Abstract
Tumor-associated macrophages (TAMs) are major constituents of the tumor microenvironment in solid tumors and have been implicated as mediators of tumor progression, invasion and metastasis. Correspondingly, accumulation of TAMs is associated with unfavorable clinical outcomes in numerous types of solid tumors. E-selectin is a hallmark of inflammation and a key adhesion molecule that accommodates the initial contact of circulating immune cells with the inflamed vessel surface. Currently, the association between E-selectin and TAMs is not fully elucidated; therefore, the present study investigated the association between vessel inflammation, TAM infiltration, and clinical outcome in breast cancer. A total of 53 procedure-naïve invasive breast cancer cases were immunohistochemically analyzed for the presence of cluster of differentiation (CD)68+ TAMs, E-selectin+ vessels and tumor inflammation. The association between CD68 and E-selectin expression, and tumor inflammation as well as overall survival was evaluated using Kaplan-Meier survival curves and multivariable Cox's proportional hazards regression analysis. The abundance of TAMs was identified to be positively associated with tumor inflammation, estrogen receptor and E-selectin expression levels. A greater prevalence of TAMs and tumor inflammation was significantly associated with shorter overall survival times. E-selectin expression levels were significantly higher in tumor vessels among elderly patients, but were not associated with overall survival. The abundance of TAMs was associated with the presence of E-selectin-expressing inflamed tumor vessels and tumor inflammation, as well as overall survival in patients with invasive breast carcinoma.
Keywords: E-selectin, cluster of differentiation 68, tumor-associated macrophages, breast cancer, inflammation, overall survival, immnohistochemistry
Introduction
Inflammation is a hallmark of cancer, not only triggering tumor development, but also promoting tumor progression, therapy resistance and metastasis (1,2). Breast cancer is a common type of malignancy, with >1.67 million new cases and 522,000 mortalities reported in 2012 worldwide (3). Approximately 30% of females with breast cancer experience recurrence within 5 years, with a 50% chance of developing distant metastases (4,5). Cancer is aptly described as a wound that never heals since solid tumors are chronically inflamed and immune cells in concert with other stromal components influence cancer cell behavior. Immune infiltrates represent a significant component of the mass in solid tumors (6) and aid in regulating cancer cell growth, angiogenesis and invasion via the production of an array of cytokines, reactive oxygen species, and proteases (7–10). Tumor-associated macrophages (TAMs) are a prominent component, serving a central role in promoting tumor growth and metastasis (1). Accordingly, a greater abundance of TAMs has been associated with metastasis and poor prognosis in numerous types of solid tumors including breast (11,12), lung (13,14), prostate (15), colorectal, and pancreatic cancer (16–19). TAMs are recognized as potent producers of growth factors (transforming growth factor-β, fibrobast growth factors and epidermal growth factor), pro-angiogenic factors [vascular endothelial growth factor, tumor necrosis factor-α (TNF-α), interleukin (IL)-8, matrix metalloproteinase and platelet derived growth factor], proteases (cathepsin and serine proteases) and cytokines (IL-10), which profoundly affect epithelial cancer cell growth, angiogenesis, local invasion, extracellualr matrix degradation, epithelial-mesenchymal transition, metastasis, therapy response, and immunosuppression (20–22).
Macrophages originate from peripheral blood mononuclear cells derived from bone marrow and are recruited into the tumor via colony stimulating factor 1 and C-X-C motif chemokine ligand 12, released from cancer cells or the tumor microenvironment (23). For successful tissue migration, circulating immune cells undergo a sequential multistep adhesion cascade initiated by adhesion to the vessel surface (24–28). Vascular expression of selectin family member proteins aids physical interaction with counter-receptor ligands expressed on immune cells, including sialyl Lewisx (sLex), sialyl LewisA (sLeA), cluster of differentiation (CD)44, cutaneous lymphocyte-associated antigen and P-selectin glycoprotein ligand-1 (29–31). E-selectin (also known as CD62E, endothelial cell leukocyte adhesion-1 or Leukocyte-endothelial cell adhesion molecule 2) is exclusively expressed on the luminal surface of inflamed vessels and serves a role in the catch bond that switches from rolling adhesion to integrin-mediated firm adhesion (32). Thus, elevated vascular E-selectin expression levels have been reported in a range of solid tumors, including breast (11,12), lung (13,14) and pancreatic (16,18) cancer. E-selectin expression often synchronizes with an abundance of sLex- or sLeA-positive immune infiltrates in the tumor (33,34). The present study investigated the abundance of tumor vascular E-selectin and macrophage marker CD68 expression levels in order to understand the association between inflamed tumor vessels and TAM infiltration, as well as their role in breast cancer prognosis.
Materials and methods
Tumors
Surgical whole mounts from a total of 100 human breast carcinoma specimens from females diagnosed between January 1987 and December 1988 from the pathology archives at Thomas Jefferson University (Philadelphia, USA) were used in the present study. The average age of patients at the time of surgery was 61.8±16.4 years. Cases with tissue containing ductal carcinoma in situ (DCIS) only, inflammatory breast cancer or other concurrent malignancies were excluded from the present study. Cases with no reactivity to vimentin staining were eliminated from the study. Only cases with fully annotated information regarding demographics, estrogen receptor (ER) expression, histology grade and overall survival (OS) were used in final analyses.
Immunohistochemistry
For quality control, cases were first immunohistochemically stained with anti-vimentin monoclonal antibody (cat. no. 550513; BD Biosciences, San Jose, CA, USA) at a 1:250 dilution overnight at 4°C. Those without vimentin reactivity were removed from the study. Double immunohistochemistry was performed using formalin-fixed paraffin-embedded tumor sections (4 µm thickness). Briefly, following deparaffinization and rehydration, antigen retrieval was performed using Envision Flex Target Retrieval Solution (pH 6.1; Dako; Santa Clara, CA, USA) in a pressure cooker for 20 min at 102°C. Endogenous peroxidase and nonspecific epitopes were blocked with 0.3% hydrogen peroxide in absolute methanol for 30 min at room temperature and 5% normal horse serum and 1% normal goat serum (Sigma-Aldrich; St. Louis, MO, USA) for 1 h at room temperature. Sections were incubated with mouse anti-E-selectin monoclonal antibody at 1:100 (cat. no. MO20039; Neuromics, Inc., Minneapolis, MN, USA) overnight at room temperature. Following washing with PBS and subsequent blocking with 5% normal horse serum and 1% normal goat serum for 5 min at room temperature, the slides were incubated with pre-dilute secondary horseradish peroxidase (HRP)-polymer conjugated anti-mouse IgG (cat. no. K4001; Dako) for 30 min at room temperature. HRP was detected using 3–3′-diaminobenzidine (DAB; Biocare Medical LLC, Paheco, CA, USA) substrate for 10 min at room temperature and enhanced using DAB Sparkle (Biocare Medical LLC) for 1 min at room temperature. Residual antibodies were eluted using Denaturing solution (Biocare Medical LLC) at a 1:3 dilution for 3 min at room temperature to ensure no cross reaction between the first and second staining. Slides were blocked with 5% normal horse serum and 1% normal goat serum for 5 min at room temperature and then incubated with mouse anti-CD68 monoclonal antibody at 1:25 (cat. no. M0876; Dako) overnight at 4°C. Following a brief wash with PBS, the slides were incubated with pre-dilute secondary alkaline phosphatase-polymer conjugated MACH2 anti-mouse IgG (cat. no. MALP521; Biocare Medical LLC) for 1 h at room temperature and then visualized using Fast-Red (Biocare Medical LLC) for 7 min, followed by counterstain with Mayer Hematoxylin (Dako) for 4 min both at room temperature. The slides were air-dried and mounted. As a negative control, breast carcinoma tissues were immunostained with the secondary IgG only.
Pathologic evaluation
All immunohistochemically stained slides were evaluated by a board certified surgical pathologist (Department of Pathology, School of Medicine, University of Oklahoma Health Sciences Center, Oklahoma City, OK, USA) for breast pathology. The tumors were classified and graded according to the protocol from of the College of American Pathologists (Protocol for the Examination of Specimens from Patients with Invasive Carcinoma of the Breast according to InvasiveBreast 3.3.0.0.) (35). Tumor inflammation was defined as positive or negative, as characterized by the presence of lymphocyte clusters. Immunohistochemical reaction to E-selectin was graded using intensity as a score of 0, 1+, 2+ or 3+ for no, weak, moderate or strong reaction in endothelial cells within the tumor, respectively. Immunohistochemical staining of CD68 was quantitatively categorized as a score of 0, 1+, 2+ or 3+ for no CD68+ cells, ≤10 CD68+ cells, 11–20 CD68+ cells or ≥21 CD68+ cells in the observing field at ×200 magnification within the tumor, respectively. All images were viewed under a light microscope (DM2500; Leica, Buffalo Grove, IL, USA) and images were captured using digital cooling color camera (DFC450; Leica).
Statistical analysis
The Fisher's exact test was used to analyze the association between age and tumor pathological parameters using CD68 and E-selectin expression levels. Spearman's rho was used to determine the correlation between CD68+ TAMs, and E-selectin expression level and tumor inflammation. The Kaplan-Meier method was used to estimate OS as a function of time, and differences were analyzed using the log-rank test. Cox proportional hazards regression analysis was used for multivariable analysis of prognostic factors in relation to OS. The statistical software SAS, version 9.4 (SAS Institute Inc., Cary, NC, USA) was used to perform statistical analyses. GraphPad Prism version 6 (Graphpad Software, Inc., La Jolla, CA, USA) was used to generate Kaplan-Meier curves. P<0.05 was considered to indicate a statistically significant difference.
Results
Tumor characteristics
Following exclusions, a total of 53 invasive breast cancer cases that had been first-time diagnosed by surgical resection in 1986–1988 at Thomas Jefferson University were used in the present study. Tumor characteristics of these patients are presented in Table I. Cases were categorized as either high (score 3+) or low (score 0–2+) CD68 expression level according to the abundance of CD68+ TAMs in the tumor core and high (score 3+), or low (score 0–2+) expression level of E-selectin on vessels in the tumor area. CD68+ TAMs were significantly associated with tumor inflammation (P=0.005) and ER status (P=0.037). E-selectin expression level was associated with age (P=0.016) and was significantly higher among females over the age of 60 years (Table I).
Table I.
Association between clinicopathologic parameters, and CD68 and E-selectin expression in procedure-naïve invasive breast carcinoma tissues.
| CD68 expression | E-selectin expression | ||||||
|---|---|---|---|---|---|---|---|
| Clinicopathologic parameters | Total no. cases | Low | High | P-value | Low | High | P-value |
| All cases | 53 | 34 | 19 | 41 | 12 | ||
| Age | 0.349 | 0.016a | |||||
| ≤60 | 20 | 14 | 6 | 19 | 1 | ||
| >60 | 33 | 20 | 13 | 22 | 11 | ||
| Tumor inflammation | 0.005a | 0.540 | |||||
| (−) | 25 | 21 | 4 | 19 | 6 | ||
| (+) | 28 | 13 | 15 | 22 | 6 | ||
| Nottingham histological grade | 0.561 | 0.455 | |||||
| Grade I | 3 | 8 | 4 | 3 | 0 | ||
| Grade II + III | 50 | 26 | 15 | 38 | 12 | ||
| ER status | 0.037a | 0.521 | |||||
| Negative | 11 | 4 | 7 | 9 | 2 | ||
| Positive | 42 | 30 | 12 | 32 | 10 | ||
Statistically significant difference following Fisher's exact probability test. CD68, cluster of differentiation 68.
Pattern of CD68+ TAM infiltration and E-selectin+ vessel inflammation in breast tumors
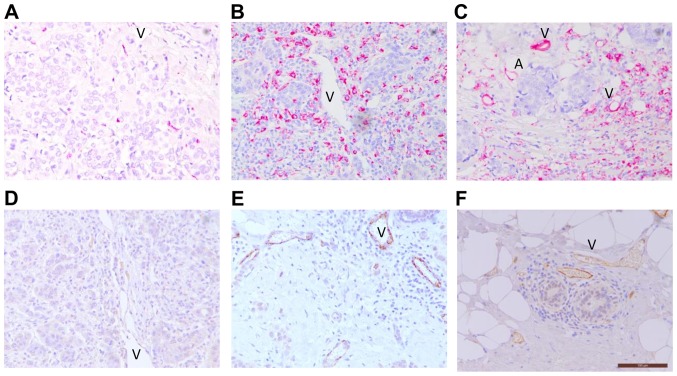
Representative images of high and low expression levels of CD68+ TAMs, and E-selectin+ inflamed vessels are presented in Fig. 1. Various expression levels of CD68+ TAMs were consistently present in the tumor stroma and core of all 53 cases (Fig. 1A-C). Considerable, multilayered CD68+ TAM deposition in the necrotic area of the tumors was observed. CD68+ TAMs were also abundant in the mammary fat adjacent to the tumor, as well as around the luminal surface of vessels (Fig. 1C). E-selectin was predominantly expressed on vessels within the tumor stroma (Fig. 1D and E). No positive signal for E-selectin was detected in other tumor components, including cancer cells, fibroblasts, immune infiltrates or the apical side of the vessels. The size of E-selectin-expressing vessels varied from small capillaries to large vessels in the stroma and fat adjacent to the invasive front of tumors. Vessel structure was well retained within the stroma but often compressed, crushed or even absent in the tumor core. Overall, 88.7% of the breast carcinoma cases exhibited E-selectin expression on their tumor vessels. Consistent with inflammation of the adipose tissue adjacent to the tumor, E-selectin expression level was also high in vessels of the neighboring peripheral adipose tissue. Of note, vascular E-selectin expression level was present in the stroma surrounding the mammary duct in invasive carcinomas that retained a ductal structure (Fig. 1F). A similar pattern of E-selectin expressing vessels in the stroma around the mammary duct in DCIS only cases was also revealed (data not shown), suggesting the presence of peritumoral inflammation at the pre-invasive stage.
Figure 1.
TAM and E-selectin expression levels in breast tumor tissues. (A-C) Single immunohistochemical staining of CD68+ TAMs for analysis of the distribution at various areas of the invasive breast carcinoma tissues (red). (D-F) Differential E-selectin expression in vessel surface in breast carcinomas (brown). The images are representative at a final magnification of ×200. Scale bar indicates 100 µm. V, vessels; A, adipocytes; CD68, cluster of differentiation 68.
Association between vessel inflammation and TAM infiltration
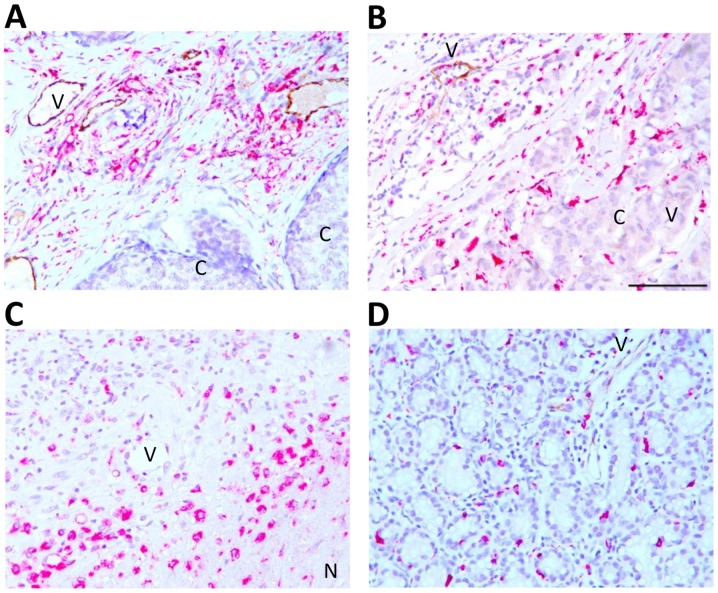
Double immunohistochemistry for CD68 (pink) and E-selectin (brown) was performed to evaluate their association. CD68+ TAMs were abundant in close proximity to E-selectin-expressing vessels in the tumor stroma and peripheral tissue adjacent to the tumor (Fig. 2A and B). CD68+ TAMs were sparsely present in carcinoma cell rich areas; however, E-selectin expression was limited to the surrounding inflamed area and absent or weakly present in the tumor core (Fig. 2B). CD68+ TAMs were also highly abundant in necrotic areas, but E-selectin was absent within and adjacent to the necrotic core (Fig. 2C). TAMs and E-selectin were present at the location where the ductal structure was retained (Fig. 2D). Association between the abundance of CD68+ TAMs and E-selectin+ vessels was evaluated using a 4-level scoring scale (0, 1+, 2+, 3+) for the expression level of each marker. High abundance of CD68+ TAMs and E-selectin+ vessels (3+/3+) was demonstrated in 7.5% of the overall analyzed samples. CD68+ TAMs and E-selectin expression levels were positively correlated (r=0.30, P=0.030; Table II). Additionally, CD68+ TAMs were significantly correlated with tumor inflammation (r=0.54, P=0.001; Table II).
Figure 2.
Double immunohistochemistry of TAM and E-selectin. (A and B) Spatial association of CD68+ TAM and E-selectin in the tumor stroma of invasive breast carcinoma tissues. (C) Absence of E-selectin expressing vessels in necrotic area. (D) Presence of CD68+ TAM and E-selectin in stroma of non-carcinoma area of invasive carcinoma tissue. Brown indicates E-selectin and red indicates TAM. The images are representative at a final magnification of ×200. Scale bar indicates 100 µm. V, vessels; C, cancer cells; N, necrotic area; CD68, cluster of differentiation 68.
Table II.
Association between CD68+ TAMs, and E-selectin expressing vessels and tumor inflammation in procedure-naïve invasive breast carcinoma tissues.
| E-selectin | Inflammation | |||||
|---|---|---|---|---|---|---|
| CD68 | 0 | 1+ | 2+ | 3+ | − | + |
| 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 1+ | 3 | 5 | 4 | 2 | 13 | 1 |
| 2+ | 1 | 6 | 10 | 3 | 8 | 12 |
| 3+ | 2 | 2 | 8 | 7 | 4 | 15 |
| *P=0.030, r=0.302 | *P<0.001, r=0.541 | |||||
E-selectin: 0, no immunohistochemical reaction; 1+, weakly; 2+, moderately; 3+, strong reactions in endothelial cells. CD68: 0, no CD68+ cells; 1+, ≤10 CD68+ cells; 2+, 11–20 CD68+ cells; 3+ ≥21 CD68+ cells. CD68, cluster of differentiation 68; TAMs, tumor-associated macrophages.
Abundance of markers and clinical outcome
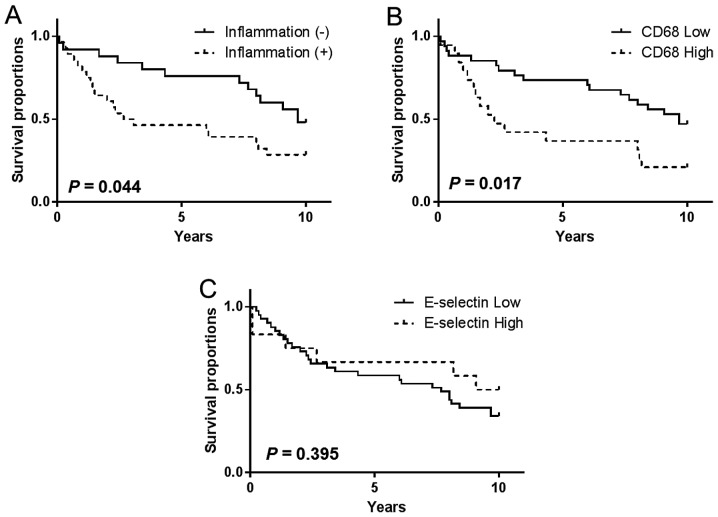
OS was determined and graphically presented using the Kaplan-Meier method, and a Cox proportional hazards regression model was used for multivariable analysis of the association between clinicopathological parameters and marker expression level with OS. Tumor inflammation was significantly associated with OS among patients with breast cancer [hazard ratio (HR), 2.00; confidence interval (CI) 95%, 1.03–4.06; P=0.044; Fig. 3A]. However, inflammation in the tumor periphery lacked association with OS (HR, 1.20; CI 95%, 0.560–2.61; P=0.629; data not shown). Compared with tissue samples with lower expression levels of CD68+ TAMs, higher expression levels of CD68+ TAMs in the tumor core were significantly associated with shorter OS at the 10-year follow-up (HR, 2.23; CI 95%, 1.12–5.54; P=0.017; Fig. 3B). Conversely, CD68+ TAMs in the tumor periphery were not significantly associated with OS (HR, 1.39; CI 95%, 0.59–3.50; P=0.420; data not shown). Although the abundance of E-selectin+ vessels and CD68+ TAMs were positively correlated, E-selectin expression level alone did not impact OS at the tumor (HR, 0.71; CI 95%, 0.32–1.57; P=0.395; Fig. 3C) or periphery. Kaplan-Meier analysis demonstrated that OS was unaffected by age for CD68, E-selectin or tumor inflammation statuses (data not shown). Finally, multivariable analysis of procedure-naïve breast tumor tissues revealed that the presence of abundant CD68+ TAMs was an independent predictor of OS (HR, 2.37; 95% CI, 1.02–5.36; P=0.045) following adjustment for ER status, tumor inflammation and E-selectin expression level (Table III).
Figure 3.
Overall survival by prevalence of (A) tumor inflammation, (B) TAM and (C) E-selectin. Kaplan-Meier survival curves according to tumor inflammation, CD68+ TAMs and E-selectin+ vessels in 53 invasive patients with breast cancer. TAM, tumor-associated macrophage; CD68, cluster of differentiation 68.
Table III.
Multivariable Cox proportional hazard regression analysis of overall survival.
| Variables | P-value | Hazard ratio | 95% CI |
|---|---|---|---|
| Tumor inflammation (ref. positive) | 0.326 | 1.48 | 0.68–3.26 |
| ER status (ref. negative) | 0.341 | 1.50 | 0.65–3.42 |
| E-selectin expression (ref. high) | 0.112 | 0.46 | 0.18–1.20 |
| CD68 expression (ref. high) | 0.045a | 2.34 | 1.02–5.36 |
Statistically significant. CI, confidence interval; ER, estrogen receptor; CD68, cluster of differentiation.
Discussion
Tissue infiltration by circulating leukocytes occurs in response to tissue damage and injury. In the context of solid tumors, cell death arises from intrinsic and extrinsic inducers, initiating an inflammatory cascade in an attempt to scavenge debris, and repair damaged tissue. Intrinsic cell death is hypoxia-derived necrosis or DNA-damage-associated apoptosis, whereas extrinsic cell death is associated with external stimuli, including chemotherapy, biopsy, surgery or radiation therapy. The standard of care for breast cancer has altered significantly over the past 3 decades (35). Diagnostic needle biopsy became popular in the 1990s (36) and neoadjuvant chemotherapy for relatively large, locally advanced tumors emerged in the 2000s (37). Both procedures provoke inflammation accompanied by cell death in the tumor and neighboring peripheral tissue. Thus, it is likely that recent surgically resected tumors contain inflammation induced by extrinsic stress along with naturally occurring intrinsic cell death. Although external stress naïve tumors can be analyzed using biopsy samples, such samples contain only small and limited amounts of tissue, making the capture of the overall tumor environment difficult. Analysis of naïve tumors collected by excisional biopsy essentially eliminates locally advanced tumors since this method is typically only used in early stage small-sized tumors. Thus, in order to understand the association of vessel inflammation and TAM infiltration in intrinsic tumor inflammation, the present study specifically targeted surgically resected, procedure-naïve breast tumor tissue samples collected between 1986 and 1988, when needle biopsy was not yet broadly adopted as the standard of care.
The involvement of E-selectin in cancer has long been recognized, as evidenced by histopathological studies; however, its clinical implications have been controversial (11,12,38). The results of the present study revealed that 88.7% of procedure-naïve breast tumors expressed E-selectin in the vessels within the tumor. Previously, the prevalence of E-selectin-positive vessels has been reported as 55.7% (n=113) (11) and 77.6% (n=22) (12) in frozen breast tumor tissue sections. A previous study by Charpin et al (11) demonstrated a positive association between E-selectin expression level, and vascular cell adhesion molecule-1, very late antigen-2 and CD44 expression levels, and a negative association with E-cadherin expression level. However, the latter was postiviely associated with ER-negative breast cancer, which may be due to the release of higher expression levels of IL-1 and TNF-α from ER-negative compared with ER-positive breast cancer cells. The present study and the study by Charpin et al (11) did not determine an association between E-selectin expression and ER status, but both studies identified an association between E-selectin, and CD68+ TAMs or CD44+ immune infiltrates, a common marker for immune cells.
TAMs are classified as either pro-inflammatory M1 or pro-tumorigenic M2 macrophages (36), although it is yet to be determined which of these is more clinically important for prognosis (37,39). Unlike T-lymphocytes, whose phenotypes are classified by differentiation, the TAM phenotype is plastic and determined by its surrounding microenvironment (40). For example, the M1 phenotype can switch to M2 in response to T helper 2-released cytokines, including IL-13 and IL-4 (41). Accordingly, the overall composition and balance of immune subsets determines the pro-tumorigenic potential and fate of the tumor. TAMs were present in all procedure-naïve invasive breast carcinoma samples in the present study and their spatial distribution pattern, as well as abundance, differed among cases. TAMs were a predominant component near the necrotic core, in adipose tissue adjacent to the tumor and in the tumor stroma. Further investigation of TAM accumulation and phenotypic distribution at different locations may improve the understanding of their clinical implications.
E-selectin turnover is short, and it is shed into the circulation as a circulating form of E-selectin, soluble (s)E-selectin (42). sE-selectin has been used as a surrogate marker for vessel inflammation since sE-selectin expression levels appear to be associated with the vascular E-selectin present on the surface of the endothelial cells (43–48). For example, the sE-selectin expression level was significantly higher among patients with metastatic breast cancer compared with that of healthy counterparts (33.5 vs. 21.8 ng/ml; P<0.01), as well as in patients with liver metastasis compared with those without (55.3 vs. 26.0 ng/ml; P<0.0001). Thus, increased expression levels of sE-selectin were associated with reduced overall survival in breast cancer (49). Similarly, pre-surgical sE-selectin expression levels were higher in patients with colorectal cancer (43 ng/ml) compared with patients with benign diseases (43 ng vs. 31 ng/ml) and were positively associated with carcinoembryonic antigen tumor marker and poorer prognosis (both P<0.001) (50). However, a study of microarray data from 1,809 breast cancer patients with no previous treatment history revealed that E-selectin expression level was associated with longer survival times (HR, 0.67; CI 95%, 0.54–0.83; P=0.001) (51). In the present study, E-selectin expression level in breast tumor tissue samples was more abundant in females >60 years compared with those ≤60. Elevated E-selection expression level among elderly females may be attributed to age-associated inflammation due to comorbidities, since sE-selectin expression levels are reported to be high in chronic inflammatory conditions, including arthritis (52,53), diabetes (52), atherosclerosis (54) and alcoholism (55). However, E-selectin expression level in the tumor was not associated with OS following age adjustment in the present study (data not shown). The survival implications of E-selectin expression may require integration of area-specific expression (tumor or necrosis vs. stroma), type of survival (overall vs. disease specific) and comorbidity status. In conclusion, tumor inflammation and E-selectin expression levels were identified to be positively correlated with TAMs, and the abundance of TAMs present in the tumor was an independent prognostic factor in invasive breast tumors.
Acknowledgements
The present study was supported by the National Institutes of Health (grant no. 1R01CA160271-01A1). The authors would like to thank Lynsie Morris for the technical assistance provided.
References
- 1.Pollard JW. Tumour-educated macrophages promote tumour progression and metastasis. Nat Rev Cancer. 2004;4:71–78. doi: 10.1038/nrc1256. [DOI] [PubMed] [Google Scholar]
- 2.Ruffell B, Coussens LM. Macrophages and therapeutic resistance in cancer. Cancer Cell. 2015;27:462–472. doi: 10.1016/j.ccell.2015.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ferlay J, Soerjomataram I, Ervik M, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int Agency Res Cancer. 2014 doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 4.Newman EA, Newman LA. Lymphatic mapping techniques and sentinel lymph node biopsy in breast cancer. Surg Clin North Am. 2007;87(viii):353–364. doi: 10.1016/j.suc.2007.01.013. [DOI] [PubMed] [Google Scholar]
- 5.Redig AJ, McAllister SS. Breast cancer as a systemic disease: A view of metastasis. J Intern Med. 2013;274:113–126. doi: 10.1111/joim.12084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/S0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 7.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 9.Mueller MM, Fusenig NE. Friends or foes-bipolar effects of the tumour stroma in cancer. Nat Rev Cancer. 2004;4:839–849. doi: 10.1038/nrc1477. [DOI] [PubMed] [Google Scholar]
- 10.Rakoff-Nahoum S. Why cancer and inflammation? Yale J Biol Med. 2006;79:123–130. [PMC free article] [PubMed] [Google Scholar]
- 11.Charpin C, Bergeret D, Garcia S, Andrac L, Martini F, Horschowski N, Choux R, Lavaut MN. ELAM selectin expression in breast carcinomas detected by automated and quantitative immunohistochemical assays. Int J Oncol. 1998;12:1041–1048. doi: 10.3892/ijo.12.5.1041. [DOI] [PubMed] [Google Scholar]
- 12.Nguyen M, Corless CL, Kräling BM, Tran C, Atha T, Bischoff J, Barsky SH. Vascular expression of E-selectin is increased in estrogen-receptor-negative breast cancer: A role for tumor-cell-secreted interleukin-1 alpha. Am J Pathol. 1997;150:1307–1314. [PMC free article] [PubMed] [Google Scholar]
- 13.Müller AM, Weichert A, Müller KM. E-cadherin, E-selectin and vascular cell adhesion molecule: Immunohistochemical markers for differentiation between mesothelioma and metastatic pulmonary adenocarcinoma? Virchows Arch. 2002;441:41–46. doi: 10.1007/s00428-001-0563-z. [DOI] [PubMed] [Google Scholar]
- 14.Staal-van den Brekel AJ, Thunnissen FB, Buurman WA, Wouters EF. Expression of E-selectin, intercellular adhesion molecule (ICAM)-1 and vascular cell adhesion molecule (VCAM)-1 in non-small-cell lung carcinoma. Virchows Arch. 1996;428:21–27. doi: 10.1007/BF00192923. [DOI] [PubMed] [Google Scholar]
- 15.Bhaskar V, Law DA, Ibsen E, Breinberg D, Cass KM, DuBridge RB, Evangelista F, Henshall SM, Hevezi P, Miller JC, et al. E-selectin up-regulation allows for targeted drug delivery in prostate cancer. Cancer Res. 2003;63:6387–6394. [PubMed] [Google Scholar]
- 16.Eichbaum MH, de Rossi TM, Kaul S, Bastert G. Serum levels of soluble E-selectin are associated with the clinical course of metastatic disease in patients with liver metastases from breast cancer. Oncol Res. 2004;14:603–610. doi: 10.3727/0965040042707916. [DOI] [PubMed] [Google Scholar]
- 17.Leek RD, Landers RJ, Harris AL, Lewis CE. Necrosis correlates with high vascular density and focal macrophage infiltration in invasive carcinoma of the breast. Br J Cancer. 1999;79:991–995. doi: 10.1038/sj.bjc.6690158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tozeren A, Kleinman HK, Grant DS, Morales D, Mercurio AM, Byers SW. E-selectin-mediated dynamic interactions of breast- and colon-cancer cells with endothelial-cell monolayers. Int J Cancer. 1995;60:426–431. doi: 10.1002/ijc.2910600326. [DOI] [PubMed] [Google Scholar]
- 19.Tsutsui S, Yasuda K, Suzuki K, Tahara K, Higashi H, Era S. Macrophage infiltration and its prognostic implications in breast cancer: The relationship with VEGF expression and microvessel density. Oncol Rep. 2005;14:425–431. [PubMed] [Google Scholar]
- 20.Leek RD, Harris AL. Tumor-associated macrophages in breast cancer. J Mammary Gland Biol Neoplasia. 2002;7:177–189. doi: 10.1023/A:1020304003704. [DOI] [PubMed] [Google Scholar]
- 21.Leek RD, Talks KL, Pezzella F, Turley H, Campo L, Brown NS, Bicknell R, Taylor M, Gatter KC, Harris AL. Relation of hypoxia-inducible factor-2 alpha (HIF-2 alpha) expression in tumor-infiltrative macrophages to tumor angiogenesis and the oxidative thymidine phosphorylase pathway in human breast cancer. Cancer Res. 2002;62:1326–1329. [PubMed] [Google Scholar]
- 22.Lewis CE, Pollard JW. Distinct role of macrophages in different tumor microenvironments. Cancer Res. 2006;66:605–612. doi: 10.1158/0008-5472.CAN-05-4005. [DOI] [PubMed] [Google Scholar]
- 23.Joyce JA, Pollard JW. Microenvironmental regulation of metastasis. Nat Rev Cancer. 2009;9:239–252. doi: 10.1038/nrc2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Berg EL, Robinson MK, Mansson O, Butcher EC, Magnani JL. A carbohydrate domain common to both sialyl Le(a) and sialyl Le(X) is recognized by the endothelial cell leukocyte adhesion molecule ELAM-1. J Biol Chem. 1991;266:14869–14872. [PubMed] [Google Scholar]
- 25.Ley K, Laudanna C, Cybulsky MI, Nourshargh S. Getting to the site of inflammation: The leukocyte adhesion cascade updated. Nat Rev Immunol. 2007;7:678–689. doi: 10.1038/nri2156. [DOI] [PubMed] [Google Scholar]
- 26.Luster AD, Alon R, von Andrian UH. Immune cell migration in inflammation: Present and future therapeutic targets. Nat Immunol. 2005;6:1182–1190. doi: 10.1038/ni1275. [DOI] [PubMed] [Google Scholar]
- 27.Welply JK, Keene JL, Schmuke JJ, Howard SC. Selectins as potential targets of therapeutic intervention in inflammatory diseases. Biochim Biophys Acta. 1994;1197:215–226. doi: 10.1016/0304-4157(94)90006-X. [DOI] [PubMed] [Google Scholar]
- 28.Zetter BR. Adhesion molecules in tumor metastasis. Semin Cancer Biol. 1993;4:219–229. [PubMed] [Google Scholar]
- 29.Phillips ML, Nudelman E, Gaeta FC, Perez M, Singhal AK, Hakomori S, Paulson JC. ELAM-1 mediates cell adhesion by recognition of a carbohydrate ligand, sialyl-Lex. Science. 1990;250:1130–1132. doi: 10.1126/science.1701274. [DOI] [PubMed] [Google Scholar]
- 30.Picker LJ, Kishimoto TK, Smith CW, Warnock RA, Butcher EC. ELAM-1 is an adhesion molecule for skin-homing T cells. Nature. 1991;349:796–799. doi: 10.1038/349796a0. [DOI] [PubMed] [Google Scholar]
- 31.Shimizu Y, Shaw S, Graber N, Gopal TV, Horgan KJ, Van Seventer GA, Newman W. Activation-independent binding of human memory T cells to adhesion molecule ELAM-1. Nature. 1991;349:799–802. doi: 10.1038/349799a0. [DOI] [PubMed] [Google Scholar]
- 32.Thomas W. Catch bonds in adhesion. Annu Rev Biomed Eng. 2008;10:39–57. doi: 10.1146/annurev.bioeng.10.061807.160427. [DOI] [PubMed] [Google Scholar]
- 33.Hidalgo A, Peired AJ, Wild MK, Vestweber D, Frenette PS. Complete identification of E-selectin ligands on neutrophils reveals distinct functions of PSGL-1, ESL-1, and CD44. Immunity. 2007;26:477–489. doi: 10.1016/j.immuni.2007.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Matsuura N, Narita T, Hiraiwa N, Hiraiwa M, Murai H, Iwase T, Funahashi H, Imai T, Takagi H, Kannagi R. Gene expression of fucosyl- and sialyl-transferases which synthesize sialyl Lewisx, the carbohydrate ligands for E-selectin, in human breast cancer. Int J Oncol. 1998;12:1157–1164. doi: 10.3892/ijo.12.5.1157. [DOI] [PubMed] [Google Scholar]
- 35.Lester SCI, Bose S, Chen YY, Connolly JL, de Baca ME, Fitzgibbons PL, Hayes DF, Kleer C, O'Malley FP, Page DL, et al. Protocol for the examination of specimens from patients with invasive carcinoma of the breast. Arch Pathol Lab Med. 2009;133:1515–1538. doi: 10.5858/133.10.1515. [DOI] [PubMed] [Google Scholar]
- 36.Martinez FO, Gordon S. The M1 and M2 paradigm of macrophage activation: Time for reassessment. F1000Prime Rep. 2014;6:13. doi: 10.12703/P6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang QW, Liu L, Gong CY, Shi HS, Zeng YH, Wang XZ, Zhao YW, Wei YQ. Prognostic significance of tumor-associated macrophages in solid tumor: A meta-analysis of the literature. PLoS One. 2012;7:e50946. doi: 10.1371/journal.pone.0050946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wei J, Cui L, Liu F, Fan Y, Lang R, Gu F, Guo X, Tang P, Fu L. E-selectin and Sialyl Lewis X expression is associated with lymph node metastasis of invasive micropapillary carcinoma of the breast. Int J Surg Pathol. 2010;18:193–200. doi: 10.1177/1066896908320832. [DOI] [PubMed] [Google Scholar]
- 39.Mei J, Xiao Z, Guo C, Pu Q, Ma L, Liu C, Lin F, Liao H, You Z, Liu L. Prognostic impact of tumor-associated macrophage infiltration in non-small cell lung cancer: A systemic review and meta-analysis. Oncotarget. 2016;7:34217–34228. doi: 10.18632/oncotarget.9079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lawrence T, Natoli G. Transcriptional regulation of macrophage polarization: Enabling diversity with identity. Nat Rev Immunol. 2011;11:750–761. doi: 10.1038/nri3088. [DOI] [PubMed] [Google Scholar]
- 41.DeNardo DG, Barreto JB, Andreu P, Vasquez L, Tawfik D, Kolhatkar N, Coussens LM. CD4(+) T cells regulate pulmonary metastasis of mammary carcinomas by enhancing protumor properties of macrophages. Cancer Cell. 2009;16:91–102. doi: 10.1016/j.ccr.2009.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pigott R, Dillon LP, Hemingway IH, Gearing AJ. Soluble forms of E-selectin, ICAM-1 and VCAM-1 are present in the supernatants of cytokine activated cultured endothelial cells. Biochem Biophys Res Commun. 1992;187:584–589. doi: 10.1016/0006-291X(92)91234-H. [DOI] [PubMed] [Google Scholar]
- 43.Cowley HC, Heney D, Gearing AJ, Hemingway I, Webster NR. Increased circulating adhesion molecule concentrations in patients with the systemic inflammatory response syndrome: A prospective cohort study. Crit Care Med. 1994;22:651–657. doi: 10.1097/00003246-199404000-00022. [DOI] [PubMed] [Google Scholar]
- 44.Fassbender K, Mössner R, Motsch L, Kischka U, Grau A, Hennerici M. Circulating selectin- and immunoglobulin-type adhesion molecules in acute ischemic stroke. Stroke. 1995;26:1361–1364. doi: 10.1161/01.STR.26.8.1361. [DOI] [PubMed] [Google Scholar]
- 45.Gearing AJ, Hemingway I, Pigott R, Hughes J, Rees AJ, Cashman SJ. Soluble forms of vascular adhesion molecules, E-selectin, ICAM-1, and VCAM-1: Pathological significance. Ann N Y Acad Sci. 1992;667:324–331. doi: 10.1111/j.1749-6632.1992.tb51633.x. [DOI] [PubMed] [Google Scholar]
- 46.Gearing AJ, Newman W. Circulating adhesion molecules in disease. Immunol Today. 1993;14:506–512. doi: 10.1016/0167-5699(93)90267-O. [DOI] [PubMed] [Google Scholar]
- 47.Koch AE, Turkiewicz W, Harlow LA, Pope RM. Soluble E-selectin in arthritis. Clin Immunol Immunopathol. 1993;69:29–35. doi: 10.1006/clin.1993.1146. [DOI] [PubMed] [Google Scholar]
- 48.Leeuwenberg JF, Smeets EF, Neefjes JJ, Shaffer MA, Cinek T, Jeunhomme TM, Ahern TJ, Buurman WA. E-selectin and intercellular adhesion molecule-1 are released by activated human endothelial cells in vitro. Immunology. 1992;77:543–549. [PMC free article] [PubMed] [Google Scholar]
- 49.Hebbar M, Révillion F, Louchez MM, Vilain MO, Fournier C, Bonneterre J, Peyrat JP. The relationship between concentrations of circulating soluble E-selectin and clinical, pathological, and biological features in patients with breast cancer. Clin Cancer Res. 1998;4:373–380. [PubMed] [Google Scholar]
- 50.Ferroni P, Roselli M, Spila A, D'Alessandro R, Portarena I, Mariotti S, Palmirotta R, Buonomo O, Petrella G, Guadagni F. Serum sE-selectin levels and carcinoembryonic antigen mRNA-expressing cells in peripheral blood as prognostic factors in colorectal cancer patients. Cancer. 2010;116:2913–2921. doi: 10.1002/cncr.25094. [DOI] [PubMed] [Google Scholar]
- 51.Gyorffy B, Lanczky A, Eklund AC, Denkert C, Budczies J, Li Q, Szallasi Z. An online survival analysis tool to rapidly assess the effect of 22,277 genes on breast cancer prognosis using microarray data of 1,809 patients. Breast Cancer Res Treat. 2010;123:725–731. doi: 10.1007/s10549-009-0674-9. [DOI] [PubMed] [Google Scholar]
- 52.Çakar M, Balta Ş, Şarlak H, Akhan M, Demirkol S, Karaman M, Ay SA, Kurt Ö, Çayci T, İnal S, Demirbaş Ş. Arterial stiffness and endothelial inflammation in prediabetes and newly diagnosed diabetes patients. Arch Endocrinol Metab. 2015;59:407–413. doi: 10.1590/2359-3997000000061. [DOI] [PubMed] [Google Scholar]
- 53.Klimiuk PA, Fiedorczyk M, Sierakowski S, Chwiecko J. Soluble cell adhesion molecules (sICAM-1, sVCAM-1, and sE-selectin) in patients with early rheumatoid arthritis. Scand J Rheumatol. 2007;36:345–350. doi: 10.1080/03009740701406460. [DOI] [PubMed] [Google Scholar]
- 54.Kvasnicka T, Kvasnicka J, Ceská R, Grauova B, Vrablík M. Increasing plasma levels of soluble cell adhesion molecules (sE-Selectin, sP-Selectin and sICAM-1) in overweight adults with combined hyperlipidemia. Sb Lek. 2001;102:473–477. [PubMed] [Google Scholar]
- 55.Sacanella E, Estruch R, Badía E, Fernández-Sola J, Nicolás JM, Urbano-Márquez A. Chronic alcohol consumption increases serum levels of circulating endothelial cell/leucocyte adhesion molecules E-selectin and ICAM-1. Alcohol Alcohol. 1999;34:678–684. doi: 10.1093/alcalc/34.5.678. [DOI] [PubMed] [Google Scholar]