Abstract
MicroRNAs (miRNAs) are small non-coding RNAs, 8–23 nucleotides in length, which regulate gene expression at the post-transcriptional level. The present study was performed to analyze the association between microRNA-21 and cisplatin resistance in epithelial ovarian cancer (EOC) SKOV3 and SKOV3/DDP cells. In this experiment, the resistance of SKOV3 and SKOV3/DDP cells to cisplatin was evaluated using the MTT assay. Reverse transcription-quantitative polymerase chain reaction analysis was used to assess miRNA-21 levels and phosphatase and tensin homolog (PTEN) mRNA levels. Western blotting was used to assess PTEN protein levels. miRNA-21 mimics or inhibitors were transfected into SKOV3 and SKOV3/DDP cells. Prior to transfection, higher expression levels of miRNA-21 were observed in SKOV3/DDP cells compared with SKOV3 cells. Following transfection with miRNA-21 mimics, SKOV3 cells demonstrated increased sensitivity to cisplatin compared with negative control cells. Following transfection with the miRNA-21 inhibitor, SKOV3/DDP cells demonstrated decreased sensitivity to cisplatin compared with negative control cells. Furthermore, PTEN mRNA expression levels in SKOV3 cells transfected with miRNA-21 mimics was significantly lower compared with negative control cells. These results suggested that miRNA-21 may regulate cisplatin resistance by negatively targeting PTEN in EOC.
Keywords: microRNA-21, Epithelial ovarian cancer, cisplatin, drug-resistance, PTEN
Introduction
Epithelial ovarian cancer (EOC) is one of the most common malignant gynecologic tumors. Debulking surgery followed by a combination of platinum and taxane based chemotherapy are widely used treatments for EOC at present. Although overall survival rates have increased slightly over the past 25 years, 5-year survival remains <50% (1). The high mortality rate of ovarian cancer is due to late-stage diagnosis and resistance to platinum-based chemotherapy. However, the mechanisms underlying cisplatin resistance in EOC remain to be fully understood.
MicroRNAs (miRNAs) are small non-coding RNAs of 8–23 nucleotides that post-transcriptionally regulate gene expression. Multiple previous reports have indicated that dysregulation of miRNA target genes promotes drug resistance, and inhibition of miRNAs may reverse drug resistance (2,3). miRNA-21 is overexpressed in multiple types of cancer, and promotes the initiation of cancer, progression and drug-resistance (4–9). miRNA-21 impacts tumorigenesis by negatively regulating several targets. Phosphatase and tensin homolog (PTEN) is a tumor suppressor molecule. Inactivating mutations and deletions of the PTEN gene have been observed in multiple types of cancer. Notably, bioinformatics tools have demonstrated that the 3′-untranslated region of the PTEN gene harbors a putative binding site for miRNA-21 (10). miRNA-21 expression has been revealed to be markedly increased in ovarian cancer compared with benign ovarian tumor tissues (11). miRNA-21 expression was also demonstrated to be increased in drug-resistant ovarian cancer compared with drug-sensitive ovarian cancer serum. In the present study, miRNA-21 mimics, inhibitors and negative control were transfected in to SKOV3 or SKOV3/DDP cells. The PTEN gene was hypothesized to be regulated by miRNA-21 in ovarian cancer cisplatin resistance.
Materials and methods
Cell lines and cell culture
The SKOV3 human ovarian cancer cell line was purchased from Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences (Shanghai, China). The SKOV3/DDP human ovarian cancer cell line was purchased from the Affiliated Hospital of Qingdao University (Qingdao, China). The cells were cultured in RPMI-1640 medium supplemented with 10% fetal bovine serum (both from Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA), 100 U/ml penicillin and 100 µg/ml streptomycin, in a humidified cell incubator with an atmosphere of 5% CO2 and a temperature of 37°C. Cells from the exponential growth phase were used for the following experiments. The treatment groups of cells were as follows: SKOV3/DDP group; SKOV3 group; the negative control group (no drug); the blank control group (no cells). Cells were cultured for 24 h in a humidified cell incubator with an atmosphere of 5% CO2 and a temperature of 37°C.
A total of 200 µl of 4×104 cells/ml SKOV3/DDP, SKOV3 or cell-free medium were seeded in a 96-well plate. Cisplatin (Qilu Pharmaceutical Co., Ltd., Jinan, China) to a concentration of 0, 3.125, 6.25, 12.5, 25, 50, 100 or 200 µmol/l was added to each well. The cells were incubated in a humidified cell incubator with an atmosphere of 5% CO2 and a temperature of 37°C for 48 h. A total of 20 µl MTT was added to each well with a concentration of 5 mg/ml. Following a 4-h incubation at 37°C, the supernatant was discarded using pipettes. A total of 150 µl DMSO was added to each well.
A microplate reader was used to analyze the absorbance of each well at an optical density of 490 nm. The cells inhibitory rate (IR) (%)=[(1-(group value-blank control group value)/(negative control group value-blank control group value)]x100%. Subsequently, the half-maximal inhibitory concentration (IC50) was determined. Resistant factor (RF)=IC50 of SKOV3 DDP/IC50 of SKOV3 (12).
RNA isolation
Total RNA was extracted and isolated from 2×105 cells/ml using the mirVana miRNA isolation kit (Ambion; Thermo Fisher Scientific, Inc., Waltham, MA, USA). The quality and quantity of the RNA samples were assessed by standard electrophoresis and spectrophotometric methods (260/280 absorbance).
Transfection
The miRNA-21 mimics and miRNA-21 inhibitor were purchased from Shanghai GenePharma Co., Ltd (Shanghai, China). SKOV3 and SKOV3/DDP cells (2×105 cells/ml) were counted and seeded onto 6-well plates the day prior to transfection to ensure 50% cell confluence on the day of transfection. Transfection of 10 nM miRNA-21 mimics into SKOV3 and transfection of 10 nM miRNA-21 inhibitors, diluted in medium, into SKOV3/DDP cells were performed using Lipofectamine 2000 (Invitrogen; Thermo Fisher Scientific, Inc.) in accordance with the manufacturer's protocol. Lipofectamine was used alone as a negative control. The miRNA-21 mimics/inhibitors were used at a final concentration of 100 nM. Reverse transcription-quantitative polymerase chain reaction (RT-qPCR) and western blot analysis were performed 48 h following transfection.
RT-qPCR analysis
miRNA-21 and PTEN mRNA expression levels were detected by stem-loop RT-qPCR. RT was performed using SYBR Premix Ex Taq (Takara Bio, Inc., Otsu, Japan) at 16°C for 30 min, 42°C for 30 min and 85°C for 5 min. qPCR was performed using SYBR Premix Ex Taq (Takara Bio, Inc.) according to the manufacturer's protocol, with 1.33 µl cDNA from the RT reaction. The thermocycler conditions were, an initial 15 min at 95°C, then 40 cycles of 15 sec at 95°C and 60 sec at 60°C. The primers for miRNA-21 were as follows: Stem-loop RT, 5′-GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACTCAACA-3′; forward, 5′GCCCGCTAGCTTATCAGACTGATG-3′ and reverse, 5′-GTGCAGGGTCCGAGGT-3′. The primers for U6 were as follows: Stem loop RT, 5′-GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACAAAAATATG-3′; forward, 5′-GCGCGTCGTGAAGCGTTC-3′ and reverse, 5′-GTGCAGGGTCCGAGGT-3′. GAPDH was used to normalize PTEN mRNA expression levels. The forward and reverse primer sequences for PTEN mRNA were as follows: 5′-GAGGGATAAAACACCATG-3′ and 5′-AGGGGTAGGATGTGAACCAGTA-3′, respectively. The forward and reverse primer sequences for GAPDH were 5′-AACTTTGGCATTGTGGAAGG-3′ and 5′-ACACATTGGGGGTAGGAACA-3′, respectively. All the experiments were performed in triplicate. The relative expression ratios of miRNA-21 and PTEN mRNA in SKOV3 and SKOV3/DDP cell lines was calculated using the 2−ΔΔCq method (13).
Western blot analysis
A total of 4×104 cultured cells were lysed using RIPA buffer (Pierce; Thermo Fisher Scientific, Inc.) in the presence of a Protease Inhibitor Cocktail (Pierce; Thermo Fisher Scientific, Inc.). The protein concentration of the lysates was measured using a BCA Protein Assay kit (Pierce; Thermo Fisher Scientific, Inc.). Equivalent amounts of protein (0.5 mg/ml) were resolved and mixed with 5X Lane Marker Reducing Sample Buffer (Pierce; Thermo Fisher Scientific, Inc.), electrophoresed on 12.5% SDS-acrylamide gel, and transferred to Immobilon-P transfer membranes (Merck KGaA, Darmstadt, Germany). The membranes were blocked with 5% non-fat milk in Tris-buffered saline at 4°C overnight and then incubated with a rabbit anti-human PTEN monoclonal antibody (cat. no., ab32199; dilution, 1:400; Abcam, Cambridge, UK) at 4°C overnight followed by horseradish peroxidase (HRP)-conjugated secondary antibody (cat. no., ab151499; dilution, 1:100; Abcam) at room temperature for 1 h. Signals were detected using Immobilon western chemiluminescent HRP substrate (Merck KGaA). β-actin (cat. no., ab8227; dilution, 1:100; Abcam) served as the loading control.
Statistical analysis
Independent and paired t-tests were used to compare the data. All analyses were performed using SPSS19.0 software (IBM SPSS, Armonk, NY, USA) and all tests were two-tailed. P<0.05 was considered to indicate a statistically significant difference.
Results
miRNA-21 regulates the sensitivity of SKOV3 and SKOV3/DDP cells to cisplatin
The RT-qPCR results revealed that SKOV3/DDP cells had a higher endogenous miRNA-21 expression level than SKOV3 cells (P<0.05; Fig. 1). The IC50 of control SKOV3 cells was 5.205 µmol/l cisplatin, of SKOV3 cells with the miRNA-21 mimic, 25.763 µmol/l, of control SKOV3/DDP cells, 21.914 µmol/l, and of SKOV3/DDP cells with the inhibitor, 15.524 µmol/l. Thus, the relative cisplatin resistance index of SKOV3 cells transfected with the miRNA-21 mimic was 4.9 compared to the control cells, of the SKOV3/DDP cells compared with SKOV3/DDP cells with the inhibitor, 0.7, and of the control SKOV3/DDP cells relative to the control SKOV3 cells, 4.2. The sensitivity of SKOV3 cells transfected with miRNA-21 mimics to cisplatin was significantly increased compared with the negative control cells (P<0.05; Fig. 2). In addition, SKOV3/DDP cells transfected with miRNA-21 inhibitors were significantly less resistant to cisplatin compared with that of the negative control cells (19.0; P<0.05; Fig. 2).
Figure 1.
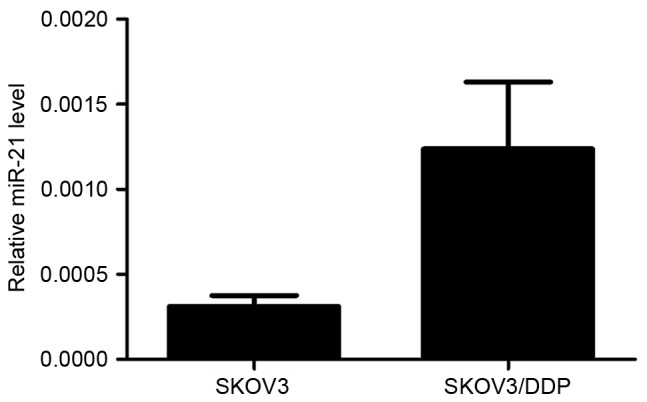
Reverse transcription-quantitative polymerase chain reaction analysis revealed that SKOV3/DDP cells had higher endogenous miRNA-21 expression levels than SKOV3 cells. miRNA, microRNA.
Figure 2.
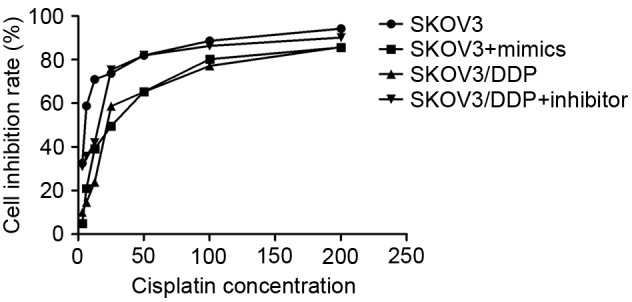
Results of the MTT cell viability assay. Resistance of SKOV3 cells transfected with miRNA-21 mimics to cisplatin was increased compared with negative control cells. SKOV3/DDP cells transfected with miRNA-21 inhibitors demonstrated decreased resistance to cisplatin compared with negative control cells. miRNA, microRNA.
miRNA-21 downregulates expression of PTEN mRNA and protein in SKOV3 cells
RT-qPCR revealed that, following transfection with miRNA-21 mimics, PTEN mRNA expression levels in the SKOV3 cell line were decreased compared with negative control cells (P<0.05; Fig. 3). When the SKOV3/DDP cell line was transfected with miR-21 inhibitors, PTEN mRNA expression levels were not significantly altered. When miR-21 was transfected into SKOV3 cells, western blot analysis revealed that PTEN protein levels were visibly lower. It was also demonstrated that when miR-21 was transfected into SKOV3/DPP cells line, PTEN protein levels were visibly increased (Fig. 4.).
Figure 3.
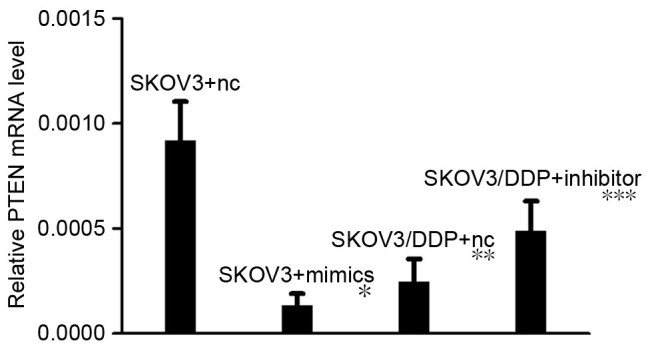
PTEN mRNA expression levels in SKOV3 and SKOV3/DPP cells following transfection with miR-21 mimics and inhibitors, as assessed by reverse transcription-quantitative polymerase chain reaction. A two paired-samples t-test was used. *SKOV3 + nc vs. SKOV3 + mimics: P<0.05; **SKOV3 + nc vs. SKOV3/DDP + nc: P>0.05; ***SKOV3/DDP + nc vs. SKOV3/DDP + Inhibitor: P>0.05. PTEN, phosphatase and tensin homolog; nc, negative control.
Figure 4.
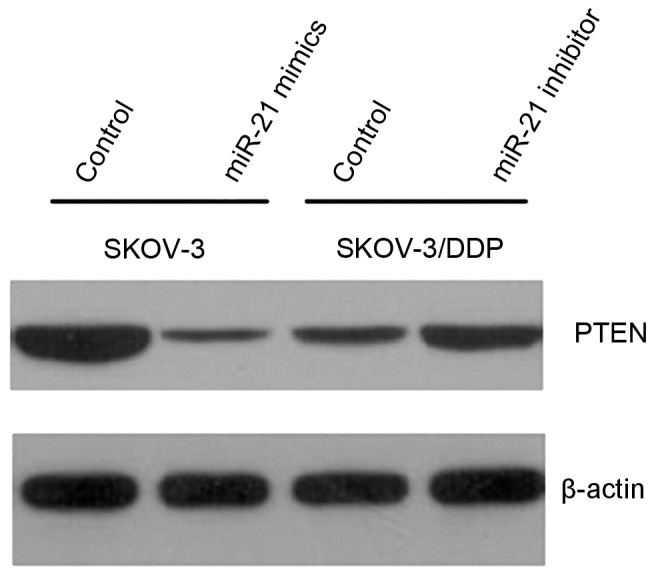
PTEN protein expression levels in SKOV3 and SKOV3/DPP cells following transfection with miR-21 mimics and inhibitors, as assessed by western blotting. PTEN, phosphatase and tensin homolog; miR, microRNA.
Discussion
In the present study, the drug resistance index of SKOV3/DDP cells relative to SKOV3 cells was demonstrated to be 4.2 using the MTT assay. Thus, the present study selected the appropriate cells for experimental study. Although platinum-based chemotherapy has improved the prognosis of ovarian cancer, drug-resistance remains the main obstacle to successful treatment. The present study revealed that multiple miRNAs participate in ovarian cancer drug-resistance, including miRNA-130a and miRNA-374a (14). miRNA-152 and miRNA-185 were demonstrated to be significantly downregulated in SKOV3/DDP and A2780/DDP cells (14). Previous studies have demonstrated that miRNA-21 is involved in drug resistance in multiple types of cancer, including gastric, breast and lung cancer, via regulation of PTEN (7,8,15). In the present study, the expression level of miRNA-21 in SKOV3/DDP cells was significantly higher than in SKOV3 cells, as detected by qPCR. It was demonstrated that miR-21 may participate in ovarian cancer drug-resistance. In the present study, miRNA-21 mimics were transfected into SKOV3 cells, and miRNA-21 inhibitors were transfected into SKOV3/DDP cells. Following transfection, the upregulation of miRNA-21 in SKOV3 cells transfected with the miRNA-21 mimics resulted in an increased cisplatin drug resistance. The downregulation of miRNA-21 in SKOV3/DDP cells transfected with the miRNA-21 inhibitor resulted in decreased cisplatin drug resistance. Thus, the present study further demonstrated miRNA-21 participated in cisplatin resistance in EOC. PTEN mRNA expression levels in SKOV3 cells transfected with the miRNA-21 mimics was significantly decreased compared with negative control cells (cells treated with Lipofectamine alone). However, PTEN mRNA expression levels in SKOV3/DDP cells transfected with the miRNA-21 inhibitors revealed no significant increase. The effect of miR-21 regulated the expression of PTEN mRNA was not clear. The upregulation of miRNA-21 in SKOV3 cells was concurrent with the downregulation of PTEN protein in these cells. The downregulation of miRNA-21 in SKOV3/DDP cells was also concurrent with the upregulation of PTEN protein in these cells. The present study demonstrated that miRNA-21 may have regulated cisplatin resistance by negatively targeting PTEN protein in EOC.
In conclusion, the present study demonstrated that miRNA-21 may have participated in cisplatin resistance in EOC. Furthermore, miRNA-21 may have regulated cisplatin resistance by negatively targeting PTEN protein in EOC. Future study should additionally consider cells with a higher resistance factor in order to further the study of cisplatin resistance. The association between the PTEN/PI3K/Akt signaling pathway, miRNA-21 and cisplatin resistance also requires further study.
Acknowledgements
The present study was supported by the National Natural Science Foundation of China (grant no. 81101973/H1621).
References
- 1.Vargas-Hernández VM, Moreno-Eutimio MA, Acosta-Altamirano G, Vargas-Aguilar VM. Management of recurrent epithelial ovarian cancer. Gland Surg. 2014;3:198–202. doi: 10.3978/j.issn.2227-684X.2013.10.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen PS, Su JL, Hung MC. Dysregulation of microRNAs in cancer. J Biomed Sci. 2012;19:90. doi: 10.1186/1423-0127-19-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Garofalo M, Croce CM. MicroRNAs as therapeutic targets in chemoresistance. Drug Resist Updat. 2013;16:47–59. doi: 10.1016/j.drup.2013.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Song WF, Wang L, Huang WY, Cai X, Cui JJ, Wang LW. MiR-21 upregulation induced by promoter zone histone acetylation is associated with chemoresistance to gemcitabine and enhanced malignancy of pancreatic cancer cells. Asian Pac J Cancer Prev. 2013;14:7529–7536. doi: 10.7314/APJCP.2013.14.12.7529. [DOI] [PubMed] [Google Scholar]
- 5.Hong L, Han Y, Zhang Y, Zhang H, Zhao Q, Wu K, Fan D. MicroRNA-21: A therapeutic target for reversing drug resistance in cancer. Expert Opin Ther Targets. 2013;17:1073–1080. doi: 10.1517/14728222.2013.819853. [DOI] [PubMed] [Google Scholar]
- 6.Wang ZX, Lu BB, Wang H, Cheng ZX, Yin YM. MicroRNA-21 modulates chemosensitivity of breast cancer cells to doxorubicin by targeting PTEN. Arch Med Res. 2011;42:281–290. doi: 10.1016/j.arcmed.2011.06.008. [DOI] [PubMed] [Google Scholar]
- 7.Yang SM, Huang C, Li XF, Yu MZ, He Y, Li J. miR-21 confers cisplatin resistance in gastric cancer cells by regulating PTEN. Toxicology. 2013;306:162–168. doi: 10.1016/j.tox.2013.02.014. [DOI] [PubMed] [Google Scholar]
- 8.Komatsu S, Ichikawa D, Kawaguchi T, Miyamae M, Okajima W, Ohashi T, Imamura T, Kiuchi J, Konishi H, Shiozaki A, et al. Circulating miR-21 as an independent predictive biomarker for chemoresistance in esophageal squamous cell carcinoma. Am J Cancer Res. 2016;6:1511–1523. [PMC free article] [PubMed] [Google Scholar]
- 9.Shi GH, Ye DW, Yao XD, Zhang SL, Dai B, Zhang HL, Shen YJ, Zhu Y, Zhu YP, Xiao WJ, Ma CG. Involvement of microRNA-21 in mediating chemo-resistance to docetaxel in androgen-independent prostate cancer PC3 cells. Acta Pharmacol Sin. 2010;31:867–873. doi: 10.1038/aps.2010.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Milella M, Falcone I, Conciatori F, Incani U Cesta, Del Curatolo A, Inzerilli N, Nuzzo CM, Vaccaro V, Vari S, Cognetti F, Ciuffreda L. PTEN: Multiple Functions in Human Malignant Tumors. Front Oncol. 2015;5:24. doi: 10.3389/fonc.2015.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lou Y, Yang X, Wang F, Cui Z, Huang Y. MicroRNA-21 promotes the cell proliferation, invasion and migration abilities in ovarian epithelial carcinomas through inhibiting the expression of PTEN protein. Int J Mol Med. 2010;26:819–827. doi: 10.3892/ijmm_00000530. [DOI] [PubMed] [Google Scholar]
- 12.Mosmann T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 13.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 14.Li N, Yang L, Wang H, Yi T, Jia X, Chen C, Xu P. MiR-130a and MiR-374a Function as novel regulators of cisplatin resistance in human ovarian cancer A2780 Cells. PLoS One. 2015;10:e0128886. doi: 10.1371/journal.pone.0128886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu ZL, Wang H, Liu J, Wang ZX. MicroRNA-21 (miR-21) expression promotes growth, metastasis, and chemo- or radioresistance in non-small cell lung cancer cells by targeting PTEN. Mol Cell Biochem. 2013;372:35–45. doi: 10.1007/s11010-012-1443-3. [DOI] [PubMed] [Google Scholar]


