Abstract
Eighty two patients of leukaemia consisting of 25 cases of acute lymphocytic leukaemia, 38 cases of acute myeloid leukaemia, 14 cases of chronic myeloid leukaemia and 5 cases of chronic lymphocytic leukaemia were evaluated for central nervous system (CNS) involvement. Speech disorders, cranial nerve palsies, encephalopathy, ataxia, intracranial haemorrhage, peripheral neuropathy and spinal cord involvement were the main neurological findings detected in 23 (28.1%) cases. All except one were subjected to autopsy after death. Leukaemic infiltrations (36.6%) and intracranial haemorrhage (26.8%) were the prominent CNS autopsy findings. In addition, demyelination with astrocytosis (9.7%) and gliosis (2.4%) were seen. In all, 45 (54.9%) of the patients showed CNS involvement at autopsy. Thus a large number of CNS lesions were missed clinically and detected only on autopsy.
KEY WORDS: Intracranial haemorrhage, Leukemia, Metastasis
Introduction
Leukaemic involvement of the central nervous system can increase morbidity and mortality in all types of leukaemia. Complications like intracranial haemorrhage which may be clinically evident may hasten the death of a leukaemic patient even though he is well controlled with chemotherapy. Many other affections of the CNS such as leukaemic infiltrations, nodules, demyelination or gliosis may be clinically silent and revealed only on autopsy. Recognition of these by modern non-invasive techniques and their subsequent treatment may lead to a more comfortable and prolonged life in many patients with leukaemia.
Material and Methods
All patients with leukaemia admitted and treated at Command Hospital, Southern Command, Pune during a 11 year period (1983–1993) were included in this study. The diagnosis and morphological classification of leukaemia were done as per the French-American-British (FAB) classification [1]. Each case was examined clinically with detailed records of history, physical and systemic examination including evaluations of central nervous system. Haematological investigations including peripheral blood and bone marrow smear examinations were done repeatedly with Leishman's stain, apart from the cytochemical stains periodic acid schiff (PAS), myeloperoxidase alkaline phosphatase and chloracetate esterase [2] to establish the morphological diagnosis and classification. Patients with clinically evident neurological signs and symptoms were subjected to electroencephalogram and computed tomography (CT) scan, whenever the condition of the patients permitted these investigations. All patients were treated with the standard regime for acute myeloid leukaemia (AML), acute lymphatic leukaemia (ALL), chronic myeloid leukaemia (CML) or chronic lymphatic leukaemia (CLL) [3]. Patients who died were subjected to postmortem examination for detection of central nervous system lesions. Gross microscopic findings were noted. For light microscopy, a minimum of seven blocks were made, one each from the frontal lobe, parietotemporal lobe, internal capsule, midbrain, medulla and cerebellum. Haematoxylin and eosin, luxol fast blue, PTAH, and Bielchowskky's silver stain were employed wherever required for histopathological studies [4].
Observations
The clinical and haematological evaluation resulted in a morphological diagnosis of ALL in 25 [30.5%) patients, AML in 38 (46.3%) patients, CML in 14 (17.1%) patients and CLL in 5 (6.1%) patients. Neurological complications were evident clinically in 23 (28.1%) patients. These included dysarthric speech (1.2%), cranial nerve palsies (7.3%), encephalopathy (6.1%), intracranial haemorrhage (10.9%) and spinal cord involvement (2.4%). The last one included sensory loss below T-10 in one patient and cauda equina syndrome in another. The cranial nerves affected were both VI nerves in 2 cases and one case each of unilateral III, VI, XI and bilateral VII nerve involvement.
Postmortem examination was done in all patients except in one case of ALL. As many as 45 patients (54.9%) were found to have neurological involvement. These 45 patients included 23 in whom CNS involvement was clinically overt. Of the 45, 38 (84.4%) were males and 7 (15.5%) were females. Their ages ranged between 12 years to 59 years with a mean age of 33.6 years. Majority of the patients belonged to the 3rd and 4th decades of life (Table 1). Haematological diagnoses in these autopsied patients were ALL in 15 patients, CLL in a solitary case, AML in 24 patients and CML in 5 patients. Postmortem findings are detailed in Table 2. Infiltrative lesions were mainly seen in ALL and AML, 25 patients out of the total of 30 belonging to these acute leukaemias. Meningeal infiltration (Fig 1) was seen in all 30 patients, whereas perivascular infiltration (Fig 2) and leukaemic nodules were seen in 17 (ALL-11, CLL-2, AML-3, CML-1) and 12 (ALL-4, CLL-1, AML-5, CML-2) patients respectively. Clinically 9 (10.9%) patients had evidence of raised intracranial tension with convulsion and coma suggesting intracranial haemorrhage. Autopsy revealed haemorrhage in 22 patients – 16 having intracranial haemorrhage and the rest 6 subarachnoid haemorrhage. Fifteen of these 22 patients suffered from AML. Infiltrative lesions were common where there was an increase of leukocyte and platelet counts whereas haemorrhage was detected more often in patients with diminished platelet counts. Leukocyte count was over 1,00,000 per cumm in 20 cases out of 30 with infiltrations. Nineteen patients out of 22 with haemorrhage had platelet counts below 10,000 per cumm.
TABLE 1.
Age distribution – leukaemia with autopsy proved CNS involvement
| Age group in years | No. of cases with CNS involvement on autopsy | ||||
|---|---|---|---|---|---|
| ALL (25) | CLL (5) | AML (38) | CML (14) | Total (82) | |
| 11 – 20 | 2 (5) | — | — | — | 2 (5) |
| 21 – 30 | 10 (13) | — | 17 (23) | 0 (1) | 27 (37) |
| 31 – 40 | 3 (7) | 0 (1) | 6 (12) | 4 (9) | 13 (29) |
| 41 – 50 | — | 1 (3) | 1 (3) | 1 (4) | 3 (10) |
| 51 – 60 | — | 0 (1) | — | — | 0 (1) |
| Total | 15 (25) | 1 (5) | 24 (38) | 5 (14) | 45 (82) |
Figures in parentheses indicate total number of cases in that group.
TABLE 2.
Neurological lesions detected on autopsy
| Sl No. | Neurological lesions |
Types of Leukaemia | Total | |||
|---|---|---|---|---|---|---|
| ALL (25) | CLL (5) | AML (38) | CML (14) | |||
| 1. | Infiltrative lesions | 13 | 01 | 12 | 04 | 30 |
| 2. | Intracerebral haemorrhage | 03 | — | 11 | 02 | 16 |
| 3. | Subarachnoid haemorrhage | 02 | — | 04 | — | 06 |
| 4. | Demyelination with astrocytosis | 03 | — | 04 | 01 | 08 |
| 5. | Gliosis | 01 | — | 01 | — | 02 |
| Grand total | 15 | 01 | 24 | 05 | 45 | |
Fig. 1.
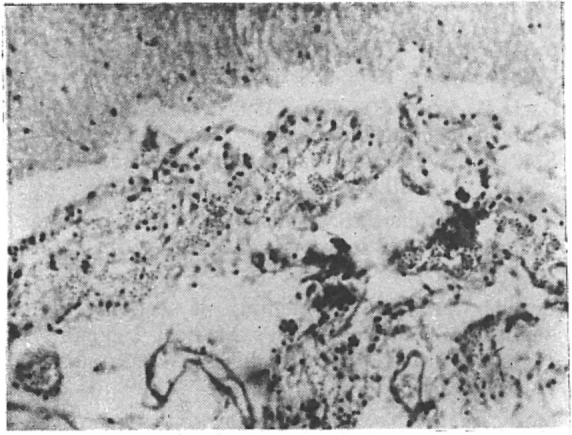
Meningeal infiltration with leukemic Cells.
Fig. 2.
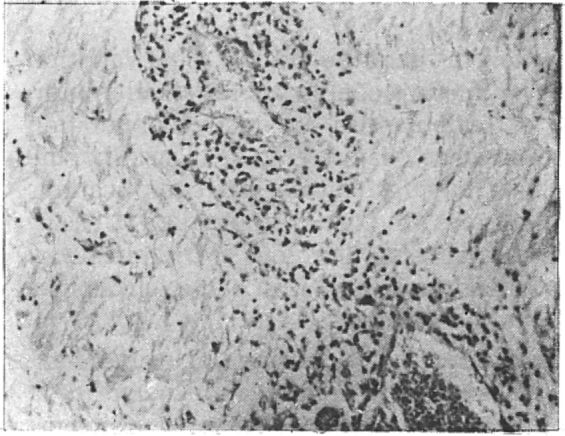
Perivascular infiltration with leukemic cells.
The remaining 36 (43.9%) patients who did not show any postmortem evidence of neurological complications, had no clinical neurological abnormality before death.
Discussion
Leukaemias are malignancies of haemopoetic system with varying clinical and haematological manifestations. High and rapidly rising concentrations of circulating blast cells in acute leukaemias may lead to infiltration in arteriolar endothelium resulting in leukoblast thrombi (leukostasis) and secondary haemorrhage [5]. Bleeding is seen in almost all systems of the body including the central nervous system. Sudden severe haemorrhage may also occur due to disseminated intravascular coagulation, especially in cases with acute promyelocytic leukaemia of M3 variety [6]. Thrombocytopenia secondary to excessive leukocytosis and sometimes following chemotherapy has also been recognised as one of the main causes of bleeding in leukaemias [7].
Leukaemic infiltrations are found almost all over the central nervous system including the meninges [8]. This complication is seen more often in children – indeed the so called meningeal leukaemia stands for infiltrative lesions in all layers of the meninges and can manifest with cranial nerve palsies and raised intracranial tension [9]. Leukaemic cell proliferation in the pia-arachnoid layer may block cerebrospinal fluid circulation and produce hydrocephalus in children [10].
In this study, neurological complications were detected clinically and on autopsy in 28.1% and 54.9% of cases respectively. This emphasizes the fact that a significant number of intracranial lesions do not manifest during life. The postmortem incidence of central nervous system complications seen in 45 out of 82 (54.9%) patients in this series is high in comparison to the range from 19.35% to 49% reported in literature [11, 12, 13]. This variation may be due to a comparatively smaller number of patients and a selective population studied by us. Majority (39 of 45 cases) of the patients having neurological complications had either AML (24 cases) or ALL (15 cases) as the diagnosis. Neurological complications are indeed mostly seen in acute leukaemias as reported by others [14, 15].
Infiltrative lesion was the single most common neurological complication noted in 30 out of 45 cases in our series. Neurological infiltrative lesions in leukaemia are reported to be more common in children [3, 9]. We have studied only 5 patients below the age of 20 years, 2 of them showing leukaemic infiltrations in the central nervous system. We had mainly treated adult patients hence the increased incidence of leukaemic infiltrations in these patients in our series should not be given significance. Majority of the patients with leukaemic infiltrations had high leukocyte count (usually above 1,00,000 per cumm) as has been reported by others [11]. Haemorrhage was the next common complication seen in 22 out of 45 patients. Most of these 22 had thrombocytopenia and not high leukocyte count. Thrombocytopenia and leukostasis have been the main causes of bleeding in the central nervous system in leukaemic patients [5, 6, 7].
The present series has shown that many neurological complications can be missed during life and can be detected only on autopsy. Efforts should be made to anticipate these complications and either prevent these or control their progress by various therapeutic measures including intrathecal methotrexate and craniospinal irradiation.
REFERENCES
- 1.Winstein JH, Clarkson B. The acute and chronic leukaemias. In: Wijngarden JB, Smith LA, editors. Textbook of Medicine, 18th Edn. WB Saunders; Philadelphia: 1988. pp. 988–1007. [Google Scholar]
- 2.Dacie JV, Lewis SM. Blood cell cytochemistry and supplementary techniques. In: Dacie JV, Lewis SM, editors. Practical Haematology, 6th ed. Churchill Livingstone; Edinburgh: 1984. pp. 84–116. [Google Scholar]
- 3.Evans AE, Gilbert ES, Zandastra R. The increasing incidence of CNS leukaemia in childhood. Cancer. 1970;26:404–409. doi: 10.1002/1097-0142(197008)26:2<404::aid-cncr2820260222>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 4.Culling CFA. Routine staining of sections. In: Raphael SS, editor. Lynch's Medical Laboratory Technology, 3rd ed. WB Saunders; Philadelphia: 1976. pp. 916–933. [Google Scholar]
- 5.Mckee LC, Jr, Collins RD. Intravascular leukocyte thrombi and aggregates as a cause of morbidity and mortality in leukaemia. Medicine. 1974;53:463–469. doi: 10.1097/00005792-197411000-00006. [DOI] [PubMed] [Google Scholar]
- 6.McKenna RW, Parkin J, Bloomfield CD, Dorothy RS, Brunning RD. Acute promyelocytic leukaemia, a study of 39 cases with identification of a hyperbasophilic microgranular variant. Br J Haemat. 1982;50:201–214. doi: 10.1111/j.1365-2141.1982.tb01910.x. [DOI] [PubMed] [Google Scholar]
- 7.Hersh EM, Bodey CP, Nies BA, Freireich EJ. Causes of death in acute leukaemia. JAMA. 1965;193:105–109. doi: 10.1001/jama.1965.03090020019005. [DOI] [PubMed] [Google Scholar]
- 8.Handerson ES, Afshani E. Clinical manifestations and diagnosis. In: Henderson ES, Lister TA, editors. Leukaemia. 5th ed. WB Saunders; Philadelphia: 1990. pp. 299–309. [Google Scholar]
- 9.Firkin F, Chesterman C, Penington D, Rush B. Leukaemia. In: Firkin F, editor. De Grouchy's Clinical haomatology in medical practice, 5th ed. Oxford University Press; New York: 1991. pp. 236–277. [Google Scholar]
- 10.Getaz EP, Miller GJ. Spinal cord involvement in chronic lymphocytic leukaemia. Cancer. 1979;43:1858–1861. doi: 10.1002/1097-0142(197905)43:5<1858::aid-cncr2820430539>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 11.Moore EW, Thomas LB, Shaw RK, Froiroich EJ. The central nervous system in acute leukaemia. Arch Intern Med. 1960;105:451–468. [PubMed] [Google Scholar]
- 12.Phair JP, Anderson RE, Namiki H. The central nervous system in leukaemia. Ann Intern Med. 1964;61:863–875. doi: 10.7326/0003-4819-61-5-863. [DOI] [PubMed] [Google Scholar]
- 13.Grouch SN, Sayer OP, Heck FJ. Central haemorrhage in leukaemia. Archives of Neurology & Psychiatry. 1960;2:439–448. doi: 10.1001/archneur.1960.03840100077011. [DOI] [PubMed] [Google Scholar]
- 14.Dawson DM, Rosenthal DS, Maloney WC. Neurological complications in acute leukaemias in adults – changing rates. Ann Intern Med. 1973;79:541–548. doi: 10.7326/0003-4819-79-4-541. [DOI] [PubMed] [Google Scholar]
- 15.Silverstein A. Intracranial haemorrhage in patients with bleeding tendencies. Neurology. 1961;11:310–316. [Google Scholar]


