Abstract
Objectives
Our objective was to examine gender differences in clinical presentation, management and prognosis of atrial fibrillation (AF) in a contemporary cohort.
Methods
In 6412 patients, 39.7% women, of the PREvention oF thromboembolic events – European Registry in Atrial Fibrillation, we examined gender differences in symptoms, risk factors, therapies and 1-year incidence of adverse outcomes.
Results
Men with AF were on average younger than women (mean±SD: 70.1±10.7 vs 74.1±9.7 years, p<0.0001). Women more frequently had at least one AF-related symptom at least occasionally compared with men (95.4% in women, 89.8% in men, p<0.0001). Prescription of oral anticoagulation was similar, with an increase of non-vitamin K antagonist oral anticoagulants from 5.9% to 12.6% in women and from 6.2% to 12.6% in men, p<0.0001 for both.
Men were more frequently treated with electrical cardioversion and ablation (20.6% and 6.3%, respectively) than women (14.9% and 3.3%, respectively), p<0.0001. Women had 65% (OR: 0.35; 95% CI (0.22 to 0.56)) lower age-adjusted and country-adjusted odds of coronary revascularisation, 40% (OR: 0.60; (0.38 to 0.93)) lower odds of acute coronary syndrome and 20% (OR: 0.80; (0.68 to 0.96)) lower odds of heart failure at 1 year. There were no statistically significant gender differences in 1-year stroke/transient ischaemic attack/arterial thromboembolism and major bleeding events.
Conclusion
In a ‘real-world’ European AF registry, women were more symptomatic but less likely to receive invasive rhythm control therapy such as electrical cardioversion or ablation. Further study is needed to confirm that these differences do not disadvantage women with AF.
Keywords: Atrial fibrillation, gender differences, European registry
Introduction
Atrial fibrillation (AF) is one of the most frequent cardiovascular diseases and a common comorbidity in older adults in both men and women.1 Gender differences in AF risk factor distribution, comorbidities, clinical presentation and cardiovascular outcomes in individuals with AF have been discussed in the literature in different settings and with conflicting results.2 3 In prior studies, women with AF carried a higher risk of adverse events such as stroke.2 The higher risk in women is taken into account in current clinical risk algorithms such as the CHA2DS2–VASc (congestive heart failure, hypertension, age ≥75 years, diabetes mellitus, stroke, vascular disease, age 65–74 years, sex category) score.3 Additionally, women appear to have a lower bleeding risk than men.4 Less evidence is available on the impact of gender on the complex interaction between AF and heart failure5 and acute coronary syndromes,6 which have been increasingly recognised as serious sequelae of AF. In addition, gender-specific clinical predictors of maintenance of sinus rhythm or adequate rate control are sparsely investigated.
Some data suggest gender differences in treatment and response to interventions.4 5 Over the last years, many changes have been witnessed, including an increased prevalence of AF, the introduction of novel therapies and the release of new guidelines.7 In particular, non-vitamin K antagonist oral anticoagulants (NOACs) are increasingly used for stroke prevention in AF.6 Limited data exist on the impact of gender on prescription and continuation of NOACs since the implementation of new AF guidelines. Guidelines recommend that physicians should offer effective diagnostic tools and therapeutic management to women and men equally.7
The PREvention oF thromboembolic events – European Registry (PREFER) in AF is a prospective, observational cohort8 implemented to describe the management of patients with AF in Europe after the release of the 2010 European Society of Cardiology Guidelines for the Management of Atrial Fibrillation.9 In this study, we focus on gender differences in baseline risk factors, disease history and AF symptoms, together with therapeutic approaches and 1-year incidence of major outcomes. Our aim is to highlight potential treatment discrepancies in women and men that could impact the prognosis of patients with AF.
Methods
Study sample
Between January 2012 and 2013 the PREFER in AF registry enrolled 7243 patients aged 18 years and older with physician-verified AF across seven Western European countries (France, Germany, Austria, Switzerland, Italy, Spain and the UK).8 Eighty-nine per cent of the study participants were treated by cardiologists and recruited from both physician offices and hospitals. One-year follow-up data were obtained from 6412 patients by physician-administered questionnaire and medical chart information. The last follow-up was performed in January 2014. Information on individuals without valid follow-up information is provided in online supplementary table 1.
heartjnl-2016-310406supp001.pdf (219.1KB, pdf)
Clinical evaluations
AF had to be present on an ECG or in the readout of an implanted device (pacemaker/defibrillator) within the preceding 12 months. Demographic data, clinical variables, disease history and treatment were ascertained by the treating physician. Symptoms related to AF were assessed using the European Heart Rhythm Association (EHRA) score10 and included symptoms such as palpitations, fatigue, dizziness, dyspnoea, chest pain and anxiety. The scoring ranged from never, occasional (less than once per month), intermediate (once per month to almost daily) to frequent (at least daily). An individual’s EHRA score was assessed by the treating physician as the maximum score of any of the six individual symptom categories. We also assessed the EHRA score in individuals with new-onset AF, which was defined as AF diagnosed fewer than 90 days prior to enrolment. Lack of guideline compliance indicates lack of treatment with oral anticoagulant in the previous 12 months despite guideline indication without contraindication. Adequate heart rate control was defined as a heart rate between 60 and 100 bpm during the clinic visit. The risk of thromboembolic and bleeding events in patients with AF was assessed by calculating the CHA2DS2VASc score and HAS-BLED (hypertension, abnormal renal/liver function, stroke, bleeding history or predisposition, labile international normalised ratio, elderly, drugs/alcohol concomitantly) score.11
Statistical analyses
Data analysis was performed as a substudy of the PREFER in AF registry on the 6412 patients with follow-up information. Complete case analysis was performed, and missing data were assumed to be missing at random. The proportion of women and men lost to follow-up was compared by Fisher’s exact test. Variables are presented as number (percentage) or mean (±SD), as appropriate. Differences by gender across EHRA score categories for each EHRA symptom and across type of anticoagulation therapy were calculated using Freeman-Halton’s extension Fisher’s exact test. Gender differences in dichotomous baseline of characteristics and the 1-year incidence of four major medical sequelae of AF (ischaemic stroke/transient ischaemic attack (TIA)/arterial thromboembolic events, acute coronary syndrome (myocardial infarction and unstable angina pectoris), coronary revascularisation, heart failure and major bleeding events) were examined by multiple logistic regression adjusted for age and country.
One-year predictive models
An initial round of variable selection was performed, where highly collinear variables and variables with a high number of missing values and/or few events were removed. Subsequently, predictor variables for each logistic regression model were selected by stepwise regression (p=0.20 for selection and p<0.05 to remain in the model). The list of included predictor variables can be found in online supplementary table 2. Age, gender and country were forced into the regression models. For the outcome heart failure (chronic heart insufficiency and reduced left ventricular ejection fraction), patients with the condition at baseline were excluded from the analysis. Effect estimates for each model are presented separately for women and men. The predictive ability of the derived regressions models compared with the CHA2DS2–VASc and HAS-BLED scores for each outcome was assessed by calculating the area under the receiver operating characteristic curve (AUC), with overoptimism corrected by repeated split sample validation (logistic regression and then AUC computation in randomly split halves of the sample, repeated 100 times).
Analyses were conducted using SAS software V.9.4 (Cary, North Carolina, USA), with a two-tailed significance value of 0.05. Graphics were produced with R V.3.3.0 using the ggplot2 package.12 13
Results
Baseline patient characteristics
Forty per cent of study participants were women, who were on average 4 years older than men (74.1±9.7 years vs 70.1±10.7 years, respectively). The body mass index was comparable in both genders (28.2±4.7 SD in men vs 27.6±5.4 SD in women), and the systolic blood pressure was lower in men than in women (130.5±16.1 SD in men vs 132.7±17.4 SD in women). There were numerous differences in the burden of cardiovascular risk factors and comorbidities according to gender (table 1). Hyperthyroidism, valvular heart disease and antiarrhythmic drug intake were more prevalent in women than in men. Most other cardiovascular risk factors and prevalent disease were more often observed in men. Adequate heart control was comparable in women and men. There was no statistically significant gender difference in the proportion of individuals lost to follow-up, p=0.27.
Table 1.
Baseline characteristics of the PREFER in AF study participants by gender
| Variables | Women (n=2546) | Men (n=3866) | Women-to-men OR* | 95% CI |
| Risk factors | ||||
| Age, years (SD) | 74.1 (9.7) | 70.1 (10.7) | ||
| Body mass index, kg/m² (SD) | 27.6 (5.4) | 28.2 (4.7) | ||
| Systolic blood pressure, mm Hg (SD) | 132.7 (17.4) | 130.5 (16.1) | ||
| Ever smoking, n (%) | 452 (18.2) | 1958 (51.9) | 0.21 | (0.19 to 0.24) |
| Alcohol excess (=8 units/week), n (%) | 14 (0.6) | 149 (3.9) | 0.16 | (0.09 to 0.27) |
| Lack of guideline compliance in anticoagulant therapy, n (%) | 79 (3.1) | 83 (2.2) | 1.17 | (0.85 to 1.62) |
| EHRA score>2, n (%) | 1564 (62.1) | 1899 (49.6) | 1.68 | (1.51 to 1.87) |
| CHA2DS2−VASc ≥2†, n (%) | 2235 (95.2) | 2823 (78.9) | 5.55 | (4.26 to 7.22) |
| HAS-BLED ≥2, n (%) | 1483 (70.8) | 2072 (65.4) | 0.85 | (0.74 to 0.97) |
| Disease history | ||||
| Diabetes mellitus, n (%) | 529 (21.0) | 893 (23.3) | 0.82 | (0.73 to 0.93) |
| Dyslipidaemia, n (%) | 1035 (41.6) | 1745 (46.2) | 0.80 | (0.72 to 0.88) |
| Chronic renal insufficiency, n (%) | 321 (12.9) | 521 (13.8) | 0.73 | (0.63 to 0.85) |
| Chronic hepatic disease, n (%) | 53 (2.1) | 72 (1.9) | 1.00 | (0.70 to 1.45) |
| Hyperthyroidism, n (%) | 130 (5.2) | 137 (3.6) | 1.50 | (1.17 to 1.93) |
| Chronic obstructive pulmonary disease, n (%) | 252 (10.0) | 475 (12.4) | 0.68 | (0.57 to 0.80) |
| Major gastrointestinal/cerebrovascular/ other bleeding events, n (%) | 98 (3.9) | 168 (4.4) | 0.80 | (0.62 to 1.04) |
| Prevalent cardiovascular disease (CHD, peripheral arterial disease, myocardial infarction), n (%) |
467 (18.9) | 1194 (31.7) | 0.41 | (0.36 to 0.47) |
| Stent insertion, n (%) | 140 (5.6) | 527 (13.9) | 0.33 | (0.27 to 0.40) |
| Heart valve dysfunction, n (%) | 1106 (44.2) | 1375 (36.1) | 1.25 | (1.12 to 1.39) |
| Variables | Women (n=2546) | Men (n=3866) | Women-to-men OR* | 95% CI |
| Heart valve replacement, n (%) | 150 (6.0) | 201 (5.3) | 1.09 | (0.88 to 1.37) |
| Heart failure, n (%) | 653 (26.5) | 1150 (30.6) | 0.72 | (0.64 to 0.80) |
| Previous ischaemic stroke/TIA/other ischaemic thromboembolic event, n (%) | 406 (16.2) | 555 (14.5) | 1.04 | (0.90 to 1.20) |
| Sinus rhythm at baseline, n (%) | 823 (32.5) | 1134 (29.5) | 1.37 | (1.22 to 1.53) |
| Adequate heart rate control (60–100 bpm) at baseline, n (%) | 1077 (51.7) | 1605 (51.0) | 0.98 | (0.87 to 1.09) |
| Medication | ||||
| Antiplatelet agents, non-steroidal anti-inflammatory drugs,‡ n (%) | 572 (22.6) | 978 (25.5) | 0.82 | (0.73 to 0.93) |
| Antiarrhythmic drugs, n (%) | 1594 (62.6) | 2257 (58.4) | 1.31 | (1.18 to 1.46) |
Mean and SD and number and percentages are presented.
*Univariate ORs for gender were obtained by logistic regression adjusted for age and country.
†CHA2DS2–VASc score included the extra point for female gender.
‡Medication as used in the HAS-BLED score. Lack of guideline compliance indicates lack of treatment with oral anticoagulant in the previous 12 months despite guideline indication without contraindication.
AF, atrial fibrillation; CHA2DS2–VASc, congestive heart failure, hypertension, age ≥75 years, diabetes mellitus, stroke, vascular disease, age 65–74 years, sex category; CHD, coronary heart disease; EHRA, European Heart Rhythm Association; HAS-BLED, hypertension, abnormal renal/liver function, stroke, bleeding history or predisposition, labile international normalised ratio, elderly, drugs/alcohol concomitantly; PREFER, PREvention oF thromboembolic events - European Registry; TIA, transient ischaemic attack.
Atrial fibrillation symptoms
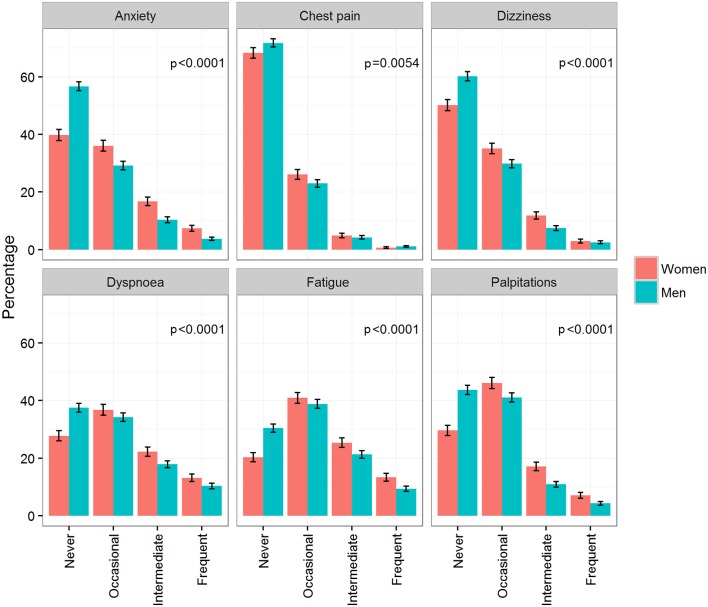
In men, 90% experienced symptoms, compared with 95% in women. Male study participants reported less severe symptoms and showed a lower frequency of symptoms compared with women (figure 1). In both genders, the most common symptoms were fatigue, dyspnoea and palpitations. At least occasional fatigue was reported by 70% of men and 80% of women. The least common symptom was chest pain (28% in men and 32% in women). The proportions of symptom distribution did not change markedly over the follow-up period (data not shown). In patients with new-onset AF, symptoms were also distributed similarly with women having consistently more frequent symptoms (see online supplementary table 3).
Figure 1.
Symptoms according to the European Heart Rhythm Association classification at baseline by gender. Percentages and 95% CI are provided. p Values are derived from Freeman-Halton’s extension of Fisher’s exact test.
Anticoagulation therapies
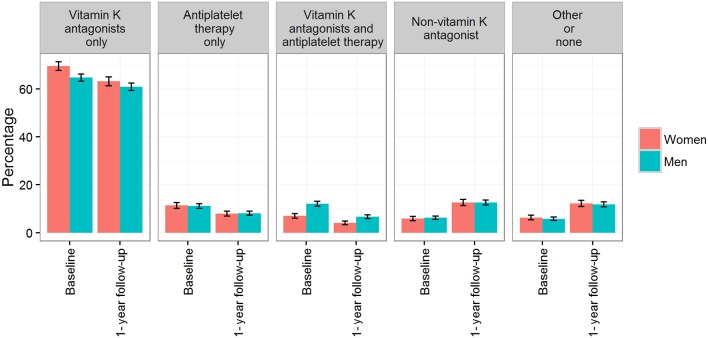
Anticoagulation therapy was prescribed in 94% of both women and men. The majority of patients (>60%) were prescribed vitamin K antagonists at both baseline and 1-year follow-up (figure 2). At both timepoints, more women than men were prescribed vitamin K antagonists only (baseline: 70% vs 65%; follow-up: 63% vs 61%), and conversely, more men than women were on a combined vitamin K antagonist and antiplatelet therapy regimen (baseline: 12% vs 7.0%; follow-up: 7% vs 4%). A twofold increase in the use of NOACs was demonstrated in both women and men, from about 6% at baseline to 13% at the 1-year follow-up.
Figure 2.
Prevalence of anticoagulation therapy in patients with atrial fibrillation at baseline and after 1-year follow-up by gender. Percentages and 95% CI are provided. p Values are derived from Freeman-Halton’s extension of Fisher’s exact test; baseline, p<0.0001; follow-up, p=0.001.
Therapies to restore sinus rhythm
Pharmacological and electrical cardioversion attempts were the most common interventions to restore sinus rhythm (table 2). At baseline, women were more likely to have undergone a pharmacological cardioversion attempt compared with men (OR 1.24; 95% CI 1.08 to 1.41). Men had more frequently received electrical cardioversion, catheter ablation (pulmonary vein isolation) or surgical ablation therapy compared with women. The point estimates for these ORs were similar at 1-year follow-up, although CIs were wider due to the smaller number of procedures. Treatment varied by country, but the proportion of women and men receiving a specific therapy was comparable across European countries (data not shown).
Table 2.
ORs for gender for baseline prevalence and 1-year incidence for treatments to restore sinus rhythm
| Treatment | Baseline | One-Year follow-up | ||||||||||
| Women | Men | Women vs men | Women | Men | Women vs men | |||||||
| N | % | N | % | OR | 95% CI | N | % | N | % | OR | 95% CI | |
| Pharmacological cardioversion | 511 | 20.2 | 716 | 18.6 | 1.24 | (1.08 to 1.41) | 132 | 5.4 | 180 | 4.8 | 1.25 | (0.99 to 1.59) |
| Electrical cardioversion | 379 | 14.9 | 795 | 20.6 | 0.78 | (0.68 to 0.90) | 144 | 5.9 | 329 | 8.8 | 0.82 | (0.67 to 1.01) |
| Ablation (pulmonary vein isolation) | 84 | 3.3 | 243 | 6.3 | 0.72 | (0.56 to 0.94) | 68 | 2.8 | 170 | 4.5 | 0.88 | (0.66 to 1.19) |
| Surgical therapy for atrial fibrillation | 10 | 0.4 | 36 | 0.9 | 0.45 | (0.22 to 0.93) | 6 | 0.2 | 19 | 0.5 | 0.52 | (0.20 to 1.31) |
ORs are age and country adjusted.
Incidence of major outcomes
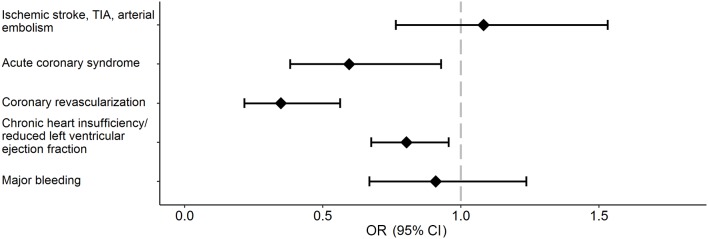
There was no evidence that men and women differed in the two outcomes: stroke/TIA/arterial thromboembolic events and major bleeding events (OR 1.08, 95% CI (0.76 to 1.53) and OR 0.91, (0.67 to 1.24), respectively) (figure 3). Women had lower age-adjusted and country-adjusted odds of coronary revascularisation (OR 0.35, 95% CI (0.22 to 0.56)), lower odds of acute coronary syndrome (OR 0.60, (0.38 to 0.93)), and lower odds of heart failure (OR 0.80, (0.68 to 0.96)).
Figure 3.
Age-adjusted and country-adjusted women-to-men ORs for 1-year major outcomes. An OR below 1 indicates a lower risk of developing the outcome in women. TIA, transient ischaemic attack.
One-year predictive models of major outcomes
The stepwise regression results for each outcome are presented in online supplementary tables 4–8. Overall, there was no evidence that the strength of association between selected predictors and outcomes differed markedly between genders.
The predictive ability (AUC index) of our models performed similar to the established CHA2DS2–VASc and HAS-BLED scores for stroke/TIA/arterial thromboembolic events and major bleeding (online supplementary table 9) in both genders. Overall, the predictive abilities of all models were moderate (AUC range: 0.58–0.69).
Discussion
Main Findings
In a prospective European registry of patients with AF, women presented with different clinical baseline characteristics including disease history, AF symptoms, therapies to restore sinus rhythm and 1-year incidence of major cardiovascular outcomes. Women were older on average and showed a lower risk of acute coronary syndrome, coronary revascularisation events and development of heart failure compared with men over the 1-year follow-up. There was no evidence of gender differences for arterial thromboembolic events or bleeding. We report possible differences between gender in the strength of association of clinical predictors and outcomes, suggesting that a gender-specific risk assessment and intervention strategies could be of advantage.
Atrial fibrillation symptoms
Our findings that women with AF reported more symptoms than men are in line with recent reports that fairly consistently described a higher subjective symptom burden in women.14–16 Truly asymptomatic AF, that is, no symptom history or current symptoms, was associated with the male gender in a prior observational registry study.17 Whether differences in illness perception and coping strategies or different major drivers causing AF underlie the lower symptom prevalence in men warrants further study.
Therapies to restore sinus rhythm
Despite more AF symptoms in women, men received more rhythm control interventions at baseline and also during the 1-year follow-up; for example, both the prevalence and 1-year incidence of electrical cardioversion, pulmonary vein isolation or surgical therapy for AF were lower in women, whereas antiarrhythmic drug use was slightly higher. Recent publications show a similar pattern.16 The reason why women with AF were less likely to receive non-pharmacological interventions to maintain sinus rhythm remains unclear. Besides symptom burden, clinical factors such as the presence of heart disease and other comorbidities and patient preferences enter the decision process for catheter-based ablation therapy.18
In our cohort, women presented more often with valvular dysfunction but otherwise presented fewer risk factors, except for age, which may explain the observed more conservative treatment pattern. Data on other cardiovascular interventions suggest that women undergo a less aggressive treatment than men. In non-ST elevation myocardial infarction, women were treated invasively less frequently despite higher risk of adverse events19 or received cardiac devices (eg, cardiac resynchronisation therapy), less often than men.20 Current evidence suggests that AF treatments are as effective in women as in men,21 supported by the current guideline recommendations that state that catheter or surgical ablation techniques should be regarded as equally effective in women and men.7 The success rates of sinus rhythm restoration appear to be similar in both genders.22 A possible explanation for the treatment differences may be patient choice. Women tend to refuse multiple ablation procedures even after initial success, which results in a lower overall maintenance of sinus rhythm due to fewer repeat procedures compared with men.22 To which extent physician preferences and recommendations play a role in treatment differences remains to be examined.
Anticoagulation therapies
As recommended in the guidelines for AF treatment, anticoagulation therapy was prescribed in the majority of patients, without any seeming difference between women or men. Similar results were reported in the Global Anticoagulant Registry in the Field–AF registry in 2014.17 Thus, positive changes can be demonstrated compared with earlier studies, which showed that women were less likely to receive oral anticoagulants compared with men.23 The uptake of NOACs during the follow-up was also similar in both genders, with a doubling from about 6% at baseline to 12% after 1-year follow-up. Considering evidence from a secondary analysis of NOAC trials24 25 and a recent meta-analysis,4 women appear to benefit from NOAC similarly to men. They may even have a higher net clinical benefit due to the high residual stroke risk in women treated with warfarin but lower stroke rates under NOAC therapy. Published data on the incidence of bleeding events are mostly from the warfarin era and have remained controversial regarding gender differences.26 Whether the absence of gender differences in bleeding risk in our patient cohort may be explained by the broader application of NOACs during the 1-year follow-up or relate to a genuine lack of gender differences should be studied in the future.
Incidence of major outcomes
A higher risk of stroke and other arterial thromboembolic events in women has been reported, most recently in a large meta-analysis, which however showed substantial heterogeneity among studies.2 Women in our study had 8% higher age-adjusted and country-adjusted odds of stroke/TIA/arterial thromboembolic events compared with men—an effect size much smaller than suggested by the meta-analysis. Due to the relatively short follow-up period of this study, the event numbers were small, and the uncertainty around our estimated effects were relatively large. At baseline, women accumulated a higher CHA2DS2–VASc score on average due to the extra point for female gender, which did not directly translate in a higher stroke risk during follow-up. Thus, the general addition of one point for gender, regardless of age, needs to be appraised carefully in practice. Women with a CHA2DS2–VASc score of 1 are usually at a very low risk of stroke.27
Additionally, our results are derived from a cohort with a high proportion of adequate anticoagulation treatment. Underuse of anticoagulation in women despite relevant stroke risk has been suggested as another explanation for gender disparities in arterial thromboembolic outcomes.15 26 The increased prescription of NOACs, with a possible more effective stroke risk reduction in women, may have contributed to smaller gender differences in stroke incidence.
AF has been recognised as a risk factor for myocardial infarction.6 28 Whether gender differences exist has remained controversial. Whereas earlier reports did not show differences in myocardial infarction incidence,26 29 a recent population-based study observed a stronger association between AF and myocardial infarction in women.30 Our current data, which included the whole spectrum of acute coronary syndromes, indicate that women were at a lower short-term risk than men. Similarly, the outcome of coronary revascularisation was more often seen in men than in women.
Heart failure is an important complication in individuals with AF. In the current analyses, we observed a higher incidence of heart failure in men over the 1-year follow-up period. We have previously described possible gender differences in the susceptibility to heart failure in AF when examining subtypes of heart failure, that is, heart failure with preserved and reduced ejection fraction.5 Men had a higher incidence of heart failure with reduced ejection fraction, and women tended to have a higher risk of developing heart failure with preserved ejection fraction.
Clinical predictors of major outcomes revealed similar predictive ability in both genders, with overall moderate discriminatory ability. We observed possible differences in the strength of association in men compared with women. If validated, such differences may help estimate the risk factor burden and target AF treatment gender specifically. Despite being recently used to assess risk across diverse outcomes in patients with AF, the CHA2DS2–VASc and HAS-BLED scores performed similarly to our predictive models.
Limitations
As common in registries, the accuracy and completeness of data may be limited, despite a high degree of standardisation and training in data acquisition across enrolling centres, which may lead to bias. The results were obtained in individuals that sought medical attention due to the rhythm disorder and may thus not be generalisable to all patients with AF. Over the 1-year time span of the registry, the number of serious medical outcomes was also limited compared with the number of predictor variables examined. The PREFER in AF registry enrolled patients in seven Western European countries. Therefore, patient characteristics, AF management and incidence of adverse events may not be generalisable to all European countries and other regions of the world. Such possible differences have been shown within the registry and are likely to be more pronounced for a comparison with other countries.8 Whereas differences in treatment choice were observed across countries in our cohort, the relative proportions of women and men undergoing a specific treatment were comparable.
In summary, we demonstrated gender differences in the clinical presentation, treatment and major clinical outcomes in a large, contemporary cohort, which call for gender-individualised research and care. Guideline-recommended anticoagulation medication for AF was prescribed in both men and women, with a good uptake of NOACs in both genders. Women were less likely to receive non-pharmacological therapies to restore sinus rhythm despite being more symptomatic. The observed gender disparities require detailed pathophysiological and clinical workup and may provide the opportunity to develop gender-specific preventive and therapeutic strategies for a disease that is reaching epidemic proportions worldwide.
Key messages.
What is already known on this subject?
There are significant gender differences in the epidemiology of atrial fibrillation. Data on clinical presentation, atrial fibrillation- specific interventions, and treatment and outcomes in a contemporary European cohort are limited.
What might this study add?
Women were more symptomatic (95.4% vs 89.8% in men). Prescription of oral anticoagulation therapy was comparable. Men were more aggressively treated (electrical cardioversion 20.6% vs 14.9%; ablation 6.3% vs 3.3%). Women had 40% lower odds of developing acute coronary syndrome and 20% lower odds of heart failure.
How might this impact on clinical practice?
Gender-specific treatment of atrial fibrillation and prevention of adverse events need to be explored to improve atrial fibrillation care and outcomes.
Acknowledgments
This analysis of the PREFER in AF registry was initiated by the Thrombosis Exchange Meeting in AF, TEAM in AF, funded and sponsored by Daiichi Sankyo Europe. We thank all the participants for their time and efforts with the establishment of the registry.
Footnotes
Contributors: RBS designed the analysis, interpreted the data and wrote the manuscript. LP designed the analysis, performed the statistical analysis and critically reviewed and revised the manuscript. FMO interpreted the data, wrote parts of the manuscript and critically reviewed the manuscript. ML designed the study, obtained the funding and critically reviewed the manuscript. NR wrote parts of the manuscript, interpreted the data and critically reviewed the manuscript. SB critically reviewed the manuscript. HD critically reviewed the manuscript. DK interpreted the data and critically revised the manuscript. RDC designed the study, obtained the funding and critically reviewed the manuscript. PK interpreted the data, designed the study, obtained the funding and critically reviewed the manuscript.
Funding: This project has received funding from the European Research Council under the European Union’s Horizon 2020 Research and Innovation Programme (grant agreement number 648131). This work was performed in the context of the Junior Research Alliance symAtrial project funded by the German Ministry of Research and Education (BMBF 01ZX1408A) e:Med – Systems Medicine program (RBS). RBS is funded by Deutsche Forschungsgemeinschaft (German Research Foundation) Emmy Noether Program SCHN 1149/3–1 (RBS). The PREFER in AF registry has been funded by Daiichi Sankyo Europe.
Competing interests: The PREFER in AF study sponsor via a contract research organisation (SSS International Clinical Research GmbH, Munich, Germany) was Daiichi Sankyo Europe GmbH, Munich. The study has an independent scientific steering committee. RDC reports that his institution received research grant support from Boehringer-Ingelheim, Bayer, Bristol-Myers Squibb/Pfizer and Roche and honoraria for lectures and/or consulting from Boehringer-Ingelheim, Bayer and Bristol-Myers Squibb/Pfizer, Daiichi Sankyo, Lilly, AstraZeneca, Merck and Novartis. PK receives further research support from the European Union (grant agreement number 633193 (CATCH ME)), British Heart Foundation (FS/13/43/30324), Medical Research Council (UK), Leducq Foundation, German Centre for Heart Research and several drug and device companies active in atrial fibrillation and has received honoraria from several such companies. He is listed as an inventor on two pending patents (WO 2015140571 and WO 2016012783) filed by the University of Birmingham. DK reports grants from Menarini outside the submitted work but during the conduct of the study and professional development support from Daiichi Sankyo.
Ethics approval: Approvals were obtained from leading and local ethics committees as required by national regulations in Austria, Germany, Switzerland, Italy, Spain and the UK before the start of enrolment at the sites. For France, no specific approval process was applicable for non-interventional studies.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Chugh SS, Havmoeller R, Narayanan K, et al. . Worldwide epidemiology of atrial fibrillation: a Global Burden of Disease 2010 Study. Circulation 2014;129:837–47. 10.1161/CIRCULATIONAHA.113.005119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Emdin CA, Wong CX, Hsiao AJ, et al. . Atrial fibrillation as risk factor for cardiovascular disease and death in women compared with men: systematic review and meta-analysis of cohort studies. BMJ 2016;532:h7013. 10.1136/bmj.h7013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lip GY, Nieuwlaat R, Pisters R, et al. . Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the euro heart survey on atrial fibrillation. Chest 2010;137:263–72. 10.1378/chest.09-1584 [DOI] [PubMed] [Google Scholar]
- 4. Pancholy SB, Sharma PS, Pancholy DS, et al. . Meta-analysis of gender differences in residual stroke risk and major bleeding in patients with nonvalvular atrial fibrillation treated with oral anticoagulants. Am J Cardiol 2014;113:485–90. 10.1016/j.amjcard.2013.10.035 [DOI] [PubMed] [Google Scholar]
- 5. Schnabel RB, Rienstra M, Sullivan LM, et al. . Risk assessment for incident heart failure in individuals with atrial fibrillation. Eur J Heart Fail 2013;15:843–9. 10.1093/eurjhf/hft041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Soliman EZ, Lopez F, O'Neal WT, et al. . Atrial Fibrillation and risk of ST-segment-elevation versus non-ST-segment-slevation myocardial infarction: the Atherosclerosis risk in communities (ARIC) study. Circulation 2015;131:1843–50. 10.1161/CIRCULATIONAHA.114.014145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kirchhof P, Benussi S, Kotecha D, et al. . ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS: The task force for the management of atrial fibrillation of the European Society of Cardiology (ESC)Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESCEndorsed by the European Stroke Organisation (ESO). Eur Heart J 2016;50:e1–e88. [DOI] [PubMed] [Google Scholar]
- 8. Kirchhof P, Ammentorp B, Darius H, et al. . Management of atrial fibrillation in seven European countries after the publication of the 2010 ESC Guidelines on atrial fibrillation: primary results of the PREvention oF thromboemolic events--European Registry in Atrial Fibrillation (PREFER in AF). Europace 2014;16:6–14. 10.1093/europace/eut263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Camm AJ, Kirchhof P, Lip GY, et al. . European Heart Rhythm Association. European Association for Cardio-Thoracic Surgery. Guidelines for the management of atrial fibrillation: the task force for the management of atrial fibrillation of the European Society of Cardiology (ESC). Eur Heart J 2010;31:2369–429. 10.1093/eurheartj/ehq278 [DOI] [PubMed] [Google Scholar]
- 10. Kirchhof P, Auricchio A, Bax J, et al. . Outcome parameters for trials in atrial fibrillation: recommendations from a consensus conference organized by the German Atrial Fibrillation Competence NETwork and the European Heart Rhythm Association. Europace 2007;9:1006–23. 10.1093/europace/eum191 [DOI] [PubMed] [Google Scholar]
- 11. Lip GY. Implications of the CHA(2)DS(2)-VASc and HAS-BLED Scores for thromboprophylaxis in atrial fibrillation. Am J Med 2011;124:111–4. 10.1016/j.amjmed.2010.05.007 [DOI] [PubMed] [Google Scholar]
- 12. R Core Team. R Foundation for Statistical Computing R: A language and environment for statistical computing. . Vienna, Austria: 2016. [Google Scholar]
- 13. Wickham H. ggplot2. Elegant Graphics for Data Analysis. New York: BMJ Publishing Group, 2009. [Google Scholar]
- 14. Potpara TS, Marinkovic JM, Polovina MM, et al. . Gender-related differences in presentation, treatment and long-term outcome in patients with first-diagnosed atrial fibrillation and structurally normal heart: the Belgrade atrial fibrillation study. Int J Cardiol 2012;161:39–44. 10.1016/j.ijcard.2011.04.022 [DOI] [PubMed] [Google Scholar]
- 15. Dagres N, Nieuwlaat R, Vardas PE, et al. . Gender-related differences in presentation, treatment, and outcome of patients with atrial fibrillation in Europe: a report from the Euro Heart Survey on Atrial Fibrillation. J Am Coll Cardiol 2007;49:572–7. 10.1016/j.jacc.2006.10.047 [DOI] [PubMed] [Google Scholar]
- 16. Lip GY, Laroche C, Boriani G, et al. . Sex-related differences in presentation, treatment, and outcome of patients with atrial fibrillation in Europe: a report from the Euro Observational Research Programme Pilot survey on Atrial Fibrillation. Europace 2015;17:24–31. 10.1093/europace/euu155 [DOI] [PubMed] [Google Scholar]
- 17. Boriani G, Laroche C, Diemberger I, et al. . Asymptomatic atrial fibrillation: clinical correlates, management, and outcomes in the EORP-AF Pilot General Registry. Am J Med 2015;128:509–18. 10.1016/j.amjmed.2014.11.026 [DOI] [PubMed] [Google Scholar]
- 18. Calkins H, Kuck KH, Cappato R, et al. . HRS/EHRA/ECAS expert consensus statement on catheter and surgical ablation of atrial fibrillation: recommendations for patient selection, procedural techniques, patient management and follow-up, definitions, endpoints, and research trial design. Heart Rhythm 2012;9:632–96.22386883 [Google Scholar]
- 19. Blomkalns AL, Chen AY, Hochman JS, et al. . CRUSADE Investigators. CRUSADE Investigators. Gender disparities in the diagnosis and treatment of non-ST-segment elevation acute coronary syndromes: large-scale observations from the CRUSADE (Can Rapid Risk Stratification of Unstable Angina Patients Suppress Adverse Outcomes With Early Implementation). J Am Coll Cardiol 2005;45:832–7. 10.1016/j.jacc.2004.11.055 [DOI] [PubMed] [Google Scholar]
- 20. Sridhar AR, Yarlagadda V, Parasa S, et al. . Cardiac Resynchronization Therapy: US Trends and Disparities in Utilization and Outcomes. Circ Arrhythm Electrophysiol 2016;9:103 13 00 00. 10.1161/CIRCEP.115.003108 [DOI] [PubMed] [Google Scholar]
- 21. Forleo GB, Tondo C, De Luca L, et al. . Gender-related differences in catheter ablation of atrial fibrillation. Europace 2007;9:613–20. 10.1093/europace/eum144 [DOI] [PubMed] [Google Scholar]
- 22. Winkle RA, Mead RH, Engel G, et al. . Long-term results of atrial fibrillation ablation: the importance of all initial ablation failures undergoing a repeat ablation. Am Heart J 2011;162:193–200. 10.1016/j.ahj.2011.04.013 [DOI] [PubMed] [Google Scholar]
- 23. DeWilde S, Carey IM, Emmas C, et al. . Trends in the prevalence of diagnosed atrial fibrillation, its treatment with anticoagulation and predictors of such treatment in UK primary care. Heart 2006;92:1064–70. 10.1136/hrt.2005.069492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Vinereanu D, Stevens SR, Alexander JH, et al. . Clinical outcomes in patients with atrial fibrillation according to sex during anticoagulation with apixaban or warfarin: a secondary analysis of a randomized controlled trial. Eur Heart J 2015;36:3268–75. 10.1093/eurheartj/ehv447 [DOI] [PubMed] [Google Scholar]
- 25. Avgil Tsadok M, Jackevicius CA, Rahme E, et al. . Sex differences in dabigatran use, safety, and effectiveness in a population-based cohort of patients with atrial fibrillation. Circ Cardiovasc Qual Outcomes 2015;8:593–9. 10.1161/CIRCOUTCOMES.114.001398 [DOI] [PubMed] [Google Scholar]
- 26. Humphries KH, Kerr CR, Connolly SJ, et al. . New-onset atrial fibrillation: sex differences in presentation, treatment, and outcome. Circulation 2001;103:2365–70. 10.1161/01.CIR.103.19.2365 [DOI] [PubMed] [Google Scholar]
- 27. Friberg L, Skeppholm M, Terént A. Benefit of anticoagulation unlikely in patients with atrial fibrillation and a CHA2DS2-VASc score of 1. J Am Coll Cardiol 2015;65:225–32. 10.1016/j.jacc.2014.10.052 [DOI] [PubMed] [Google Scholar]
- 28. Soliman EZ, Safford MM, Muntner P, et al. . Atrial fibrillation and the risk of myocardial infarction. JAMA Intern Med 2014;174:107–14. 10.1001/jamainternmed.2013.11912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chao TF, Huang YC, Liu CJ, et al. . Acute myocardial infarction in patients with atrial fibrillation with a CHA2DS2-VASc score of 0 or 1: a nationwide cohort study. Heart Rhythm 2014;11:1941–7. 10.1016/j.hrthm.2014.08.003 [DOI] [PubMed] [Google Scholar]
- 30. Soliman EZ, Safford MM, Muntner P, et al. . Atrial fibrillation and the risk of myocardial infarction. JAMA Intern Med 2014;174:107–14. 10.1001/jamainternmed.2013.11912 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
heartjnl-2016-310406supp001.pdf (219.1KB, pdf)