Abstract
Objective
Patients with rheumatoid arthritis (RA) are at increased cardiovascular risk. Recent studies suggest that high-density lipoprotein (HDL) may lose its protective vascular phenotype in inflammatory conditions. However, the effects of common anti-inflammatory treatments on HDL function are not yet known.
Methods
We compared the function of HDL in 18 patients with RA and 18 matched healthy controls. Subsequently, patients were randomised to (methotrexate+infliximab (M+I) (5 mg/kg)) or methotrexate+placebo (M+P) infusions for 54 weeks. At week 54 and thereafter, all patients received infliximab therapy until completion of the trial (110 weeks), enabling assessment of the impact of 1 year of infliximab therapy in all patients. HDL functional properties were assessed at baseline, 54 weeks and 110 weeks by measuring the impact on endothelial nitric oxide (NO) bioavailability and superoxide production (SO), paraoxonase activity (PON-1) and cholesterol efflux.
Results
All HDL vascular assays were impaired in patients compared with controls. After 54 weeks, NO in response to HDL was significantly greater in patients who received M+I compared with those who received M+P. Endothelial SO in response to HDL was reduced in both groups, but PON-1 and cholesterol efflux remained unchanged. All vascular measures improved compared with baseline after ≥1 infliximab therapy in the analysis at 110 weeks. No significant trend was noted for cholesterol efflux.
Conclusions
HDL function can be improved with anti-inflammatory treatment in patients with RA. The M+I combination was superior to the M+P alone, suggesting that the tumour necrosis factor-α pathway may have a role in HDL vascular properties.
Introduction
Cardiovascular (CV) disease remains the leading cause of morbidity and mortality in patients with rheumatoid arthritis (RA).1 Conventional risk factors do not account fully for this, and increased levels of inflammation associated with RA may be an important determinant of CV outcomes.2 In a study of 651 patients, an elevated inflammatory state was associated with an increased CV event rate but a paradoxical reduction in circulating lipid levels.3 This suggests that the relationship between lipid levels and increased CV disease is altered in RA and may be explained, at least in part, by qualitative changes to lipoproteins as a result of the inflammatory milieu.
High-density lipoprotein (HDL) has been suggested to exert anti-atherosclerotic effects via reverse cholesterol transport and activation of protective endothelial pathways. In conditions such as RA however, HDL has been shown to acquire pro-inflammatory and pro-thrombotic phenotype that can promote atherogenesis and potentially increase CV risk.4 5 A previous study from our group demonstrated that the beneficial function of HDL can be restored after resolution of acute inflammatory stimulus, suggesting that the modification of systemic inflammation offers potential for CV risk reduction.6
In our current study, we examined the influence of standard (methotrexate (MTX)) and novel anti-inflammatory treatments (infliximab) on HDL function in patients with RA in a randomised controlled trial over 1 year. We also assessed the long-term effect of infliximab on HDL function and CV and RA risk factors. Our findings support the concept that HDL dysfunction can be improved by anti-inflammatory drugs that are widely used for RA therapy and this may confer CV benefit.
Methods
Study population and protocol
RA population
We studied 18 patients with early erosive RA who were required to have (i) a diagnosis of RA according to the American College of Rheumatology 1987 criteria, (ii) symptoms for 6 months—3 years, (iii) a minimum of two swollen metacarpophalangeal (MCP) joints despite treatment with MTX and (iv) seropositivity for IgM rheumatoid factor. In addition, eligible patients were required to have either (i) erosion of ≥1 MCP joint as demonstrated on plain radiography or as a cortical break with irregular margins (or contour) on greyscale ultrasound in both the longitudinal and transverse scanning planes or (ii) erosions of ≥2 MCP joints (cortical breaks with irregular margins/contour on greyscale ultrasound in either the transverse or the longitudinal plane associated with a strong vascular signal in power Doppler mode at the site of the cortical break).7
All patients received oral MTX for greater than or equal to at least 8 weeks, at a minimum stable dosage of 12.5 mg/week but not exceeding 17.5 mg/week. Patients being treated with oral corticosteroids must have been receiving a stable dosage (10 mg prednisolone per day) for 4 weeks.
Study 1: case–control study
Vascular properties of HDL from 18 patients with RA were compared with 18 healthy control subjects. Healthy controls had no CV risk factors by history, clinical examination and laboratory tests, and were matched to patients with RA for age and gender.
Study 2: randomised clinical trial (double-blind phase)—secondary analysis
All physicians, patients, nurses and other non-clinical members of the study team were blinded for the first year of the study. Eighteen patients with RA were randomised into one of two treatment groups by a pharmacist who did not participate in the screening visit. Eleven patients received infusions of infliximab at 5 mg/kg and seven received placebo (normal saline) infusions at weeks 0, 2 and 6, and then every 8 weeks through week 46. At the end of the first year, all patients were maintained a single-blinded study for a further year. Patients in the methotrexate+infliximab (M+I ) group received infliximab infusions at weeks 54, 56, 62 and thereafter every 8 weeks. Those in the M+I group received a placebo infusion at week 56 in order to maintain blinding and received infliximab infusions every 8 weeks until the end of the study (110 weeks) (see online supplementary appendix figure 1). As a result, all patients received a minimum of 1 year of infliximab in the two phases of the study.
heartjnl-2015-308953supp001.pdf (48.3KB, pdf)
Baseline dosages of MTX or corticosteroid were maintained during the first 18 weeks of the study. After week 18, if any patient failed to achieve a 50% reduction from baseline in the number of swollen hand and wrist joints, the weekly dose of MTX was increased by 2.5 mg once every 4 weeks until a 50% reduction from baseline in the number of swollen hand and wrist joints was achieved, until the dosage of oral MTX reached 25 mg/week or until the dose escalation was limited by toxicity. Thereafter, irrespective of their response status, patients continued to receive the maximum tolerated MTX dosage until the end of the study. The study was approved by Riverside Research Ethics Committee, and all patients provided informed consent. Clinical monitoring of the study was performed independently (Centocor, Malvern, Pennsylvania, USA).
Laboratory assays
Anthropometric and biochemical measurements
Anthropometric measurements were made and body mass index (BMI (kg/m2)) was calculated from weight and height. Blood pressure was measured in triplicate (HEM-705CP, Omron), and the average of the readings was calculated. Blood was drawn and processed after an overnight fast, and serum and plasma samples were stored at −70°C for subsequent analysis. Full blood count, lipid and glucose level measurements were made with standard biochemistry assays and C reactive protein (CRP) was measured with an immunoturbidimetric, high-sensitivity assay (Tina-quant assay performed on a Cobas Integra analyzer, Roche Diagnostics).
HDL measurements
Measurements of HDL function were carried out at baseline, 54 weeks and 110 weeks in patients with RA. Baseline measurements were compared with those from healthy controls.
HDL isolation
HDL was isolated by sequential ultracentrifugation (d=1.063–1.21 g/mL) using solid potassium bromide for density adjustment.8 All functional assays of HDL were carried out within two weeks of isolation by a blinded investigator in duplicate.
Endothelial nitric oxide bioavailability
The effect of HDL (50 μg/mL: 60 min, 37°C) on endothelial nitric oxide (NO) bioavailability (bovine aortic endothelial cells (BAECs): passage 4–7; Lonza Bioscience) was measured using a fluorescent indicator. BAECs were incubated with 4,5-diaminofluorescein diacetate (DAF-2; 1Um; Cayman Chemical), and triazolofluorescein fluorescence was measured using an excitation wavelength of 485 nm.9
Endothelial superoxide production
The effect of HDL on endothelial cell superoxide production (SO) was measured in unstimulated and tumour necrosis factor-α (TNF-α)-stimulated (5 ng/mL, R&D Systems) human aortic endothelial cells (HAECs) by erythrocyte sedimentation rate (ESR) spectroscopy. HAECs were incubated with HDL from patients and controls (50 μg/mL, 60 min, 37°C), with or without TNF-α and resuspended in Krebs-Hepes buffer (pH 7.4; Noxygen) containing diethyldithiocarbamic acid sodium salt (5 μM, Noxygen) and deferoxamine methanesulfonate salt (25 μM, Noxygen). ESR spectra were recorded after addition of the spin probe 1-hydroxy-3-methoxycarbonyl-2,2,5,5-tetramethylpyrrolidine (CMH; Noxygen; final concentration 200 μM) using a Bruker e-scan spectrometer (Bruker Biospin). The ESR instrumental settings were centre field (B0) 3495 G; field sweep width 10 G; microwave frequency 9.75 GHz; microwave power 19.91 mW; magnetic field modulation frequency 86.00 kHz; modulation amplitude 2.60 G; conversion time 10.24 ms; and number of x-scans 1020.9
Cholesterol efflux capacity
HDL for measurements of efflux capacity was extracted from serum by ApoB depletion. Whole serum was incubated for 20 min with a 20% polyethylene glycol (PEG) solution (20% PEG 8000 (sigma P2139) in 200 mM glycine (sigma G8898, pH=10)). Samples were centrifuged at 1900 g, and the supernatant was collected and stored at 4°C. J774 cells were radiolabeled for 24 hours in a medium containing 2 μCi of [3H]-cholesterol per microlitre. Addition of 0.3 mM 8-(4-chlorophenylthio)-cyclic AMP for 6 hours upregulated expression of ABCA1. An efflux medium containing 2.8% apolipoprotein B-depleted serum from each individual was added for 4 hours. To prevent cholesterol esterification during labelling, equilibration and flux, 2 μg/mL of CP113,818, a acyl-coenzyme A:cholesterol acyltransferase inhibitor was added to all mediums. Efflux capacity was quantified using liquid scintillation to measure radioactive cholesterol effluxed from the cells (medium+intracellular lipids). All assays were performed in duplicate and the final average value normalised against a baseline control for statistical analyses between time points.10
Paraoxonase-1 activity
Serum paraoxonase activities were measured by ultraviolet spectrophotometry in a 96-well plate format using paraoxon (Sigma-Aldrich, St Louis, Missouri, USA). Briefly, 50 μg/mL HDL was diluted in a reaction mixture containing 10 mM Tris hydrochloride (pH 8.0), 1 M sodium chloride and 2 mM calcium chloride. At 24°C, 1.5 mM paraoxon was added to initiate the reaction, and the increase in absorbance at 405 nm was recorded over 30 min. An extinction coefficient (at 405 nm) of 17 000 M−1 • cm−1 was used to calculate units of paraoxonase-1 (PON-1).11
Statistics
HDL studies were powered for a 1:1 randomised controlled trial on the basis of NO bioavailability using paired assessments in 35 healthy controls (mean=0.98, SD =0.13, and intraclass correlation 0.91).12 From this power calculation, a total of seven patients per group was required (power=80% and α=0.05) to detect a 5% difference in the primary outcome (NO bioavailability). Normal distribution was assessed using the Shapiro-Wilk test. All measures are reported as mean (SD) or median (IQR) for those not normally distributed. Baseline comparisons were performed using an independent t-test or Mann-Whitney U test if data was non-normally distributed.
Post hoc multivariate analysis of variance (ANOVA) modelling was performed to evaluate the effects of treatment with infliximab (vs placebo) and duration of treatment on HDL function, CV and clinical markers at 54 weeks. A repeated-measures ANOVA, with time point comparisons using Bonferroni-corrected t-tests, was performed to determine differences in HDL function, CV and clinical markers in patients who received 2 years infliximab treatment. The Greenhouse-Geisser correction for the F test was used to adjust the degrees of freedom for deviation from sphericity. Analysis was performed using GraphPad Prism analysis software. A two-sided p value of <0.05 was considered to indicate statistical significance. An additional multilevel logistic regression analysis was conducted in R to confirm our results (see online supplementary appendix 2). We fit multilevel logistic regression models for measure of HDL function using the lme4 package (http://cran.r-project.org/web/packages/lme4/index.html) in the R statistical language (http://www.r-project.org/). All coefficients were taken as random effects.
heartjnl-2015-308953supp002.pdf (142.8KB, pdf)
Results
Cross-sectional study: comparison between patients with RA and controls
The patients and controls were well matched for age, gender and BMI (table 1). Patients with RA had significantly lower HDL-cholesterol (HDL-c) levels (p=0.01), but increased triglyceride levels (p<0.01). Total cholesterol and low-density lipoprotein (LDL)-c were similar between the groups (table 1). Patients with RA had significantly higher CRP levels (p<0.01) and lower blood glucose levels compared with controls (p=0.01).
Table 1.
Baseline characteristics in rheumatoid arthritis and healthy participants
| Control n=18 |
Rheumatoid arthritis n=18 |
p Value | |
|---|---|---|---|
| Demographics | |||
| Age (years) | 55.61 (6.37) | 58.56 (11.05) | 0.20 |
| Gender (proportion female) | 10/18 | 12/18 | 0.48 (χ2 test) |
| Cardiovascular risk | |||
| BMI (kg/m2) | 26.27 (2.29) | 24.42 (4.71) | 0.14 |
| Total cholesterol (mmol/L) | 4.93 (0.66) | 5.00 (1.43) | 0.79 |
| HDL (mmol/L) | 1.35 (0.44) | 1.02 (0.19) | 0.01 |
| LDL (mmol/L) | 2.89 (0.88) | 3.01 (1.19) | 0.74 |
| Triglyceride (mmol/L) | 1.13 (0.36) | 2.19 (0.96) | <0.01 |
| Glucose (mg/dL) | 4.95 [4.70–5.82] | 4.49 [3.46–4.94] | 0.01 |
| Inflammation | |||
| CRP (mg/L) | 1.00 [1.00–1.00] | 22.74 [13.76–40.78] | <0.01 |
Values expressed as mean (SD) and median [IQR] for non-normally distributed data. Comparisons between rheumatoid patients and healthy controls were performed using the independent t-test. Categorical variables were compared using Fisher's exact χ2 test. Comparisons for non-parametric measurements were performed using Mann-Whitney test.
BMI, body mass index; CRP, C reactive protein; HDL, high-density lipoprotein; LDL, low-density lipoprotein.
HDL, which was isolated from patients with RA, showed reduced NO bioavailability and increased SO production (p<0.01 for both) in cultured endothelial cells compared with controls. Paraoxonase activity was also impaired in patients with RA (p=0.03) (figure 1).
Figure 1.

Comparison of the functional properties of high-density lipoprotein between patients with rheumatoid arthritis (RA) and healthy controls. (A) Nitric oxide bioavailability. (B) Superoxide production. (C) Paraoxonase-1 (PON-1) activity. Individual data points for each patients (n=18 in each group). Error bars represent mean and SEM.
Double-blind randomised control study: lipid levels and HDL functional properties after 1 year of treatment
Both treatment groups were balanced for CV risk factors, RA disease activity and HDL function at baseline (table 2). An increase in total cholesterol levels was observed in the infliximab arm at 54 weeks (p=0.04) and was primarily driven by an increase of HDL-c (p=0.02). M+P treatment did not alter lipid profile. CRP was significantly reduced with infliximab treatment compared with MTX alone (p=0.03), which did not demonstrate a significant change at 54 weeks. Clinical and laboratory measures of RA were improved with M+I therapy, but no changes were detected in the M+P arm (table 3).
Table 2.
Baseline characteristics of patients with RA entering randomised trial
| Placebo n=7 |
Infliximab n=11 |
p Value | |
|---|---|---|---|
| Demographics | |||
| Age (years) | 55.86 (15.89) | 61.30 (11.05) | 0.42 |
| Gender (proportion female) | 4/7 | 8/11 | 0.31 (χ2 test) |
| Cardiovascular risk | |||
| BMI (kg/m2) | 25.88 (0.55) | 24.98 (5.11) | 0.73 |
| Total cholesterol (mmol/L) | 5.05(1.42) | 5.01 (1.5) | 0.96 |
| HDL-c (mmol/L) | 1.10 (0.22) | 0.97 (0.17) | 0.20 |
| LDL-c (mmol/L) | 3.20 (0.95) | 2.90 (1.34) | 0.62 |
| Triglyceride (mmol/L) | 1.67 (1.00) | 2.52 (0.81) | 0.63 |
| Glucose (mg/dL) | 4.60 (0.70) | 4.75 (1.24) | 0.59 |
| Systolic BP (mm Hg) | 140.70 (7.27) | 132.5 (4.67) | 0.33 |
| Diastolic BP (mm Hg) | 75.71 (3.96) | 76.00 (3.70) | 0.96 |
| HDL function | |||
| Nitric oxide bioavailability (% buffer-treated cells) | 81.81 (3.97) | 84.23 (6.23) | 0.37 |
| Superoxide production (nmol/O2-/100 000 cells) | 4.37 (1.71) | 3.90 (1.66) | 0.56 |
| Paraoxonase-1 activity (μmol p-nitrophenol/L/serum/min) | 137.52 (1.71) | 148.24 (14.49) | 0.09 |
| Efflux capacity (%) | 12.25 (2.04) | 13.52 (1.26) | 0.31 |
| RA disease activity | |||
| CRP (mg/L) | 37.65 [19.48–64.31] | 26.83 [14.37–37.33] | 0.22 |
| ESR (mm/hours) | 30.00 [22.00–76.00] | 25.00 [14.00–50.50] | 0.36 |
| DAS28—CRP score | 6.08 (0.71) | 5.17 (1.13) | 0.09 |
| DAS28—ESR score | 5.59 (0.85) | 4.68 (1.56) | 0.15 |
Values expressed as mean (SD) and median [IQR] for non-normally distributed data. Comparisons between groups were performed using the independent t-test. Categorical variables were compared using Fisher's exact χ2 test. Comparisons for non-parametric measurements were performed using Mann-Whitney test.
APO4, Apolipoprotein A; BMI, body mass index; BP, blood pressure; CRP, C reactive protein; DAS28, Disease Activity Score 28; ESR, erythrocyte sedimentation rate; HDL-c, high-density lipoprotein cholesterol; LDL-c, low-density lipoprotein cholesterol; RA, rheumatoid arthritis.
Table 3.
Clinical characteristics and HDL function at 1 year
| MTX+placebo |
MTX+infliximab |
|||||
|---|---|---|---|---|---|---|
| Baseline | One year | p Value | Baseline | One year | p Value | |
| Cardiovascular risk | ||||||
| Total cholesterol (mmol/L) | 5.05 (1.42) | 5.13 (1.55) | 0.84 | 5.01 (1.5) | 6.23 (1.63) | 0.04 |
| HDL-c (mmol/L) | 1.10 (0.22) | 1.10 (0.42) | 0.14 | 1.02 [0.93–1.09] | 1.33 [1.26–1.50] | 0.02 |
| LDL-c (mmol/L) | 3.20 (0.95) | 3.18 (1.23) | 0.96 | 2.90 (1.34) | 3.65 (1.22) | 0.07 |
| Triglyceride (mmol/L) | 1.35 [1.07–1.62] | 1.26 [1.12–1.43] | 0.20 | 2.52 (0.81) | 2.48 (0.92) | 0.91 |
| Glucose (mg/dL) | 4.59 [4.28–4.76] | 4.40 [4.04–5.12] | 0.61 | 4.75 (1.24) | 4.99 (0.95) | 0.15 |
| HDL function | ||||||
| Nitric oxide bioavailability (% buffer-treated cells) |
81.81 (3.97) | 82.72 (3.43) | 0.67 | 84.23 (6.23) | 89.08 (9.31) | 0.02 |
| Superoxide production (nmol/O2-/100 000 cells) |
4.37 (1.71) | 3.63 (1.67) | 0.03 | 3.90 (1.66) | 2.98 (1.47) | 0.01 |
| Paraoxonase-1 activity (μmol p-nitrophenol/L/serum/min) |
137.52 (6.41) | 153.58 (17.88) | 0.07 | 148.24 (14.49) | 155.15 (14.86) | 0.07 |
| Efflux capacity (%) | 12.25 (2.04) | 13.25 (2.52) | 0.26 | 13.52 (1.26) | 14.01 (3.23) | 0.72 |
| RA disease activity | ||||||
| CRP (mg/L) | 37.65 [19.48–64.31] | 26.00 [13.85–36.83] | 0.09 | 26.83 [14.37–37.33] | 2.34 [0.00–6.34] | 0.03 |
| ESR (mm/hours) | 30.00 [22.00–76.00] | 27.50 [16.00–38.00] | 0.69 | 25.00 [14.00–50.50] | 20.50 [11.00–30.00] | 0.61 |
| DAS28—CRP score | 6.08 (0.71) | 5.04 (0.85) | 0.14 | 5.17 (1.13) | 3.53 (1.06) | <0.01 |
| DAS28—ESR score | 5.59 (0.85) | 4.43 (1.74) | 0.08 | 4.68 (1.56) | 2.25 (0.83) | <0.01 |
Values expressed as mean (SD) and median [IQR] for non-normally distributed data. Comparisons between groups were performed using a paired t-test. Comparisons for non-parametric measurements were performed using Wilcoxon signed-rank test.
CRP, C reactive protein; ESR, erythrocyte sedimentation rate; HDL-c, high-density lipoprotein cholesterol; LDL-c, low-density lipoprotein cholesterol; MTX, methotrexate; RA, rheumatoid arthritis.
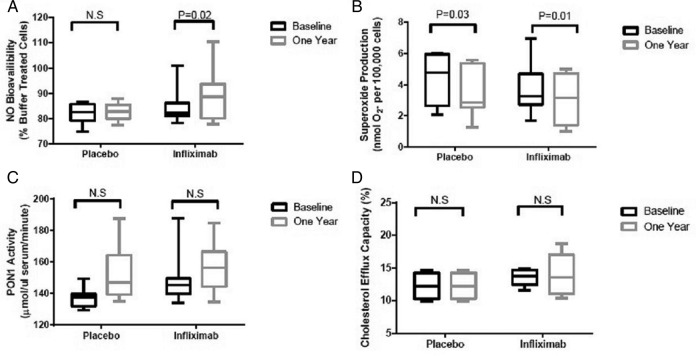
SO production was reduced from baseline following both M+P and M+I treatments (p=0.03 and 0.01). NO bioavailability was improved (p=0.02) with M+I therapy, but no effect was noted in the M+P patients. PON-1 activity increased in a non-significant manner in both groups, but cholesterol efflux capacity remained unchanged (figure 2). Between-treatment comparisons at 54 weeks did not demonstrate benefit of M+I treatment over M+P treatment for any measure of HDL function. These results were confirmed in a multilevel logistic regression model (see online supplementary appendix 2).
Figure 2.
Comparison of (A) nitric oxide (NO) bioavailability, (B) superoxide production, (C) paraoxonase-1 (PON-1) activity and (D) cholesterol efflux during placebo controlled phase of trial. Box and whisker plot represents mean and 95% CIs.
Single-blinded study
In grouped analysis of the 18 patients who received 54 weeks of M+I treatment, NO bioavailability increased compared with baseline (p<0.01). Furthermore, a reduction in SO (p<0.01) and an increase of PON-1 (both p=0.03) was seen (figure 3). No change in cholesterol efflux capacity was noted. Lipid levels were increased and inflammatory and RA disease activity scores were significantly reduced (data not shown).
Figure 3.

Trends in high-density lipoprotein function following 1 year infliximab treatment for (A) nitric oxide (NO) bioavailability, (B) superoxide production and (C) paraoxonase-1 (PON-1) activity. Individual data points for each patient (n=18). Error bars represent mean and SEM.
Two years infliximab treatment
Eleven patients received infliximab therapy for 110 weeks. Total cholesterol (p=0.08) and LDL-c (p=0.09) showed a non-significant increase at 110 weeks, but there was a significant increase in HDL-c (p=0.01). CRP and RA disease activity scores were both significantly reduced. No changes were noted for cholesterol efflux (figure 4).
Figure 4.
Comparison of (A) nitric oxide (NO) bioavailability, (B) superoxide production, (C) paraoxonase-1 (PON-1) activity and (D) cholesterol efflux after 2 years treatment with infliximab. Individual data points representative for each patient (n=11). n.s., not significant.
Repeated-measures ANOVA demonstrated a significant increase in NO bioavailability (p=0.01) and significant reductions in SO production (p=0.02) and PON-1 (p=0.01). There was no further improvement in HDL function between patients who had received 54 weeks of M+I treatment compared with 110 weeks of M+I treatment. All measures of HDL function remained impaired compared with the healthy controls (data not shown).
Discussion
This study shows that anti-inflammatory treatments can improve the antioxidant and endothelial protective properties of HDL in patients with erosive RA. These effects were not accompanied by changes in cholesterol efflux and tracked improvements in RA disease activity. Treatment with infliximab in addition to MTX for 2 years showed a benefit for a broad range of HDL functional properties including NO bioavailability, SO production and PON-1 activity. These findings suggest that there is a role for anti-inflammatory treatments, particularly those targeting the TNF-α pathway, for improving not only RA disease activity but also potentially CV risk factors in patients with RA.
The threefold increased risk of CV disease in patients with RA is not accounted for by traditional risk factors and is related to increased levels of inflammation.2 This influences a range of CV risk factors and causes quantitative and qualitative changes to lipoproteins.13 Substantial evidence has now accumulated, suggesting that inflammation can convert the normally protective HDL into a ‘particle with adverse effects on endothelial function’.4 14
We showed that HDL has several dysfunctional properties in patients with RA, which may contribute to their increased CV risk. Our findings are in agreement with previous evidence describing a pro-inflammatory HDL particle with reduced PON-1 activity.15 We have now demonstrated an impairment of the beneficial effects of HDL on endothelium with a marked decrease in the stimulation of endothelial NO and increase in SO production compared with healthy controls. Diminished production of NO may reflect endothelial dysfunction, an early step in atherogenesis, and represents a novel pathway by which HDL dysfunction can promote arterial disease in patients with RA.
We evaluated the potential for HDL function to be restored following treatment with RA anti-inflammatory therapies. MTX is commonly used as a first-line treatment in early RA. Observational studies have demonstrated reduced rates of myocardial infarction and stroke with MTX therapy and the ongoing CIRT trial is evaluating the drug in secondary CV prevention.16 17 Its exact anti-inflammatory mechanisms remain uncertain, although it has been shown to mediate increased extracellular accumulation of adenosine around connective tissue and reduce levels of CRP and interleukin-6.17 Biological inhibition of TNF-α with infliximab in addition to MTX is a more effective approach for reducing RA disease activity. TNF-α is a proximal cytokine and its blockade has well-established anti-inflammatory actions and effects on lipid metabolism.18 At 54 weeks, NO bioavailability was significantly improved in patients who received infliximab, but not with MTX. Infliximab has previously been associated with increased AKT/eNOS phosphorylation and improved endothelial function.19 20 Our study provides a novel mechanism by which infliximab can improve endothelial function and contribute to vascular protection by TNF-α inhibition.
After 1 year of our double-blinded randomised trial, SO production was significantly reduced in both arms. MTX is known to inhibit several inflammatory and oxidative stress mediators, associated with increased lipid peroxidation.21 Oxidative modification of the HDL particle alters its protective phenotype.22 Combination therapy with infliximab caused an additional reduction of SO production compared with MTX alone. Previous studies of infliximab in humans suggest that TNF-α inhibition protects against oxidative DNA damage and lipid peroxidation, and this may explain its additional efficacy in reducing SO production.23 Despite a trend towards an increased benefit of infliximab therapy at 1 year, between-treatment comparisons did not reach statistical significance.
Assay of PON-1 provided an additional measure of the antioxidative function of HDL but no significant difference was found in the double-blinded randomised study in either arm at 1 year possibly due to the small sample size. However, PON-1 activity was improved in the single-blinded study at 1 and 2 years treatment of infliximab. While the effect of MTX on PON-1 activity has not previously been reported, infliximab is known to improve PON-1 activity through the reduction of lipid peroxidation including myeloperoxidase (MPO) pathways.24 Site-specific oxidative modification of PON-1 by MPO impairs the enzymes ability to bind with APOA-1 and reduced MPO could explain the increased PON-1 activity in our patients.25
We did not demonstrate any differences in cholesterol efflux with either treatment regimen. This suggests that anti-inflammatory therapies have a more pronounced impact on the vascular properties of HDL than on its efflux capacity. These findings support previous reports from our group and indicate that the endothelial function of HDL may be more sensitive to modification than cholesterol efflux.6 However, anti-inflammatory treatments including MTX and infliximab have recently been associated with improved cholesterol efflux in patients with RA, and associated with CV disease in both healthy and at-risk populations.10 26–28 These differences may be due to methodological issues in the efflux measurement and their interpretation, and require further investigation.
Our study was not powered to assess the quantitative relationship between HDL function, inflammation and disease activity. This also limited our ability to test for between-treatment differences in the randomised study design, although combination therapy demonstrated a clear benefit in the single-blinded study. However, we have demonstrated that a range of vascular properties of HDL can be improved with anti-inflammatory drug therapy. HDL function did not return to normal levels following treatment, and this is likely to be as a result of the residual levels of inflammation even after 2 years treatment (data not shown).
The ability of anti-inflammatory treatments to modify CV risk remains an intense area of clinical research. Currently, three anti-inflammatory agents are the subject of phase III trials; MTX (CIRT), Darapladib (SOLID-TMI and STABILITY) and canakinumab (CANTOS). Recently, both the STABILITY and SOLID-TMI trials reported that darapladib failed to reduce the risk of CV death and myocardial infarction in primary and secondary prevention.29 30 The CIRT and CANTOS trials are due to report shortly, but our data suggest that monotherapy with MTX alone may not be sufficient. Inhibition of TNF-α, a broad immune effector, is more likely to suppress inflammation and may be a better strategy for CV benefit.
This study underlines the need to measure and better understand HDL function. It provides evidence for the use of anti-inflammatory treatments, particularly those modulating the TNF-α pathway, to restore the beneficial effects of HDL on the vasculature. Our findings are of relevance to many recent clinical trials evaluating both HDL elevation and anti-inflammatory therapies with the aim of reducing CV risk.
Key messages.
What is already known on this subject?
Clinical trials aimed at raising high-density lipoprotein (HDL)-cholesterol have thus far failed to improve cardiovascular (CV) outcome, and this may be explained by a dysfunctional HDL phenotype in disease.
What might this study add?
This study underlines the need to measure and better understand HDL function. It provides evidence for the use of anti-inflammatory treatments, particularly those modulating the tumour necrosis factor-α pathway, to restore the beneficial effects of HDL on the vasculature.
How might this impact on clinical practice?
Our findings are of relevance to many recent clinical trials evaluating both therapies aiming to raise HDL levels and anti-inflammatory agents with the aim of reducing CV risk.
Footnotes
Contributors: FON, MC, PC and JD made substantial contributions to the conception and design of the work. FON, EM, NP and ES made substantial contributions to the acquisition, analysis or interpretation of data for the work. FON, MC, CWMK, UL, FD, PCT and JD made substantial contribution to the work or revising it critically for important intellectual content and final approval of the version to be published.
Funding: F. Hoffmann-La Roche and the Leducq Foundation.
Competing interests: None declared.
Patient consent: Obtained.
Ethics approval: Riverside Research Ethics Committee.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Meune C, Touzé E, Trinquart L, et al. Trends in cardiovascular mortality in patients with rheumatoid arthritis over 50 years: a systematic review and meta-analysis of cohort studies. Rheumatology (Oxford) 2009;48:1309–13. 10.1093/rheumatology/kep252 [DOI] [PubMed] [Google Scholar]
- 2.del Rincón ID, Williams K, Stern MP, et al. High incidence of cardiovascular events in a rheumatoid arthritis cohort not explained by traditional cardiac risk factors. Arthritis Rheum 2001;44:2737–45. [DOI] [PubMed] [Google Scholar]
- 3.Myasoedova E, Crowson CS, Kremers HM, et al. Lipid paradox in rheumatoid arthritis: the impact of serum lipid measures and systemic inflammation on the risk of cardiovascular disease. Ann Rheum Dis 2011;70:482–7. 10.1136/ard.2010.135871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Charakida M, Besler C, Batuca JR, et al. Vascular abnormalities, paraoxonase activity, and dysfunctional HDL in primary antiphospholipid syndrome. JAMA 2009;302:1210–17. 10.1001/jama.2009.1346 [DOI] [PubMed] [Google Scholar]
- 5.McMahon M, Grossman J, FitzGerald J, et al. Proinflammatory high-density lipoprotein as a biomarker for atherosclerosis in patients with systemic lupus erythematosus and rheumatoid arthritis. Arthritis Rheum 2006;54:2541–9. 10.1002/art.21976 [DOI] [PubMed] [Google Scholar]
- 6.O'Neill FP, Riwanto M, Charakida M, et al. Structural and functional changes in HDL with low grade and chronic inflammation. Int J Cardiol 2015;188:111–16. 10.1016/j.ijcard.2015.03.058 [DOI] [PubMed] [Google Scholar]
- 7.Taylor PC, Steuer A, Gruber J, et al. Ultrasonographic and radiographic results from a two-year controlled trial of immediate or one-year-delayed addition of infliximab to ongoing methotrexate therapy in patients with erosive early rheumatoid arthritis. Arthritis Rheum 2006;54:47–53. 10.1002/art.21544 [DOI] [PubMed] [Google Scholar]
- 8.Tong H, Knapp HR, VanRollins M. A low temperature flotation method to rapidly isolate lipoproteins from plasma. J Lipid Res 1998;39:1696–704. [PubMed] [Google Scholar]
- 9.Besler C, Heinrich K, Rohrer L, et al. Mechanisms underlying adverse effects of HDL on eNOS-activating pathways in patients with coronary artery disease. J Clin Invest 2011;121:2693–708. 10.1172/JCI42946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khera AV, Cuchel M, de la Llera-Moya M, et al. Cholesterol efflux capacity, high-density lipoprotein function, and atherosclerosis. N Engl J Med 2011;364:127–35. 10.1056/NEJMoa1001689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bhattacharyya T, Nicholls SJ, Topol EJ, et al. Relationship of paraoxonase 1 (PON1) gene polymorphisms and functional activity with systemic oxidative stress and cardiovascular risk. JAMA 2008;299:1265–76. 10.1001/jama.299.11.1265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.O'Neill F, McLoughlin E, Riwanto M, et al. Reproducibility and biological variability of HDL's vascular functional assays. Atherosclerosis 2015;241:588–94. 10.1016/j.atherosclerosis.2015.06.005 [DOI] [PubMed] [Google Scholar]
- 13.González-Gay MA, González-Juanatey C. Inflammation and lipid profile in rheumatoid arthritis: bridging an apparent paradox. Ann Rheum Dis 2014;73:1281–3. 10.1136/annrheumdis-2013-204933 [DOI] [PubMed] [Google Scholar]
- 14.Hahn BH, Lourencço EV, McMahon M, et al. Pro-inflammatory high-density lipoproteins and atherosclerosis are induced in lupus-prone mice by a high-fat diet and leptin. Lupus 2010;19:913–17. 10.1177/0961203310364397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Popa C, van Tits LJ, Barrera P, et al. Anti-inflammatory therapy with tumour necrosis factor alpha inhibitors improves high-density lipoprotein cholesterol antioxidative capacity in rheumatoid arthritis patients. Ann Rheum Dis 2009;68:868–72. 10.1136/ard.2008.092171 [DOI] [PubMed] [Google Scholar]
- 16.Everett BM, Pradhan AD, Solomon DH, et al. Rationale and design of the Cardiovascular Inflammation Reduction Trial: a test of the inflammatory hypothesis of atherothrombosis. Am Heart J 2013;166:199–207. 10.1016/j.ahj.2013.03.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Westlake SL, Colebatch AN, Baird J, et al. The effect of methotrexate on cardiovascular disease in patients with rheumatoid arthritis: a systematic literature review. Rheumatology (Oxford) 2010;49:295–307. 10.1093/rheumatology/kep366 [DOI] [PubMed] [Google Scholar]
- 18.Tracey D, Klareskog L, Sasso EH, et al. Tumor necrosis factor antagonist mechanisms of action: a comprehensive review. Pharmacol Ther 2008;117:244–79. 10.1016/j.pharmthera.2007.10.001 [DOI] [PubMed] [Google Scholar]
- 19.Filho AG, Kinote A, Pereira DJ, et al. Infliximab prevents increased systolic blood pressure and upregulates the AKT/eNOS pathway in the aorta of spontaneously hypertensive rats. Eur J Pharmacol 2013;700:201–9. 10.1016/j.ejphar.2012.11.059 [DOI] [PubMed] [Google Scholar]
- 20.Hürlimann D, Forster A, Noll G, et al. Anti-tumor necrosis factor-alpha treatment improves endothelial function in patients with rheumatoid arthritis. Circulation 2002;106:2184–7. 10.1161/01.CIR.0000037521.71373.44 [DOI] [PubMed] [Google Scholar]
- 21.Wessels JA, Huizinga TW, Guchelaar HJ. Recent insights in the pharmacological actions of methotrexate in the treatment of rheumatoid arthritis. Rheumatology (Oxford) 2008;47:249–55. 10.1093/rheumatology/kem279 [DOI] [PubMed] [Google Scholar]
- 22.Undurti A, Huang Y, Lupica JA, et al. Modification of high density lipoprotein by myeloperoxidase generates a pro-inflammatory particle. J Biol Chem 2009;284:30825–35. 10.1074/jbc.M109.047605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kageyama Y, Takahashi M, Ichikawa T, et al. Reduction of oxidative stress marker levels by anti-TNF-alpha antibody, infliximab, in patients with rheumatoid arthritis. Clin Exp Rheumatol 2008;26:73–80. [PubMed] [Google Scholar]
- 24.Bacchetti T, Campanati A, Ferretti G, et al. Oxidative stress and psoriasis: the effect of antitumour necrosis factor-α inhibitor treatment. Br J Dermatol 2013;168:984–9. 10.1111/bjd.12144 [DOI] [PubMed] [Google Scholar]
- 25.Huang Y, Wu Z, Riwanto M, et al. Myeloperoxidase, paraoxonase-1, and HDL form a functional ternary complex. J Clin Invest 2013;123:3815–28. 10.1172/JCI67478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Voloshyna I, Seshadri S, Anwar K, et al. Infliximab reverses suppression of cholesterol efflux proteins by TNF-α: a possible mechanism for modulation of atherogenesis. Biomed Res Int 2014;2014:312647 10.1155/2014/312647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rohatgi A, Khera A, Berry JD, et al. HDL cholesterol efflux capacity and incident cardiovascular events. N Engl J Med 2014;371:2383–93. 10.1056/NEJMoa1409065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ronda N, Greco D, Adorni MP, et al. Newly identified antiatherosclerotic activity of methotrexate and adalimumab: complementary effects on lipoprotein function and macrophage cholesterol metabolism. N Engl J Med 2015;67:1155–64. 10.1002/art.39039 [DOI] [PubMed] [Google Scholar]
- 29.White HD, Held C, Stewart R, et al. Darapladib for preventing ischemic events in stable coronary heart disease. N Engl J Med 2014;370:1702–11. 10.1056/NEJMoa1315878 [DOI] [PubMed] [Google Scholar]
- 30.O'Donoghue ML, Braunwald E, White HD, et al. Effect of darapladib on major coronary events after an acute coronary syndrome: the SOLID-TIMI 52 randomized clinical trial. JAMA 2014;312:1006–15. 10.1001/jama.2014.11061 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
heartjnl-2015-308953supp001.pdf (48.3KB, pdf)
heartjnl-2015-308953supp002.pdf (142.8KB, pdf)