Abstract
A mutant of Salmonella typhimurium with a defect in the regulation of pyr-gene expression was obtained during a selection for mutants resistant to a combination of the two pyrimidine analogs, 5-fluorouracil and 5-fluorouridine. The mutant possesses 4-fold elevated pools of the pyrimidine nucleoside triphosphatases, UTP and CTP. The specific activities of aspartate transcarbamylase and orotate phosphoribosyltransferase are 40-fold and 7-fold higher in the mutant than in the parent strain when grown in minimal media. Furthermore, the synthesis of the two enzymes in the mutant is not repressed following addition of exogenous pyrimidines. The levels of carbamoylphosphate synthase and orotidine 5'-monophosphate decarboxylase are approximately 3-fold enhanced, while the activities of dihydroorotase and dihydroorotate oxidase appear largely unaffected by the mutation. The mutation responsible for these effects was shown to map between two known point mutations in the rpoBC gene cluster encoding the beta and beta' subunits of RNA polymerase. These observations indicate a regulatory function of RNA polymerase in the control of pyr-gene expression in S. typhimurium.
Full text
PDF
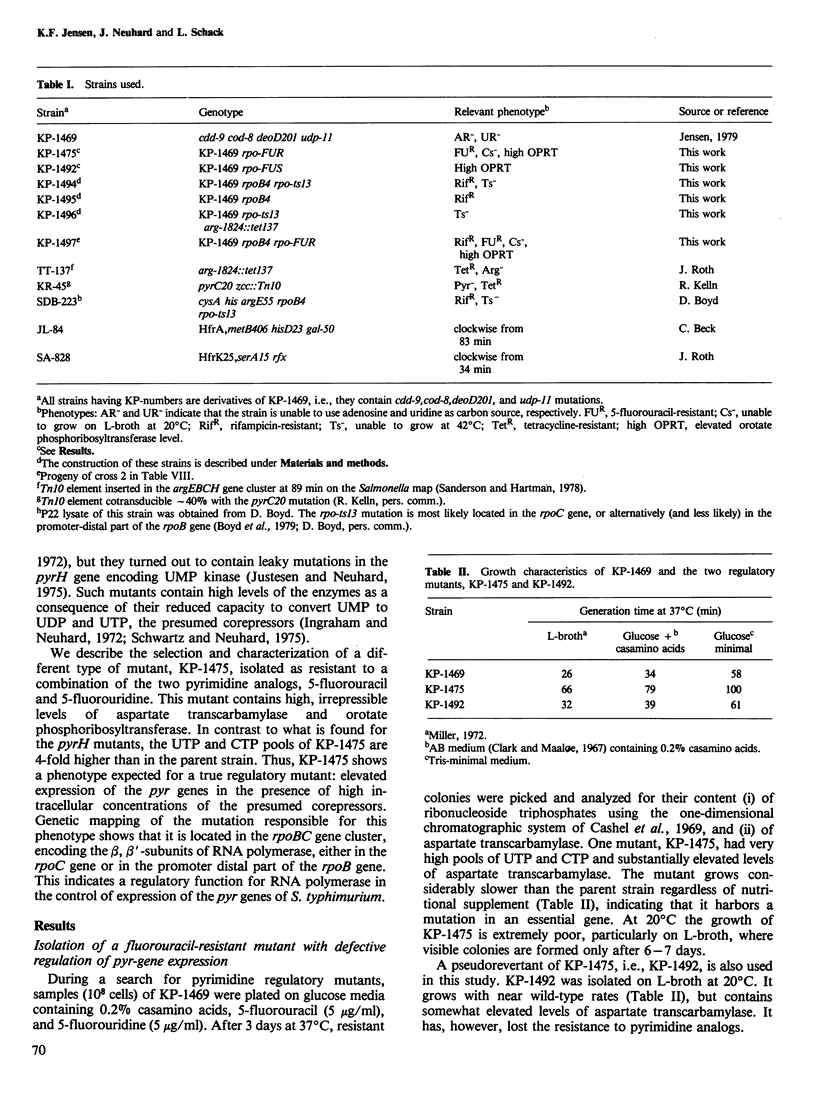
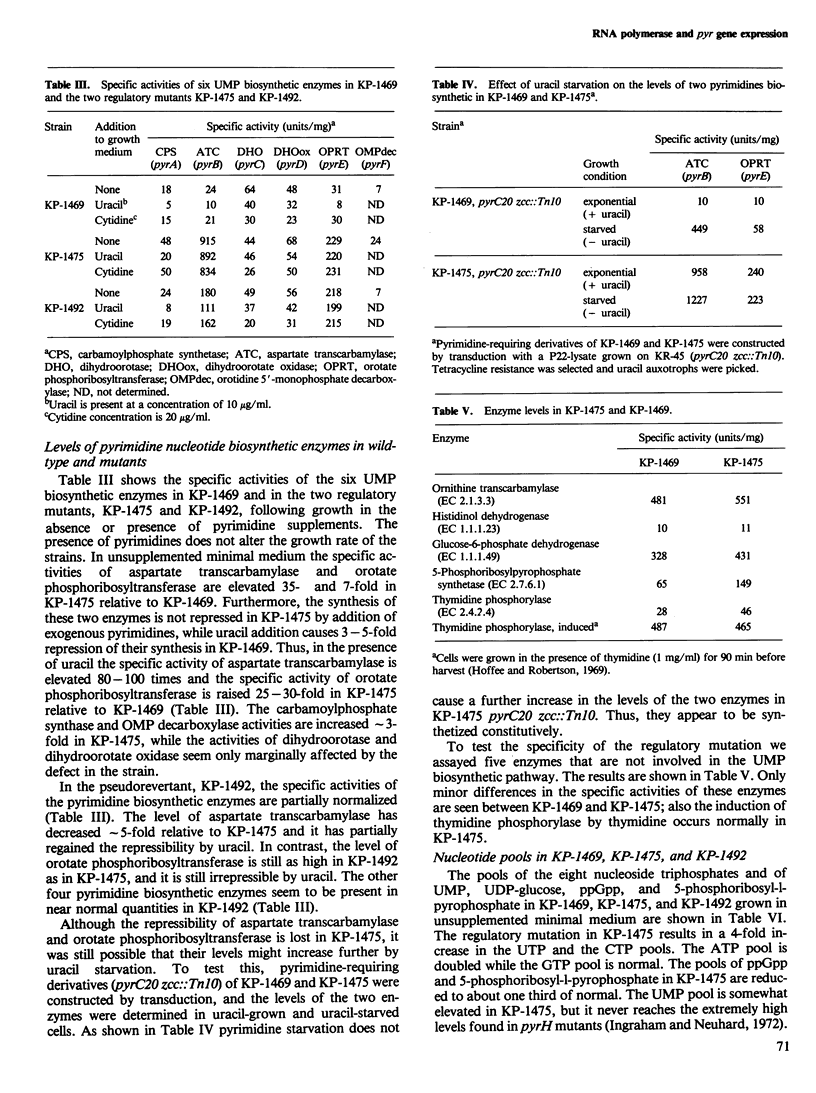
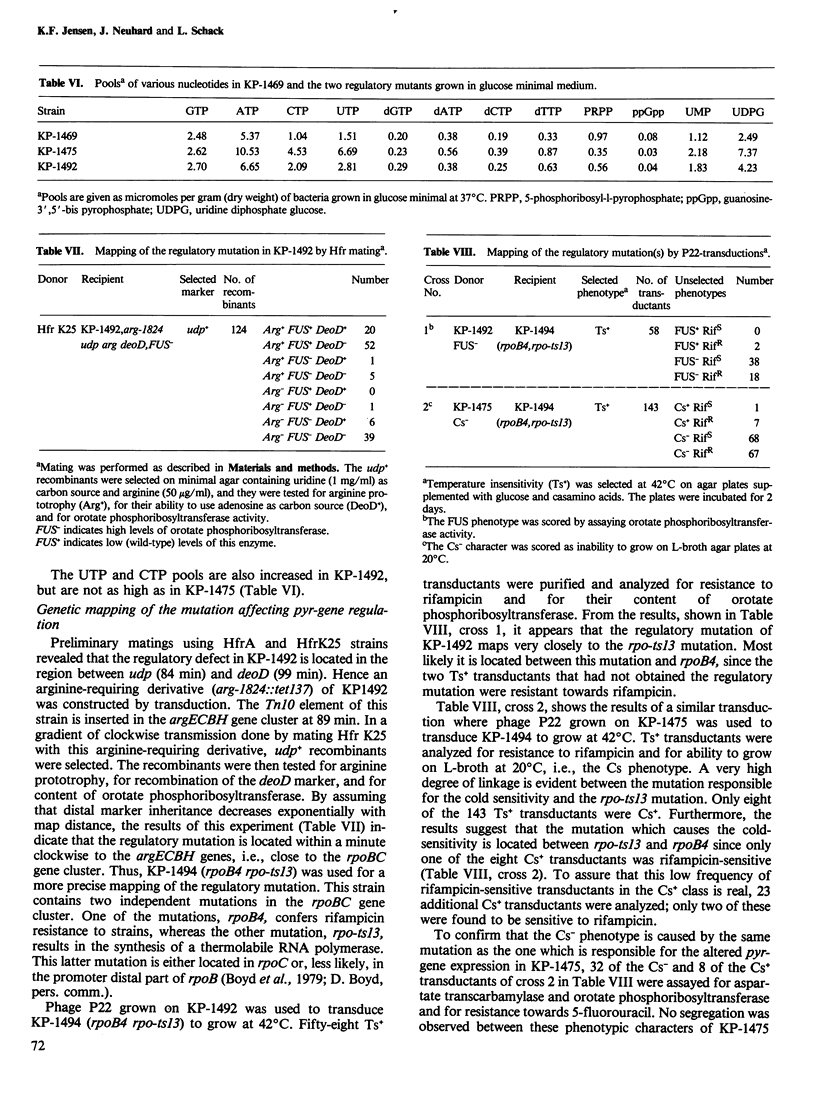


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abd-el-Al A., Ingraham J. L. Control of carbamyl phosphate synthesis in Salmonella typhimurium. J Biol Chem. 1969 Aug 10;244(15):4033–4038. [PubMed] [Google Scholar]
- Abdelal A. T., Ingraham J. L. Carbamylphosphate synthetase from Salmonella typhimurium. Regulations, subunit composition, and function of the subunits. J Biol Chem. 1975 Jun 25;250(12):4410–4417. [PubMed] [Google Scholar]
- Abdelal A. T., Kennedy E. H., Nainan O. Ornithine transcarbamylase from Salmonella typhimurium: purification, subunit composition, kinetic analysis, and immunological cross-reactivity. J Bacteriol. 1977 Mar;129(3):1387–1396. doi: 10.1128/jb.129.3.1387-1396.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BECKWITH J. R., PARDEE A. B., AUSTRIAN R., JACOB F. Coordination of the synthesis of the enzymes in the pyrimidine pathway of E. coli. J Mol Biol. 1962 Dec;5:618–634. doi: 10.1016/s0022-2836(62)80090-4. [DOI] [PubMed] [Google Scholar]
- Beck C. F., Ingraham J. L. Location on the chromosome of Salmonella typhimurium of genes governing pyrimidine metabolism. Mol Gen Genet. 1971;111(4):303–316. doi: 10.1007/BF00569782. [DOI] [PubMed] [Google Scholar]
- Boyd D. H., Porter L. M., Young B. S., Wright A. The in vitro detection of defects in temperature sensitive RNA polymerases from mutants of Salmonella typhimurium. Mol Gen Genet. 1979 Jun 20;173(3):279–287. doi: 10.1007/BF00268638. [DOI] [PubMed] [Google Scholar]
- Cashel M., Lazzarini R. A., Kalbacher B. An improved method for thin-layer chromatography of nucleotide mixtures containing 32P-labelled orthophosphate. J Chromatogr. 1969 Mar 11;40(1):103–109. doi: 10.1016/s0021-9673(01)96624-5. [DOI] [PubMed] [Google Scholar]
- Edlin G., Maaloe O. Synthesis and breakdown of messenger RNA without protein synthesis. J Mol Biol. 1966 Feb;15(2):428–434. doi: 10.1016/s0022-2836(66)80118-3. [DOI] [PubMed] [Google Scholar]
- GERHART J. C., PARDEE A. B. The enzymology of control by feedback inhibition. J Biol Chem. 1962 Mar;237:891–896. [PubMed] [Google Scholar]
- Hoffee P. A., Robertson B. C. 2-Deoxyribose gene-enzyme complex in Salmonella typhimurium: regulation of phosphodeoxyribomutase. J Bacteriol. 1969 Mar;97(3):1386–1396. doi: 10.1128/jb.97.3.1386-1396.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingraham J. L., Neuhard J. Cold-sensitive mutants of Salmonella typhimurium defective in uridine monophosphate kinase (pyrH). J Biol Chem. 1972 Oct 10;247(19):6259–6265. [PubMed] [Google Scholar]
- Jensen K. F. Apparent involvement of purines in the control of expression of Salmonella typhimurium pyr genes: analysis of a leaky guaB mutant resistant to pyrimidine analogs. J Bacteriol. 1979 Jun;138(3):731–738. doi: 10.1128/jb.138.3.731-738.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen K. F., Houlberg U., Nygaard P. Thin-layer chromatographic methods to isolate 32P-labeled 5-phosphoribosyl-alpha-1-pyrophosphate (PRPP): determination of cellular PRPP pools and assay of PRPP synthetase activity. Anal Biochem. 1979 Oct 1;98(2):254–263. doi: 10.1016/0003-2697(79)90138-6. [DOI] [PubMed] [Google Scholar]
- Justesen J., Neuhard J. pyrR identical to pyrH in Salmonella typhimurium: control of expression of the pyr genes. J Bacteriol. 1975 Sep;123(3):851–854. doi: 10.1128/jb.123.3.851-854.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassavetis G. A., Chamberlin M. J. Pausing and termination of transcription within the early region of bacteriophage T7 DNA in vitro. J Biol Chem. 1981 Mar 25;256(6):2777–2786. [PubMed] [Google Scholar]
- Kelln R. A., Kinahan J. J., Foltermann K. F., O'Donovan G. A. Pyrimidine biosynthetic enzymes of Salmonella typhimurium, repressed specifically by growth in the presence of cytidine. J Bacteriol. 1975 Nov;124(2):764–774. doi: 10.1128/jb.124.2.764-774.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- O'Donovan G. A., Gerhart J. C. Isolation and partial characterization of regulatory mutants of the pyrimidine pathway in Salmonella typhimurium. J Bacteriol. 1972 Mar;109(3):1085–1096. doi: 10.1128/jb.109.3.1085-1096.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donovan G. A., Neuhard J. Pyrimidine metabolism in microorganisms. Bacteriol Rev. 1970 Sep;34(3):278–343. doi: 10.1128/br.34.3.278-343.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piérard A., Glansdorff N., Gigot D., Crabeel M., Halleux P., Thiry L. Repression of Escherichia coli carbamoylphosphate synthase: relationships with enzyme synthesis in the arginine and pyrimidine pathways. J Bacteriol. 1976 Jul;127(1):291–301. doi: 10.1128/jb.127.1.291-301.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randerath K., Randerath E. Ion-exchange thin-layer chromatography. XIV. Separation of nucleotide sugars and nucleoside monophosphates on PEI-cellulose. Anal Biochem. 1965 Dec;13(3):575–579. doi: 10.1016/0003-2697(65)90356-8. [DOI] [PubMed] [Google Scholar]
- Sanderson K. E., Hartman P. E. Linkage map of Salmonella typhimurium, edition V. Microbiol Rev. 1978 Jun;42(2):471–519. doi: 10.1128/mr.42.2.471-519.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz M., Neuhard J. Control of expression of the pyr genes in Salmonella typhimurium: effects of variations in uridine and cytidine nucleotide pools. J Bacteriol. 1975 Mar;121(3):814–822. doi: 10.1128/jb.121.3.814-822.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz M. Thymidine phosphorylase from Escherichia coli. Properties and kinetics. Eur J Biochem. 1971 Jul 29;21(2):191–198. doi: 10.1111/j.1432-1033.1971.tb01455.x. [DOI] [PubMed] [Google Scholar]
- Yanofsky C. Attenuation in the control of expression of bacterial operons. Nature. 1981 Feb 26;289(5800):751–758. doi: 10.1038/289751a0. [DOI] [PubMed] [Google Scholar]



