibers. Physical activity determines a morphological hypertrophy of muscle due to the increase in fiber numbers, in nuclei number per fiber and fiber size. Together with muscle hypertrophy, exercise induces proliferation of endothelial cells and release of proangiogenic and anti-angiogenic factors whose balance contributes to muscle adaptation to physical exercise. SC, Satellite Cells;
Panel B: A schematic heart section is shown. Physical exercise determines cardiac hypertrophy (due to the increase of cardiomyocyte length, activation of cardiac stem cells and microvascular remodeling).
RV, Right Ventricle; LV, Left Ventricle; IGF, Insulin Like Growth Factor 1; NRG-2, Neuregulin-2; NO, Nitric Oxide; CSCs, Cardiac Stem Cells; EPCs, Endothelial Progenitor Cells. In both panels, possible molecular mediators are reported.
Different training types (aerobic, resistance) seem to have similar effects like hypertrophy even if the signaling pathways are different.
Indeed the molecular signature of physical exercise effects presents common elements both in differentiated and in quiescent stem cells like: activation of specific transcription factors, proliferative pathways, and cytokines secretion. Also mitochondrial DNA has been shown to have a role in stem cell activation. More recently, post translational modulations (miRNAs) or epigenetic (DNA modifications) have also been demonstrated.
However, although many players have been identified, we think that several points remain unresolved thus requiring new research on the effect of exercise on muscle stem cells. Finally, research has advanced in therapeutic use of exercise, but more definitive protocols of training need to be established for general healthy population to identify the best exercise strategy in terms of intensity, volume, and timetable, also taking into account the overall physical condition of the subject.
Acknowledgements
We wish to thank Cristina Micheloni and Luciana Cerasuolo (University of Parma) for administrative support. We apologize if, inadvertently, did not cite some other contributors in the field.
Abbreviations
- VO2max
Maximal Oxygen Volume.
- AMP
Adenosine MonoPhosphate.
- PKD
Protein Kinase D.
- MAPKs
Mitogen-Activated Protein Kinases.
- VEGF
Vascular Endothelial Growth Factor.
- IGF
Insulin Growth Factor.
- HRV
Heart Rate value
Footnotes
Conflict of interests: No authors report any conflict of interest.
References
- [1].World Health Organization. Global recommandation of physical activity for health 2010. :1–58. http://www.who.int/dietphysicalactivity/publications/9789241599979/en/ [PubMed] [Google Scholar]
- [2].Pedersen B. K., Saltin B.. Exercise as medicine - evidence for prescribing exercise as therapy in 26 different chronic diseases. Scand J Med Sci Sports. 2015;25:1–72. doi: 10.1111/sms.12581. [DOI] [PubMed] [Google Scholar]
- [3].Kemi OJ., Wisl⊘ff U.. Mechanisms of exercise-induced improvements in the contractile apparatus of the mammalian myocardium. Acta Physiol (Oxf) 2010;199:425–39. doi: 10.1111/j.1748-1716.2010.02132.x. [DOI] [PubMed] [Google Scholar]
- [4].Farup J., S⊘rensen H., Kj⊘lhede T.. Similar changes in muscle fiber phenotype with differentiated consequences for rate of force development: endurance versus resistance training. Hum Mov Sci. 2014;34:109–19. doi: 10.1016/j.humov.2014.01.005. [DOI] [PubMed] [Google Scholar]
- [5].Snijders T., Nederveen JP., Joanisse S., Leenders M., Verdijk LB., van Loon LJ., Parise G.. Muscle fibre capillarization is a critical factor in muscle fibre hypertrophy during resistance exercise training in older men. J Cachexia Sarcopenia Muscle. 2017;8:267–276. doi: 10.1002/jcsm.12137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Kingsley JD., Figueroa A.. Acute and training effects of resistance exercise on heart rate variability. Clin Physiol Funct Imaging. 2016;36:179–87. doi: 10.1111/cpf.12223. [DOI] [PubMed] [Google Scholar]
- [7].Isner-Horobeti ME., Dufour SP., Vautravers P., Geny B., Coudeyre E., Richard R.. Eccentric exercise training: modalities, applications and perspectives. Sports Med. 2013;43:483–512. doi: 10.1007/s40279-013-0052-y. [DOI] [PubMed] [Google Scholar]
- [8].Reid K.F., Fielding R.A.. Skeletal muscle power: a critical determinant of physical functioning in older adults. Exerc Sport Sci Rev. 2012;40:4–12. doi: 10.1097/JES.0b013e31823b5f13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Shulman G.I., Rothman D.L., Jue T., Stein P., DeFronzo R.A., Shulman R.G.. Quantitation of muscle glycogen synthesis in normal subjects and subjects with non-insulin-dependent diabetes by 13C nuclear magnetic resonance spectroscopy. N Engl J Med. 1990;322:223–228. doi: 10.1056/NEJM199001253220403. [DOI] [PubMed] [Google Scholar]
- [10].Pozefsky T., Tancredi R.G., Moxley R.T., Dupre J., Tobin J.D.. Effects of brief starvation on muscle amino acid metabolism in nonobese man. J Clin Invest. 1976;57:444–9. doi: 10.1172/JCI108295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Canepari M., Pellegrino M.A., D’Antona G., Bottinelli R.. Skeletal muscle fibre diversity and the underlying mechanisms. Acta Physiol (Oxf) 2010;199:465–76. doi: 10.1111/j.1748-1716.2010.02118.x. [DOI] [PubMed] [Google Scholar]
- [12].Schiaffino S., Dyar K.A., Ciciliot S., Blaauw B., Sandri M.. Mechanisms regulating skeletal muscle growth and atrophy. FEBS J. 2013;280:4294–314. doi: 10.1111/febs.12253. [DOI] [PubMed] [Google Scholar]
- [13].Brook M.S., Wilkinson D.J., Phillips B.E., Perez-Schindler J., Philp A., Smith K., Atherton P. J.. Skeletal muscle homeostasis and plasticity in youth and ageing: impact of nutrition and exercise. Acta Physiol (Oxf) 2016;2016;216:15–41. doi: 10.1111/apha.12532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Damas F., Phillips S., Vechin F.C., Ugrinowitsch C.. A review of resistance training-induced changes in skeletal muscle protein synthesis and their contribution to hypertrophy. Sports Med. 2015;45:801–7. doi: 10.1007/s40279-015-0320-0. [DOI] [PubMed] [Google Scholar]
- [15].Ozaki H., Loenneke J.P., Thiebaud R.S., Abe T.. Cycle training induces muscle hypertrophy and strength gain: strategies and mechanisms. Acta Physiol Hung. 2015;102:1–22. doi: 10.1556/APhysiol.102.2015.1.1. [DOI] [PubMed] [Google Scholar]
- [16].Abruzzo P.M., Esposito F., Marchionni C., di Tullio S., Belia S., Fulle S.. et al. Moderate exercise training induces ROS-related adaptations to skeletal muscles. Int J Sports Med. 2013;34:676–87. doi: 10.1055/s-0032-1323782. [DOI] [PubMed] [Google Scholar]
- [17].Lexell J., Taylor C.C., Sjöström M.. What is the cause of the ageing atrophy, Total number, size and proportion of different fiber types studied in whole vastus lateralis muscle from 15- to 83-year-old men. J Neurol Sci. 1988;84:275–94. doi: 10.1016/0022-510x(88)90132-3. https://doi.org/10.1016/0022-510X(88)90132-3 [DOI] [PubMed] [Google Scholar]
- [18].Verdijk L.B., Koopman R., Schaart G., Meijer K., Savelberg H.H., van Loon L.J.. Satellite cell content is specifically reduced in type II skeletal muscle fibers in the elderly. Am J Physiol Endocrinol Metab. 2007;292:E151–7. doi: 10.1152/ajpendo.00278.2006. [DOI] [PubMed] [Google Scholar]
- [19].Wang X., Pickrell AM., Rossi SG., Pinto M., Dillon LM., Hida A.. et al. Transient systemic mtDNA damage leads to muscle wasting by reducing the satellite cell pool. Hum Mol Genet. 2013;22:3976–86. doi: 10.1093/hmg/ddt251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Zampieri S., Mammucari C., Romanello V., Barberi L., Pietrangelo L., Fusella A.. et al. Physical exercise in aging human skeletal muscle increases mitochondrial calcium uniporter expression levels and affects mitochondria dynamics. Physiol Rep. 2016;4(24):e13005. doi: 10.14814/phy2.13005. Erratum in: Physiol Rep. 2017 Mar,5(6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Matsakas A., Macharia R., Otto A., Elashry M.I., Mouisel E., Romanello V.. et al. Exercise training attenuates the hypermuscular phenotype and restores skeletal muscle function in the myostatin null mouse. Exp Physiol. 2012;97:125–40. doi: 10.1113/expphysiol.2011.063008. [DOI] [PubMed] [Google Scholar]
- [22].Konopka A.R., Harber M.P.. Skeletal muscle hypertrophy after aerobic exercise training. Exerc Sport Sci Rev. 2014;42:53–61. doi: 10.1249/JES.0000000000000007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].McGregor R.A., Poppitt S.D., Cameron-Smith D.. Role of microRNAs in the age-related changes in skeletal muscle and diet or exercise interventions to promote healthy aging in humans. Ageing Res Rev. 2014;17:25–33. doi: 10.1016/j.arr.2014.05.001. [DOI] [PubMed] [Google Scholar]
- [24].McCarthy J.J., Esser K.A., Andrade F.H.. MicroRNA-206 is overexpressed in the diaphragm but not the hindlimb muscle of mdx mouse. Am J Physiol Cell Physiol. 2007;293:C451–7. doi: 10.1152/ajpcell.00077.2007. [DOI] [PubMed] [Google Scholar]
- [25].Davidsen P.K., Gallagher I.J., Hartman J.W., Tarnopolsky M.A., Dela F., Helge J.W.. et al. High responders to resistance exercise training demonstrate differential regulation of skeletal muscle microRNA expression. J Appl Physiol. 1985;2011;110:309–17. doi: 10.1152/japplphysiol.00901.2010. [DOI] [PubMed] [Google Scholar]
- [26].Mueller M., Breil F.A., Lurman G., Klossner S., Flück M., Billeter R., Däpp C., Hoppeler H.. Different molecular and structural adaptations with eccentric and conventional strength training in elderly men and women. Gerontology. 2011;57:528–38. doi: 10.1159/000323267. [DOI] [PubMed] [Google Scholar]
- [27].Camera D.M., Ong J.N., Coffey V.G., Hawley J.A.. Selective Modulation of MicroRNA Expression with Protein Ingestion Following Concurrent Resistance and Endurance Exercise in Human Skeletal Muscle. Front Physiol. 2016;7:87. doi: 10.3389/fphys.2016.00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Masi LN., Serdan TD., Levada-Pires AC., Hatanaka E., Silveira LD., Cury-Boaventura MF.. et al. Regulation of Gene Expression by Exercise-Related Micrornas. Cell Physiol Biochem. 2016;39:2381–2397. doi: 10.1159/000452507. [DOI] [PubMed] [Google Scholar]
- [29].Barrès R., Yan J., Egan B., Treebak J.T., Rasmussen M., Fritz T.. et al. Acute exercise remodels promoter methylation in human skeletal muscle. Cell Metab. 2012;15:405–11. doi: 10.1016/j.cmet.2012.01.001. [DOI] [PubMed] [Google Scholar]
- [30].Laker R.C., Ryall J.G.. DNA Methylation in Skeletal Muscle Stem Cell Specification, Proliferation, and Differentiation. Stem Cells Int. 2016:5725927. doi: 10.1155/2016/5725927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Gehlert S., Klinz F.J., Willkomm L., Schiffer T., Suhr F., Bloch W.. Intense Resistance Exercise Promotes the Acute and Transient Nuclear Translocation of Small Ubiquitin-Related Modifier (SUMO)-1 in Human Myofibres. Int J Mol Sci. 2016;17:pii:E646. doi: 10.3390/ijms17050646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Mauro A.. Satellite cell of skeletal muscle fibers. J Biophys Biochem Cytol. 1961;9:493–5. doi: 10.1083/jcb.9.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Yin H., Price F., Rudnicki M.A.. Satellite cells and the muscle stem cell niche. Physiol Rev. 2013;93:23–67. doi: 10.1152/physrev.00043.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Snijders T., Verdijk L.B., Beelen M., McKay B.R., Parise G., Kadi F., van Loon L.J.. A single bout of exercise activates skeletal muscle satellite cells during subsequent overnight recovery. Exp Physiol. 2012;97:762–73. doi: 10.1113/expphysiol.2011.063313. [DOI] [PubMed] [Google Scholar]
- [35].van de Vyver M., Myburgh K.H.. Cytokine and satellite cell responses to muscle damage: interpretation and possible confounding factors in human studies. J Muscle Res Cell Motil. 2012;33:177–85. doi: 10.1007/s10974-012-9303-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Walker D.K., Fry C.S., Drummond M.J., Dickinson J.M., Timmerman K.L., Gundermann D.M.. et al. PAX7+ satellite cells in young and older adults following resistance exercise. Muscle Nerve. 2012;46:51–9. doi: 10.1002/mus.23266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Cermak N.M., Snijders T., McKay B.R., Parise G., Verdijk L.B., Tarnopolsky M.A.. et al. Eccentric exercise increases satellite cell content in type II muscle fibers. Med Sci Sports Exerc. 2013;45:230–7. doi: 10.1249/MSS.0b013e318272cf47. [DOI] [PubMed] [Google Scholar]
- [38].Hanssen K.E., Kvamme N.H., Nilsen T.S., R⊘nnestad B., Ambj⊘rnsen I.K., Norheim F.. et al. The effect of strength training volume on satellite cells, myogenic regulatory factors, and growth factors. Scand J Med Sci Sports. 2013;23:728–39. doi: 10.1111/j.1600-0838.2012.01452.x. [DOI] [PubMed] [Google Scholar]
- [39].Mangan G., Bombardier E., Mitchell A.S., Quadrilatero J., Tiidus P.M.. Oestrogen-dependent satellite cell activation and proliferation following a running exercise occurs via the PI3K signalling pathway and not IGF-1. Acta Physiol (Oxf) 2014;212:75–85. doi: 10.1111/apha.12317. [DOI] [PubMed] [Google Scholar]
- [40].Oishi Y., Tsukamoto H., Yokokawa T., Hirotsu K., Shimazu M., Uchida K.. et al. Mixed lactate and caffeine compound increases satellite cell activity and anabolic signals for muscle hypertrophy. J Appl Physiol (1985) 2015;118:742–9. doi: 10.1152/japplphysiol.00054.2014. [DOI] [PubMed] [Google Scholar]
- [41].Caldow M.K., Thomas E.E., Dale M.J., Tomkinson G.R., Buckley J.D., Cameron-Smith D.. Early myogenic responses to acute exercise before and after resistance training in young men. Physiol Rep. 2015 Sep;3;9:pii: e12511. doi: 10.14814/phy2.12511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Macaluso F., Brooks N.E., van de Vyver M., Van Tubbergh K., Niesler C.U., Myburgh K.H.. Satellite cell count, VO(2max), and p38 MAPK in inactive to moderately active young men. Scand J Med Sci Sports. 2012;22:e38–44. doi: 10.1111/j.1600-0838.2011.01389.x. [DOI] [PubMed] [Google Scholar]
- [43].Frese S., Ruebner M., Suhr F., Konou T.M., Tappe K.A., Toigo M.. et al. Long-Term Endurance Exercise in Humans Stimulates Cell Fusion of Myoblasts along with Fusogenic Endogenous Retroviral Genes In Vivo. PLoS One. 2015;10:e0132099. doi: 10.1371/journal.pone.0132099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].McKenzie AI., D’Lugos AC., Saunders MJ., Gworek KD., Luden ND.. Fiber Type-Specific Satellite Cell Content in Cyclists Following Heavy Training with Carbohydrate and Carbohydrate-Protein Supplementation. Front Physiol. 2016;7:550. doi: 10.3389/fphys.2016.00550. eCollection 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Dinulovic I., Furrer R., Beer M., Ferry A., Cardel B., Handschin C.. Muscle PGC-1α modulates satellite cell number and proliferation by remodeling the stem cell niche. Skelet Muscle. 2016;6:39. doi: 10.1186/s13395-016-0111-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Friday B..B, Mitchell P.O., Kegley K.M., Pavlath G.K.. Calcineurin initiates skeletal muscle differentiation by activating MEF2 and MyoD. Differentiation. 2003;71:217–27. doi: 10.1046/j.1432-0436.2003.710303.x. [DOI] [PubMed] [Google Scholar]
- [47].Gehlert S., Bloch W., Suhr F.. Ca2+-dependent regulations and signaling in skeletal muscle: from electro-mechanical coupling to adaptation. Int J Mol Sci. 2015;16:1066–95. doi: 10.3390/ijms16011066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Dalbo V.J., Roberts M.D.. The activity of satellite cells and myonuclei during 8 weeks of strength training in young men with suppressed testosterone. Acta Physiol (Oxf) 2015;213:556–8. doi: 10.1111/apha.12411. [DOI] [PubMed] [Google Scholar]
- [49].Vahidi Ferdousi L., Rocheteau P., Chayot R., Montagne B., Chaker Z., Flamant P.. et al. More efficient repair of DNA double-strand breaks in skeletal muscle stem cells compared to their committed progeny. Stem Cell Res. 2014;13:492–507. doi: 10.1016/j.scr.2014.08.005. [DOI] [PubMed] [Google Scholar]
- [50].Taivassalo T., Fu K., Johns T., Arnold D., Karpati G., Shoubridge EA.. Gene shifting: a novel therapy for mitochondrial myopathy. Hum Mol Genet. 1999;8:1047–52. doi: 10.1093/hmg/8.6.1047. https://doi.org/10.1093/hmg/8.6.1047. [DOI] [PubMed] [Google Scholar]
- [51].Schultz E., McCormick KM.. Skeletal muscle satellite cells. Rev Physiol Biochem Pharmacol. 1994;123:213–57. doi: 10.1007/BFb0030904. https://www.ncbi.nlm.nih.gov/pubmed/8209136 [DOI] [PubMed] [Google Scholar]
- [52].Murphy JL., Blakely EL., Schaefer AM., He L., Wyrick P., Haller RG.. et al. Resistance training in patients with single, large-scale deletions of mitochondrial DNA. Brain. 2008;131:2832–40. doi: 10.1093/brain/awn252. [DOI] [PubMed] [Google Scholar]
- [53].Spendiff S., Reza M., Murphy JL., Gorman G., Blakely EL., Taylor RW.. et al. Mitochondrial DNA deletions in muscle satellite cells: implications for therapies. Hum Mol Genet. 2013;22:4739–47. doi: 10.1093/hmg/ddt327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Murach KA., Walton RG., Fry CS., Michaelis SL., Groshong JS., Finlin BS.. et al. Cycle training modulates satellite cell and transcriptional responses to a bout of resistance exercise. Physiol Rep. 2016:pii: e12973. doi: 10.14814/phy2.12973. Sep;4(18) [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Nederveen JP., Snijders T., Joanisse S., Wavell CG., Mitchell CJ., Johnston LM.. et al. Altered muscle satellite cell activation following 16 wk of resistance training in young men. Am J Physiol Regul Integr Comp Physiol. 2017;312:R–85. doi: 10.1152/ajpregu.00221.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Rowlands D.S., Nelson A.R., Raymond F., Metairon S., Mansourian R., Clarke J.. et al. Protein-leucine ingestion activates a regenerative inflammo-myogenic transcriptome in skeletal muscle following intense endurance exercise. Physiol Genomics. 2016;48:21–32. doi: 10.1152/physiolgenomics.00068.2015. [DOI] [PubMed] [Google Scholar]
- [57].Peake J.M., Della Gatta P., Suzuki K., Nieman D.C.. Cytokine expression and secretion by skeletal muscle cells: regulatory mechanisms and exercise effects. Exerc Immunol Rev. 2015;21:8–25. https://www.ncbi.nlm.nih.gov/pubmed/25826432 [PubMed] [Google Scholar]
- [58].Hiscock N., Chan M.H., Bisucci T., Darby I.A., Febbraio M.A.. Skeletal myocytes are a source of interleukin-6 mRNA expression and protein release during contraction: evidence of fiber type specificity. FASEB J. 2004;18:992–4. doi: 10.1096/fj.03-1259fje. [DOI] [PubMed] [Google Scholar]
- [59].Hoier B., Walker M., Passos M., Walker P.J., Green A., Bangsbo J.. et al. Angiogenic response to passive movement and active exercise in individuals with peripheral arterial disease. J Appl Physiol (1985) 2013;115:1777–87. doi: 10.1152/japplphysiol.00979.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Hubal M.J., Chen T.C., Thompson P.D., Clarkson P.M.. Inflammatory gene changes associated with the repeated-bout effect. Am J Physiol Regul Integr Comp Physiol. 2008;294:R1628–37. doi: 10.1152/ajpregu.00853.2007. [DOI] [PubMed] [Google Scholar]
- [61].Paulsen G., Mikkelsen U.R., Raastad T., Peake J.M.. Leucocytes, cytokines and satellite cells: what role do they play in muscle damage and regeneration following eccentric exercise? Exerc Immunol Rev. 2012;18:42–97. https://www.ncbi.nlm.nih.gov/pubmed/22876722 [PubMed] [Google Scholar]
- [62].Saclier M., Cuvellier S., Magnan M., Mounier R., Chazaud B.. Monocyte/macrophage interactions with myogenic precursor cells during skeletal muscle regeneration. FEBS J. 2013;Sep;280(17):4118–30. doi: 10.1111/febs.12166. [DOI] [PubMed] [Google Scholar]
- [63].Olfert I.M., Baum O., Hellsten Y., Egginton S.. Advances and challenges in skeletal muscle angiogenesis. Am J Physiol Heart Circ Physiol. 2016;310:H326–36. doi: 10.1152/ajpheart.00635.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Polesskaya A., Degerny C., Pinna G., Maury Y., Kratassiouk G., Mouly V.. et al. Genome-wide exploration of miRNA function in mammalian muscle cell differentiation. PLoS One. 2013;8:e71927. doi: 10.1371/journal.pone.0071927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Hashemi Gheinani A., Burkhard F.C., Rehrauer H., Aquino Fournier C., Monastyrskaya K.. MicroRNA MiR-199a-5p regulates smooth muscle cell proliferation and morphology by targeting WNT2 signaling pathway. J Biol Chem. 2015;290:7067–86. doi: 10.1074/jbc.M114.618694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Bentzinger CF., Wang YX., von Maltzahn J., Rudnicki MA.. The emerging biology of muscle stem cells: implications for cell-based therapies. Bioessays. 2013;35:231–41. doi: 10.1002/bies.201200063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].De Lisio M., Farup J., Sukiennik R.A., Clevenger N., Nallabelli J., Nelson B.. et al. The acute response of pericytes to muscledamaging eccentric contraction and protein supplementation in human skeletal muscle. J Appl Physiol (1985) 2015;119:900–7. doi: 10.1152/japplphysiol.01112.2014. [DOI] [PubMed] [Google Scholar]
- [68].Farup J., De Lisio M., Rahbek S.K., Bjerre J., Vendelbo M.H., Boppart M.D., Vissing K.. Pericyte response to contraction mode-specific resistance exercise training in human skeletal muscle. J Appl Physiol. 1985;2015;119:1053–63. doi: 10.1152/japplphysiol.01108.2014. Epub 2015 Sep 24. PubMed PMID: 26404620. [DOI] [PubMed] [Google Scholar]
- [69].Zou K., Huntsman H.D., Carmen Valero M., Adams J., Skelton J., De Lisio M.. et al. Mesenchymal stem cells augment the adaptive response to eccentric exercise. Med Sci Sports Exerc. 2015;47:315–25. doi: 10.1249/MSS.0000000000000405. [DOI] [PubMed] [Google Scholar]
- [70].Sacchetti B., Funari A., Remoli C., Giannicola G., Kogler G., Liedtke S.. et al. No Identical “Mesenchymal Stem Cells” at Different Times and Sites: Human Committed Progenitors of Distinct Origin and Differentiation Potential Are Incorporated as Adventitial Cells in Microvessels. Stem Cell Reports. 2016;6:897–913. doi: 10.1016/j.stemcr.2016.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Kostallari E., Baba-Amer Y., Alonso-Martin S., Ngoh P., Relaix F., Lafuste P., Gherardi R.K.. Pericytes in the myovascular niche promote post-natal myofiber growth and satellite cell quiescence. Development. 2015;142:1242–53. doi: 10.1242/dev.115386. [DOI] [PubMed] [Google Scholar]
- [72].Hyldahl R.D., Xin L., Hubal M.J., Moeckel-Cole S., Chipkin S., Clarkson P.M.. Activation of nuclear factor-κB following muscle eccentric contractions in humans is localized primarily to skeletal muscle-residing pericytes. FASEB J. 2011;25:2956–66. doi: 10.1096/fj.10-177105. [DOI] [PubMed] [Google Scholar]
- [73].LaBarbera K.E., Hyldahl R.D., O’Fallon K.S., Clarkson P.M., Witkowski S.. Pericyte NF-κB activation enhances endothelial cell proliferation and proangiogenic cytokine secretion in vitro. Physiol Rep. 2015;3:pii: e12309. doi: 10.14814/phy2.12309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Valero M.C., Huntsman H.D., Liu J., Zou K., Boppart M.D.. Eccentric exercise facilitates mesenchymal stem cell appearance in skeletal muscle. PLoS One. 2012;7:e29760. doi: 10.1371/journal.pone.0029760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Valero M.C., Huntsman H.D., Liu J., Zou K., Boppart M.D.. Eccentric exercise facilitates mesenchymal stem cell appearance in skeletal muscle. PLoS One. 2012;7:e29760. doi: 10.1371/journal.pone.0029760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Liese A.D., Krebs-Smith S.M., Subar A.F., George S.M., Harmon B.E., Neuhouser M.L.. et al. The Dietary Patterns Methods Project: synthesis of findings across cohorts and relevance to dietary guidance. J Nutr. 2015;145:393–402. doi: 10.3945/jn.114.205336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Wilson M.G., Ellison G.M., Cable N.T.. Basic science behind the cardiovascular benefits of exercise. Br J Sports Med. 2016;50:93–9. doi: 10.1136/bjsports-2014-306596rep. [DOI] [PubMed] [Google Scholar]
- [78].Nayor M., Vasan R.S.. Preventing heart failure: the role of physical activity. Curr Opin Cardiol. 2015;30:543–50. doi: 10.1097/HCO.0000000000000206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Després J.P.. Physical Activity, Sedentary Behaviours, and Cardiovascular Health: When Will Cardiorespiratory Fitness Become a Vital Sign? Can J Cardiol. 2016;32:505–13. doi: 10.1016/j.cjca.2015.12.006. [DOI] [PubMed] [Google Scholar]
- [80].Fernandes T., Baraúna V.G., Negrão C.E., Phillips M.I., Oliveira E.M.. Aerobic exercise training promotes physiological cardiac remodeling involving a set of microRNAs. Am J Physiol Heart Circ Physiol. 2015;309:H543–52. doi: 10.1152/ajpheart.00899.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Kwak H.B.. Aging, exercise, and extracellular matrix in the heart. J Exerc Rehabil. 2013;9:338–47. doi: 10.12965/jer.130049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Wasfy M.M., Weiner R.B.. Differentiating the athlete’s heart from hypertrophic cardiomyopathy. Curr Opin Cardiol. 2015;30:500–5. doi: 10.1097/HCO.0000000000000203.Review. [DOI] [PubMed] [Google Scholar]
- [83].Izumo S., Lompré A.M., Matsuoka R., Koren G., Schwartz K., Nadal-Ginard B., Mahdavi V.. Myosin heavy chain messenger RNA and protein isoform transitions during cardiac hypertrophy. Interaction between hemodynamic and thyroid hormone-induced signals. J Clin Invest. 1987;79:970–7. doi: 10.1172/JCI112908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Izumo S., Nadal-Ginard B., Mahdavi V.. Protooncogene induction and reprogramming of cardiac gene expression produced by pressure overload. Proc Natl Acad Sci U S A. 1988;85:339–43. doi: 10.1073/pnas.85.2.339. PMID: 2963328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].MacLellan WR., Schneider MD.. Genetic dissection of cardiac growth control pathways. Annu Rev Physiol. 2000;62:289–319. doi: 10.1146/annurev.physiol.62.1.289. [DOI] [PubMed] [Google Scholar]
- [86].McMullen J.R., Shioi T., Zhang L., Tarnavski O., Sherwood M.C., Kang P.M., Izumo S.. Phosphoinositide 3-kinase(p110alpha) plays a critical role for the induction of physiological, but not pathological, cardiac hypertrophy. Proc Natl Acad Sci U S A. 2003;100:12355–60. doi: 10.1073/pnas.1934654100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Sadoshima J., Izumo S.. The cellular and molecular response of cardiac myocytes to mechanical stress. Annu Rev Physiol. 1997;59:551–71. doi: 10.1146/annurev.physiol.59.1.551. [DOI] [PubMed] [Google Scholar]
- [88].Akazawa H., Komuro I.. Roles of cardiac transcription factors in cardiac hypertrophy. Circ Res. 2003;92:1079–88. doi: 10.1161/01.RES.0000072977.86706.23. [DOI] [PubMed] [Google Scholar]
- [89].Kwak H.B., Song W., Lawler J.M.. Exercise training attenuates age-induced elevation in Bax/Bcl-2 ratio, apoptosis, and remodeling in the rat heart. FASEB J. 2006;20:791–3. doi: 10.1096/fj.05-5116fje. [DOI] [PubMed] [Google Scholar]
- [90].Kwak H.B., Kim J.H., Joshi K., Yeh A., Martinez D.A., Lawler J.M.. Exercise training reduces fibrosis and matrix metalloproteinase dysregulation in the aging rat heart. FASEB J. 2011;25:1106–17. doi: 10.1096/fj.10-172924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Tao L., Bei Y., Zhang H., Xiao J., Li X.. Exercise for the heart: signaling pathways. Oncotarget. 2015;6:20773–84. doi: 10.18632/oncotarget.4770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Marini M., Lapalombella R., Margonato V., Ronchi R., Samaja M., Scapin C.. et al. Mild exercise training, cardioprotection and stress genes profile. Eur J Appl Physiol. 2007;99:503–10. doi: 10.1007/s00421-006-0369-4. [DOI] [PubMed] [Google Scholar]
- [93].Nizielski S.E., Arizmendi C., Shteyngarts A.R., Farrell C.J., Friedman J.E.. Involvement of transcription factor C/EBP-beta in stimulation of PEPCK gene expression during exercise. Am J Physiol. 1996 May;270;:1005–12. doi: 10.1152/ajpregu.1996.270.5.R1005. (5 Pt 2):R. PubMed PMID: 8928898. [DOI] [PubMed] [Google Scholar]
- [94].Weeks K.L., McMullen J.R.. The athlete’s heart vs. the failing heart: can signaling explain the two distinct outcomes? Physiology (Bethesda) 2011;26:97–105. doi: 10.1152/physiol.00043.2010. [DOI] [PubMed] [Google Scholar]
- [95].van Rooij E., Sutherland L.B., Liu N., Williams A.H., McAnally J., Gerard R.D.. et al. A signature pattern of stress-responsive microRNAs that can evoke cardiac hypertrophy and heart failure. Proc Natl Acad Sci U S A. 2006;103:18255–60. doi: 10.1073/pnas.0608791103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Zhao Y., Ransom J.F., Li A., Vedantham V., von Drehle M., Muth A.N.. et al. Dysregulation of cardiogenesis, cardiac conduction, and cell cycle in mice lacking miRNA-1-2. Cell. 2007;129:303–17. doi: 10.1016/j.ce11.2007.03.030. [DOI] [PubMed] [Google Scholar]
- [97].Eulalio A., Mano M., Dal Ferro M., Zentilin L., Sinagra G., Zacchigna S., Giacca M.. Functional screening identifies miRNAs inducing cardiac regeneration. Nature. 2012;492:376–81. doi: 10.1038/nature11739. [DOI] [PubMed] [Google Scholar]
- [98].Xu J., Liu Y., Xie Y., Zhao C., Wang H.. Bioinformatics Analysis Reveals MicroRNAs Regulating Biological Pathways in Exercise-Induced Cardiac Physiological Hypertrophy. Biomed Res Int. 2017;2017:2850659. doi: 10.1155/2017/2850659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Melo S.F., Barauna V.G., Júnior M.A., Bozi L.H., Drummond L.R., Natali A.J., de Oliveira E.M.. Resistance training regulates cardiac function through modulation of miRNA-214. Int J Mol Sci. 2015;16:6855–67. doi: 10.3390/ijms16046855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Baggish A.L., Hale A., Weiner R.B., Lewis G.D., Systrom D., Wang F.. et al. Dynamic regulation of circulating microRNA during acute exhaustive exercise and sustained aerobic exercise training. J Physiol. 2011;589:3983–94. doi: 10.1113/jphysiol.2011.213363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Sawada S., Kon M., Wada S., Ushida T., Suzuki K., Akimoto T.. Profiling of circulating microRNAs after a bout of acute resistance exercise in humans. PLoS One. 2013;8:e70823. doi: 10.1371/journal.pone.0070823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Leite C.F., Lopes C.S., Alves A.C., Fuzaro C.S., Silva M.V., Oliveira L.F.. et al. Endogenous resident c-Kit cardiac stem cells increase in mice with an exercise-induced, physiologically hypertrophied heart. Stem Cell Res. 2015;15:151–64. doi: 10.1016/j.scr.2015.05.011. [DOI] [PubMed] [Google Scholar]
- [103].Torella D., Ellison G.M., Méndez-Ferrer S., Ibanez B., Nadal-Ginard B.. Resident human cardiac stem cells: role in cardiac cellular homeostasis and potential for myocardial regeneration. Nat Clin Pract Cardiovasc Med. 2006;3:S8–13. doi: 10.1038/ncpcardio0409. [DOI] [PubMed] [Google Scholar]
- [104].Bergmann O., Bhardwaj R.D., Bernard S., Zdunek S., Barnabe-Heider F., Walsh S.. et al. Evidence for cardiomyocyte renewal in humans. Science. 2009;324:98–102. doi: 10.1126/science.1164680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Ellison G.M., Waring C.D., Vicinanza C., Torella D.. Physiological cardiac remodelling in response to endurance exercise training: cellular and molecular mechanisms. Heart. 2012;98:5–10. doi: 10.1136/heartjnl-2011-300639. [DOI] [PubMed] [Google Scholar]
- [106].Schuler G., Adams V., Goto Y.. Role of exercise in the prevention of cardiovascular disease: results, mechanisms, and new perspectives. Eur Heart J. 2013;34:1790–9. doi: 10.1093/eurheartj/eht111. [DOI] [PubMed] [Google Scholar]
- [107].Dimmeler S., Burchfield J., Zeiher A.M.. Cell-based therapy of myocardial infarction. Arterioscler Thromb Vasc Biol. 2008;28:208–16. doi: 10.1161/ATVBAHA.107.155317. [DOI] [PubMed] [Google Scholar]
- [108].Landers-Ramos R.Q., Sapp R.M., Jenkins N.T., Murphy A.E., Cancre L., Chin E.R.. et al. Chronic endurance exercise affects paracrine action of CD31+ and CD34+ cells on endothelial tube formation. Am J Physiol Heart Circ Physiol. 2015;309:H407–20. doi: 10.1152/ajpheart.00123.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Galli D., Vitale M., Vaccarezza M.. Bone marrow-derived mesenchymal cell differentiation toward myogenic lineages: facts and perspectives. Biomed Res Int. 2014;2014:762695. doi: 10.1155/2014/762695. [DOI] [PMC free article] [PubMed] [Google Scholar]