Abstract
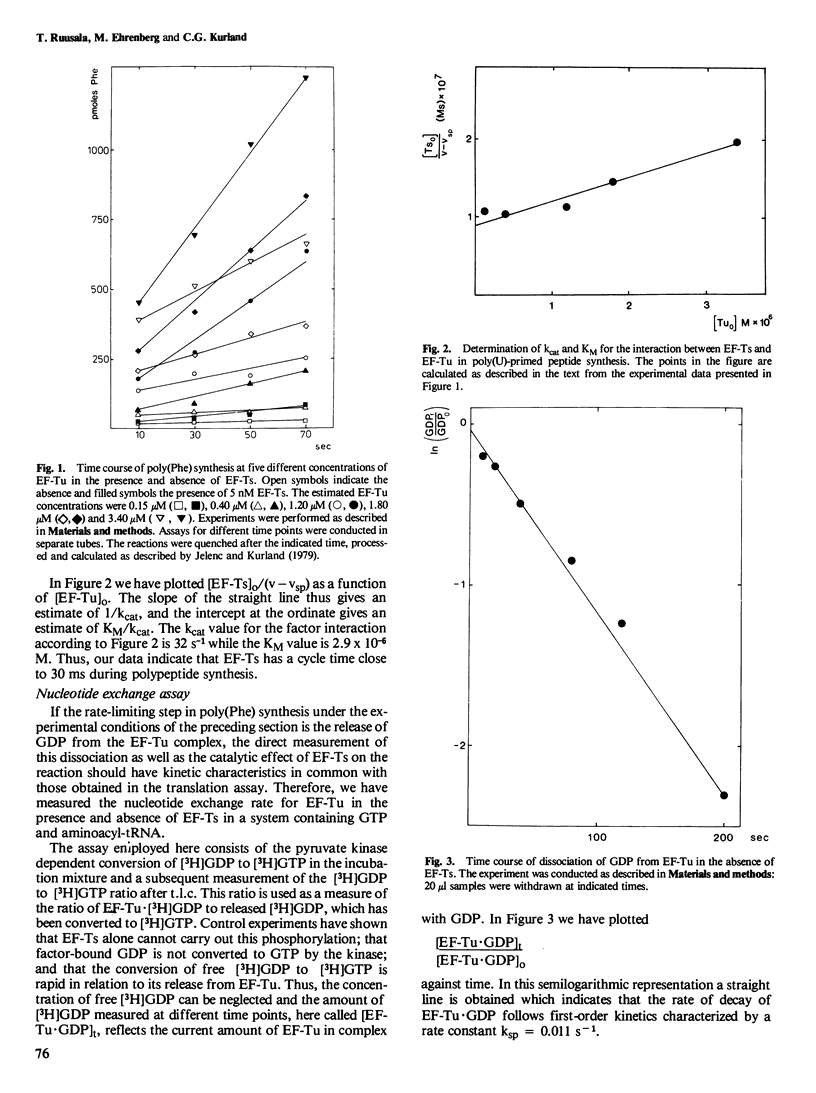
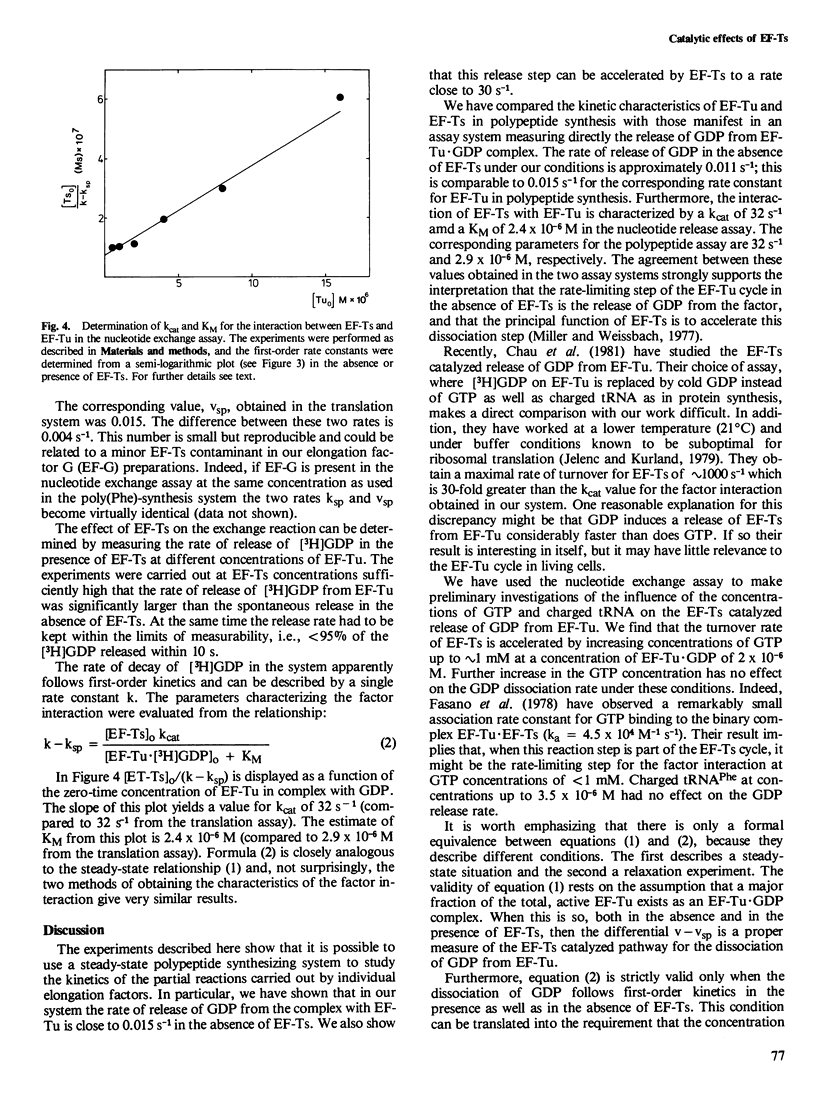
The kinetic parameters which characterize the interaction between elongation factor Tu (EF-Tu) and elongation factor Ts (EF-Ts) have been determined in a poly(uridylic acid)-primed translation system. The EF-Ts catalyzed release of GDP from EF-Tu was measured independently in a nucleotide exchange assay. We conclude that the rate-limiting step for the EF-Tu cycle in protein synthesis in the absence of EF-Ts is the release of GDP. By adding EF-Ts the time of this step is reduced from 90 s to 30 ms. Half maximal rate is obtained at an EF-Ts concentration of 2.5 x 10−6 M.
Keywords: EF-Ts, EF-Tu, kinetics, protein synthesis
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arai K. I., Kawakita M., Kaziro Y. Studies on polypeptide elongation factors from Escherichia coli. II. Purification of factors Tu-guanosine diphosphate, Ts, and Tu-Ts, and crystallization of Tu-guanosine diphosphate and Tu-Ts. J Biol Chem. 1972 Nov 10;247(21):7029–7037. [PubMed] [Google Scholar]
- Bagnara A. S., Finch L. R. Quantitative extraction and estimation of intracellular nucleoside triphosphates of Escherichia coli. Anal Biochem. 1972 Jan;45(1):24–34. doi: 10.1016/0003-2697(72)90004-8. [DOI] [PubMed] [Google Scholar]
- Chau V., Romero G., Biltonen R. L. Kinetic studies on the interactions of Escherichia coli K12 elongation factor Tu with GDP and elongation factor Ts. J Biol Chem. 1981 Jun 10;256(11):5591–5596. [PubMed] [Google Scholar]
- Fasano O., Bruns W., Crechet J. B., Sander G., Parmeggiani A. Modification of elongation-factor-Tu . guanine-nucleotide interaction by kirromycin. A comparison with the effect of aminoacyl-tRNA and elongation factor Ts. Eur J Biochem. 1978 Sep 1;89(2):557–565. doi: 10.1111/j.1432-1033.1978.tb12560.x. [DOI] [PubMed] [Google Scholar]
- Holz U., Weller S., Wagner D. Typische Implantatbrúche--Eine klinische Analyse. Unfallheilkunde. 1981 Jan;84(1):20–25. [PubMed] [Google Scholar]
- Jelenc P. C., Kurland C. G. Nucleoside triphosphate regeneration decreases the frequency of translation errors. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3174–3178. doi: 10.1073/pnas.76.7.3174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jelenc P. C. Rapid purification of highly active ribosomes from Escherichia coli. Anal Biochem. 1980 Jul 1;105(2):369–374. doi: 10.1016/0003-2697(80)90472-8. [DOI] [PubMed] [Google Scholar]
- Kennell D., Riezman H. Transcription and translation initiation frequencies of the Escherichia coli lac operon. J Mol Biol. 1977 Jul;114(1):1–21. doi: 10.1016/0022-2836(77)90279-0. [DOI] [PubMed] [Google Scholar]
- Leberman R., Antonsson B., Giovanelli R., Guariguata R., Schumann R., Wittinghofer A. A simplified procedure for the isolation of bacterial polypeptide elongation factor EF-Tu. Anal Biochem. 1980 May 1;104(1):29–36. doi: 10.1016/0003-2697(80)90272-9. [DOI] [PubMed] [Google Scholar]
- Miller D. L., Weissbach H. Interactions between the elongation factors: the displacement of GPD from the TU-GDP complex by factor Ts. Biochem Biophys Res Commun. 1970 Mar 27;38(6):1016–1022. doi: 10.1016/0006-291x(70)90341-4. [DOI] [PubMed] [Google Scholar]
- Miller D. L., Weissbach H. Studies on the purification and properties of factor Tu from E. coli. Arch Biochem Biophys. 1970 Nov;141(1):26–37. doi: 10.1016/0003-9861(70)90102-5. [DOI] [PubMed] [Google Scholar]
- Wagner E. G., Kurland C. G. Escherichia coli elongation factor G blocks stringent factor. Biochemistry. 1980 Mar 18;19(6):1234–1240. doi: 10.1021/bi00547a030. [DOI] [PubMed] [Google Scholar]



