Abstract
Objective
Many types of treatment are available for patients with rheumatoid arthritis (RA), however, some patients fail to achieve remission. This report aims to determine the safety and efficacy of using repository corticotropin injection (RCI) as an adjunctive therapy in patients with RA refractory to at least three therapeutics with different mechanisms of action.
Method
In this open-label, interventional, single-group study, patients received 80 U RCI twice weekly via subcutaneous injection over 12 weeks. Changes in the Ritchie–Camp Articular Index and health assessment questionnaire scores were monitored for changes from baseline measures.
Results
Eight patients were enrolled and consisted of seven females and one male with an average age of 64.6 years and disease duration of 20.9 years. Use of RCI resulted in significant improvement in swollen and tender joint counts. The disease activity score 28 and the physician and patient visual analog scale scores were significantly reduced at treatment week 12. The reduction in health assessment questionnaire scores did not reach statistical significance after RCI treatment. Once RCI therapy was discontinued, all improvements in disease activity score 28, physician and patient visual analog scale, and tender and swollen joint counts achieved during treatment were lost by the week 16 follow-up visit.
Conclusion
While larger clinical trials are necessary to further confirm the efficacy of RCI in patients with refractory RA, the response of patients with refractory RA in this study suggests that RCI can be an effective add-on therapy for patients who have exhausted several classes of treatments. Furthermore, this study suggests that RCI has an alternative mode of action, compared to other available antirheumatic drugs.
Keywords: refractory rheumatoid arthritis, rheumatoid arthritis, adrenocorticotropic hormone, repository corticotropin injection
Introduction
In the USA, there are ~1.5 million adults suffering from rheumatoid arthritis (RA), with women more likely to develop the disease.1 RA is a chronic, systemic autoimmune disorder that can develop at any age and is characterized by swelling, stiffness, and tenderness of the joints, as well as progressive bone and joint damage.2 The destruction of cartilage, bone, joints, and damage to other organ systems in patients with RA results in disability and an increase in mortality.2–4 Perpetuation of inflammation is mediated by a variety of cell types, including osteoclasts, fibroblast-like synoviocytes, chondrocytes, T-cells, and B-cells.5
Current therapeutic options for RA include those that target the underlying inflammatory disorder.3 Disease-modifying antirheumatic drugs (DMARDs), such as methotrexate (MTX), sulfasalazine, and hydroxychloroquine, are usually prescribed to combat the inflammation associated with RA. Corticosteroids are also frequently used in patients with RA to rapidly control the inflammation associated with the disease.6 While certain antirheumatic drugs have a long history in the clinic, the introduction of biologics in the late 1990s offered patients with RA additional therapeutic options; there are at least ten currently approved biologics, half of which are tumor necrosis factor-α (TNF-α) inhibitors.7,8 The remaining inhibitors antagonize the inflammatory response by targeting interleukin (IL)-6 receptor, receptor-activator of nuclear factor kappa B ligand, IL-1 type-1 receptors, adhesion molecules (i.e., CD20 on the surface of B-cells), and signaling required for T-cell activation.7
The American College of Rheumatology (ACR) suggests that remission in patients with established RA is an achievable goal due to the introduction of these various therapies and treatment strategies.9 Despite the availability of several effective options for treatment of patients with RA, including corticosteroids, DMARDs, and biologics, many patients have inadequate disease control. Up to 30% of all patients can experience glucocorticoid resistance, which includes those patients with inflammatory disease.10–17 Not only are patients refractory to corticosteroid treatments, but between 14% and 38% of patients do not respond to first-line biologics (i.e., anti-TNF-α therapies).18 Evaluation of the use of sequential TNF-α therapies in patients with RA via systematic review of clinical, peer-reviewed studies indicates that increasing the number of biologics used is associated with a decrease in likelihood of response.19
Repository corticotropin injection (RCI) contains a long-acting formulation of a porcine analog of adrenocorticotropic hormone (ACTH1–39, prepared in 16% gelatin).20 RCI is not considered a member of the aforementioned classes of treatments; however, ACTH was identified as a modulator of symptoms in RA over 50 years ago.21 While ACTH, a member of the melanocortin family of peptides, can have steroidogenic effects, its mechanism of action (MOA) is dependent upon melanocortin receptor activation.22 RCI is Food and Drug Administration approved as an adjunctive therapy for the treatment of rheumatic disorders, particularly in patients with acute episodes or exacerbation of disease.20 However, no completed trial exists that has assessed RCI in patients who have failed at least three therapeutic options with different biologic MOAs.
This single-center, prospective, open-label, interventional study includes patients who are refractory to at least three different therapeutics with various biologic MOAs. Patients also received all clinical standards of care. The primary objective of this study was to assess the efficacy and safety of RCI treatment in patients with refractory RA as an add-on therapy.
Methods
Patients
Adult male or female patients, aged 18–80 years, with confirmed RA based on the ACR/European League Against Rheumatism criteria23,24 were eligible to participate in this study after providing written informed consent/Health Insurance Portability and Accountability Act authorization (clinicaltrials. gov, NCT01966718). Each patient was required to have RA for at least 2 years, an ACR functional status between one and three, and been treated with at least three therapeutic agents with a different MOA for >3 months and still have active disease, defined by six tender and six swollen joints. Erythrocyte sedimentation rate (ESR) >28 or C-reactive protein (CRP) >1.2 times the normal limit was required; however, waiver of these particular criteria was at the discretion of the investigator. Each patient must have maintained a stable treatment regimen for 4 weeks prior to initiation of RCI therapy. Patients were excluded from this study if they had the following: received RCI therapy before, an allergy or intolerance to corticosteroids, uncontrolled diabetes, active infection, malignancy within the last 5 years, or an unstable disease state deemed significant by the investigator. Any impending surgery that might interfere with treatment schedules and those diagnosed with kidney disease (glomerular filtration rate <30 mL/min) were also excluded. Females who were pregnant or lactating and any patient who refused adequate methods of birth control were excluded. One patient included in this study had the criteria for malignancy waived and two patients’ ESR and CRP criteria were waived for inclusion in this study. Ultimately, eight patients were enrolled in this study (N=8).
Study design
This interventional study aimed to determine the safety and efficacy of treating patients with RA refractory to at least three therapeutics of varying MOAs with RCI as an add-on therapy. It was an open-label study that included a single group assignment. The study site involved in this trial received approval from the Western Institutional Review Board.
Treatment
RCI (80 U; H.P. Acthar® Gel; Mallinckrodt Pharmaceuticals, Hazelwood, MO, USA) was administered every 3 days for 12 weeks via subcutaneous injection. The first dose was administered by the investigator and subsequent doses were self-administered. Patients included in this study continued on stable doses of prednisone, biologics, and/or DMARDs, nonsteroidal anti-inflammatory drugs, and analgesics while being treated with RCI.
Clinical evaluation
The study included five visits and two follow-up visits after cessation of RCI therapy. Observations during these visits included the standards of care for clinical follow-up, particularly vital signs, health assessment questionnaire (HAQ) scores, joint count (full count of 68 joints), patient global visual analog scale (VAS), physician global VAS, disease activity score for RA (DAS28), and assessment of adverse events (AEs). Detailed patient histories were taken in order to ascertain improvement during the course of treatment. Primary endpoint measures included the Ritchie–Camp Articular Index25 and the 20-item HAQ score prior to treatment and at 2- to 4-week intervals. Secondary endpoint measures included acute ESR and CRP levels. Outcome measures were assessed at baseline and at weeks 0, 2, 6, and 12. After cessation of RCI therapy, measures were assessed at follow-up visits at weeks 14 and 16.
Laboratory testing
Laboratory values were assessed at baseline and each following visit. Complete blood counts, chemistry screens of 12 parameters, ESR, CRP, rheumatoid factor (IgM only), urinalysis, and anti-cyclic citrullinated peptide values were assessed.
Statistical analyses
Demographic information was summarized using descriptive statistics (i.e., mean, standard deviation, median, and range). A paired-samples t-test was performed to determine statistical significance and values of P ≤0.05 were considered significant. Patients included in assessments were those who received RCI and completed an assessment after baseline measurements. Statistical analyses were performed using GraphPad Prism 6 software.
Results
Eight patients were enrolled in this study between October 2013 and June 2015. The population consisted of seven females and one male with an average age of 64.6 years, ranging from 46 to 80 years. Patients’ mean disease duration was 20.9 years, ranging from 9 to 39 years. A complete record of baseline clinical characteristics is found in Table 1. On average, patients had a baseline tender joint count of 31.4 and a swollen joint count of 31. The HAQ scores ranged from 0.875 to 2.75, with an average of 1.78 prior to RCI treatment. Patients in this population had a mean failure of three biologics prior to their current RCI treatment regimen (Table 2). All patients had failed at least two therapies with biologic MOA and were currently failing another at the time of intervention with RCI. Concomitant medications are also listed in Table 2; during RCI treatment, all patients continued to receive MTX along with their current biologic. This patient population also maintained their prednisone dosing over the course of this study, which averaged 4.4 mg/day.
Table 1.
Demographic and clinical characteristics at baseline (N=8)
| Characteristics | Patients (N=8) |
|---|---|
| Patients (m/f) | 8 (1/7) |
| Male (%) | 12.5 |
| Age (years) | |
| Mean (SD) | 64.6 (12.1) |
| Median | 66 |
| Range | 46–80 |
| Disease duration (years) | |
| Mean (SD) | 20.9 (11.6) |
| Median | 19.5 |
| Range | 9–39 |
| Tender joint count | |
| Mean (SD) | 31.4 (16.3) |
| Median | 33.5 |
| Range | 7–50 |
| Swollen joint count | |
| Mean (SD) | 32.9 (10.74) |
| Median | 36 |
| Range | 17–48 |
| HAQ score | |
| Mean (SD) | 1.78 (0.71) |
| Median | 1.75 |
| Range | 0.875–2.75 |
| ESR (mm/h) | |
| Mean (SD) | 21.5 (16.5) |
| Median | 16 |
| Range | 7–60 |
| CRP (mg/L) | |
| Mean (SD) | 0.825 (0.6) |
| Median | 0.9 |
| Range | 0.1–1.8 |
| Patient VAS | |
| Mean (SD) | 40.1 (30.3) |
| Median | 28.5 |
| Range | 8–97 |
| Physician VAS | |
| Mean (SD) | 7.6 (2.2) |
| Median | 8.4 |
| Range | 3.8–9.9 |
| Disease activity score for RA (DAS28) | |
| Mean (SD) | 5.7 (0.84) |
| Median | 5.83 |
| Range | 4.33–6.87 |
Abbreviations: CRP, C-reactive protein; DAS28, disease activity score 28; ESR, erythrocyte sedimentation rate; f, female; HAQ, health assessment questionnaire; m, male; RA, rheumatoid arthritis; SD, standard deviation; VAS, visual analog scale.
Table 2.
DMARDs failed by each patient prior to RCI treatments and concomitant medications
| Patients | Previous treatments | Concomitant treatments | Dose of concomitant treatments |
|---|---|---|---|
| Patient 1 | Biologic | Biologic | |
| Abatacept | Certolizumab pegol | 200 mg biweekly | |
| Etanercept | DMARD | ||
| Rituximab | MTX | 0.6 mL SQ/week | |
| Tocilizumab | Corticosteroid | ||
| DMARD | Prednisone | 5 mg QD | |
| Hydroxychloroquine | Analgesics | ||
| Other DMARD | Morphine sulfur | 30 mg QD | |
| Tofacitinib citrate | Cyclobenzaprine | 5 mg QD | |
| Acetaminophen | 500 mg QD | ||
| Hydromorphone | 2 mg QD | ||
| Patient 2 | Biologic | Biologic | |
| Adalimumab | Abatacept | 125 mg/week | |
| Certolizumab pegol | DMARD | ||
| Tocilizumab | MTX | 15 mg/week | |
| Corticosteroid | |||
| Prednisone | 5 mg QD | ||
| NSAID | |||
| Celecoxib | 200 mg QD | ||
| Patient 3 | Biologic | Biologic | |
| Adalimumab | Abatacept | 125 mg/week | |
| Certolizumab pegol | DMARD | ||
| Etanercept | MTX | 15 mg/week | |
| Rituximab | Corticosteroid | ||
| Prednisone | 10 mg QD | ||
| NSAID | |||
| Meloxicam | 5 mg QD | ||
| Patient 4 | Biologic | Biologic | |
| Certolizumab pegol | Etanercept | 50 mg/week | |
| Investigational IL-1 inhibitor | DMARD | ||
| Rituximab | MTX | 12.5 mg/week | |
| Other DMARD | Analgesics | ||
| Tofacitinib citrate | Ibuprofen | 800 mg QD | |
| Acetaminophen | 500 mg QD | ||
| Patient 5 | Biologic | Biologic | |
| Certolizumab pegol | Abatacept | 250 mg every 4 weeks | |
| Other DMARD | DMARD | ||
| Tofacitinib citrate | MTX | 15 mg/week | |
| Corticosteroid | |||
| Prednisone | 10 mg QD | ||
| Patient 6 | Biologic | Biologic | |
| Etanercept | Abatacept | 750 mg every 4 weeks | |
| DMARD | DMARD | ||
| Hydroxychloroquine | MTX | 10 mg/week | |
| Other DMARD | |||
| Tofacitinib citrate | |||
| Patient 7 | Biologic | Biologic | |
| Abatacept | Rituximab | 2000 mg every 6 months | |
| Etanercept | DMARD | ||
| DMARD | MTX | 17.5 mg/week | |
| Hydroxychloroquine | Analgesics | ||
| Vicodin | 5 mg PRN | ||
| Patient 8 | Biologic | DMARD | |
| Adalimumab | MTX | 5 mg QD | |
| Tocilizumab | Other DMARD | ||
| Tofacitinib citrate | 10 mg/week | ||
| Corticosteroid | |||
| Prednisone | 5 mg QD |
Abbreviations: DMARD, disease-modifying antirheumatic drug; IL, interleukin; MTX, methotrexate; NSAID, nonsteroidal anti-inflammatory drug; PRN, as needed (latin: pro re nata); QD, daily; RCI, repository corticotropin injection; SQ, subcutaneous.
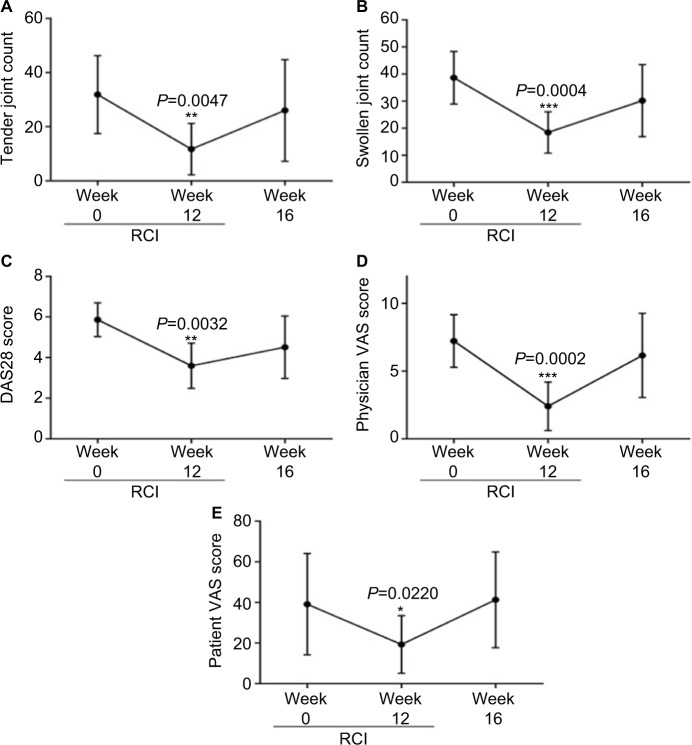
After a 12-week treatment period, the primary endpoint of decreased swollen and tender joint counts was achieved. Patients exhibited significant improvement in tender (P=0.0047) and swollen (P=0.0004) joint counts, as well as the DAS28 score (P=0.0032; Figure 1A–C; Table 3). RCI resulted in a decrease in HAQ score; however, the reduction did not reach statistical significance (Table 3). Physician and patient VAS scores significantly decreased by week 12 of RCI treatment (Figure 1D, E; Table 3). Although this cohort of patients had ESR and CRP values outside the normal range, no significant change in ESR or CRP was detected, which was likely related to the duration of treatment with biologic agents utilized in this patient population. After the study period had concluded, RCI was removed from the patients’ treatment regimen, and all improvements gained during the 12-week therapy were lost by the second follow-up visit at week 16 (Figure 1).
Figure 1.
Patients with refractory rheumatoid arthritis respond to RCI treatments.
Notes: At week 12 of RCI treatment, patients had significant improvement in tender and swollen joint counts (A and B), DAS28 score (C), and physician and patient VAS (D and E). After cessation of RCI treatment, any improvement in patient scores was lost by week 16. *P<0.05; ** P<0.01; ***P<0.001.
Abbreviations: DAS28, disease activity score 28; RCI, repository corticotropin injection; VAS, visual analog scale.
Table 3.
Clinical characteristics at week 12 of RCI treatment (N=8)
| Characteristics | Value | P-value |
|---|---|---|
| Tender joint count | 0.0047 | |
| Mean (SD) | 10.9 (9.1) | |
| Median | 9.0 | |
| Range | 0–26 | |
| Swollen joint count | 0.0004 | |
| Mean (SD) | 20.8 (9.6) | |
| Median | 20.8 | |
| Range | 6–37 | |
| Health assessment questionnaire score | 0.1249 | |
| Mean (SD) | 1.4 (0.76) | |
| Median | 1.3 | |
| Range | 0.38–2.5 | |
| ESR (mm/h) | 0.8996 | |
| Mean (SD) | 17.3 (10.3) | |
| Median | 16.0 | |
| Range | 5–32 | |
| CRP (mg/L) | 0.4776 | |
| Mean (SD) | 1.0 (1.3) | |
| Median | 0.45 | |
| Range | 0.1–3.8 | |
| Patient VAS | 0.0220 | |
| Mean (SD) | 16.9 (14.8) | |
| Median | 17 | |
| Range | 0–40 | |
| Physician VAS | 0.0002 | |
| Mean (SD) | 2.9 (2.2) | |
| Median | 2.5 | |
| Range | 0.30–6.5 | |
| Disease activity score for RA (DAS28) | 0.0032 | |
| Mean (SD) | 3.8 (1.2) | |
| Median | 3.4 | |
| Range | 2.5–5.6 |
Abbreviations: CRP, C-reactive protein; DAS28, disease activity score 28; ESR, erythrocyte sedimentation rate; RA, rheumatoid arthritis; RCI, repository corticotropin injection; SD, standard deviation; VAS, visual analog scale.
Two serious AEs (SAEs) were reported during this study; the patient was admitted to the hospital after development of thromboses in both the lungs and legs. All AEs are listed in Table 4. Two treatment-emergent AEs were reported and included injection site reaction and hypoglycemia. One patient ceased RCI treatments at week 12, missing two doses; all other reported events were transient and eventually resolved.
Table 4.
Reported adverse events in patients with RA treated with RCI
| AE | n |
|---|---|
| Any AE | 10 |
| AE | |
| Reaction at injection site | 1 |
| Hypoglycemia | 1 |
| Hip pain | 1 |
| Development of allergies | 1 |
| Pneumonia | 1 |
| Atrial fibrillation | 1 |
| Fibromyalgia | 1 |
| Hypertension | 1 |
| SAE | |
| Pulmonary embolism | 1 |
| Deep vein thrombosis | 1 |
Abbreviations: AE, adverse event; RA, rheumatoid arthritis; RCI, repository corticotropin injection; SAE, serious adverse event.
Discussion
This study demonstrates the effects of RCI treatment on patients with RA who are refractory to several classes of antirheumatic drugs, and is the only study performed among a modern patient population. At 12 weeks of treatment, patients with refractory disease responded to RCI treatment with a significant decrease in tender and swollen joint count, as well as total DAS28 scores. In particular, patient VAS scores were significantly improved at week 12, which is a component of the DAS28. This scale reflects a measure of how severe a patient perceives their disease to be, as a higher score usually correlates to increased pain felt.26 The use of RCI in this set of patients with RA led to a reduction in pain, which is one of the primary symptoms of RA and significantly affects the quality of life of the patient. Furthermore, the physician VAS was also reduced; previous literature has indicated that in instances where patient and physician assessments of disease are in concordance, patient adherence to therapy and outcomes improve.27
Although both patient and physician assessment of disease activity were in concordance and statistically signifi-cantly reduced at 12 weeks, inflammatory markers did not change significantly. The use of ESR and CRP as indicators of disease has been called into question, particularly when referring to monitoring of RA.28 In an assessment of correlation between the clinical measure of disease and ESR and CRP, it was shown that there was weak interaction between the affected joint counts and the laboratory values.28 The results presented here also support this previous work; the more pertinent measures of disease activity may be those that are clinically relevant, as opposed to those based on laboratory values.
Removal of RCI from the patients’ treatment regimen resulted in a return of the parameters of RA that had previously shown significant improvement to baseline. This suggests that patients with refractory disease may require long-term treatment with RCI.
The response of refractory patients suggests that RCI has an alternate MOA compared to corticosteroids and the available biologics, as well as MTX. ACTH exerts its steroidogenic effect by interacting with the melanocortin receptors in the adrenal cortex (MC2R), but can also have nonsteroidogenic effects by interacting with the receptors found on immune cells, osteoclasts, osteoblasts, and cells of the synovium (MC1R, MC3R, MC5R).29–34 The ability of melanocortins to have diverse moderate effects among a wide variety of cell types as opposed to significant inhibition of a specific aspect of the immune response classifies it as a pro-resolving molecule.22 In particular, ACTH is associated with both promotion of anti-inflammatory cytokine production and inhibition of proinflammatory responses, acting as a pro-resolving molecule and resulting in amelioration of leukocyte infiltration of tissues, which is pertinent to RA pathology.22,31 In vivo evidence from a mouse model of arthritis supports the use of melanocortins as anti-inflammatory peptides.33 Since melanocortins possess the ability to be pro-resolving molecules, they are attractive options for patients with inflammatory diseases. Synthetic melanocortins have been developed that are characterized by enhanced potency, improved duration of activity compared to endogenous melanocortins, and resistance to proteolytic activity.35 In addition, selective agonist peptides, selective antagonist peptides, and small-molecule ligands have been and are being developed in order to target particular melano-cortin receptors to exert control in particular pathologies; for example, MC4R has been the frequent target of development of agonists because of the association between this receptor and obesity.35
RCI has been a Food and Drug Administration-approved treatment for rheumatic diseases for over 60 years and has a long history of safe use. The majority of AEs reported during this trial were mild and resolved and/or were unrelated to RCI treatments. One patient experienced development of blood clots in the legs and lungs and this was considered an SAE; however, it is not likely related to the study drug, but rather the RA. This SAE resulted in the patient missing the last two doses of RCI during the 12th week of the study.
This particular study has several limitations that must be considered. The trial size was small; the patient cohort was eight patients. Treatment of a larger population of patients with refractory RA may provide further insight into effective treatment and dosing strategies. Additionally, this was an open-label trial and physicians and patients were aware of the status of RCI administration. As such, sampling bias cannot be completely excluded. Furthermore, patients were on different medications at the time of intervention with RCI and no washout periods or standardization of concomitant medications was undertaken. However, administration of multiple classes of treatments, such as a DMARD and a biologic, is common in patients with inadequate response to MTX monotherapy.8 This was also a single-group study and no cohort exists that was not receiving RCI in order to make a direct comparison.
Despite the limitations of this trial, RCI treatment in this group of patients with RA resulted in significant improvement. Swollen joint scores, tender joint scores, DAS28, and physician and patient VAS scores were all significantly reduced after 12 weeks. Two patients modified their concomitant medications during the follow-up period; one patient ceased taking the biologic due to development of nausea and headaches, while another patient removed MTX from the regimen due to fluctuation in lab values. Since patients with RA who are refractory to several treatments with differing MOAs are particularly difficult to treat, RCI may provide an effective treatment option for those patients based on the data herein. Larger clinical trials are required to further confirm the effectiveness of RCI treatment in patients with refractory RA. Additional trials are also necessary in order to determine the minimum effective dosing schedule for RCI administration in patients with refractory RA.
Acknowledgments
T Gillis, M Crane, C Hinkle, and N Wei received study funding from Autoimmune and Rare Disease Business Mallinckrodt Pharmaceuticals. The authors are grateful to AXON Communications for writing assistance in the development of this manuscript.
Interim findings related to the work presented here have been published as an abstract in “Abstracts Accepted for Publication” in the Annals of Rheumatic Diseases: http://ard.bmj.com/content/74/Suppl_2/1066.2.abstract (DOI: 10.1136/annrheumdis-2015-eular.2092).
Footnotes
Author contributions
All authors contributed to the study design, acquisition and interpretation of data, as well as revising and approving the content of the manuscript.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Sacks JJ, Luo YH, Helmick CG. Prevalence of specific types of arthritis and other rheumatic conditions in the ambulatory health care system in the United States, 2001–2005. Arthritis Care Res (Hoboken) 2010;62(4):460–464. doi: 10.1002/acr.20041. [DOI] [PubMed] [Google Scholar]
- 2.Davis JM, 3rd, Matteson EL, American College of Rheumatology, European League Against Rheumatism My treatment approach to rheumatoid arthritis. Mayo Clin Proc. 2012;87(7):659–673. doi: 10.1016/j.mayocp.2012.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Singh JA, Cameron DR. Summary of AHRQ’s comparative effectiveness review of drug therapy for rheumatoid arthritis (RA) in adults--an update. J Manag Care Pharm. 2012;18(4 Supp C):S1–18. doi: 10.18553/jmcp.2012.18.s4-c.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shoenfeld Y, Gerli R, Doria A, et al. Accelerated atherosclerosis in autoimmune rheumatic diseases. Circulation. 2005;112(21):3337–3347. doi: 10.1161/CIRCULATIONAHA.104.507996. [DOI] [PubMed] [Google Scholar]
- 5.Bluml S, Redlich K, Smolen JS. Mechanisms of tissue damage in arthritis. Semin Immunopathol. 2014;36(5):531–540. doi: 10.1007/s00281-014-0442-8. [DOI] [PubMed] [Google Scholar]
- 6.Guidelli GM, Barskova T, Brizi MG, et al. One year in review: novelties in the treatment of rheumatoid arthritis. Clin Exp Rheumatol. 2015;33(1):102–108. [PubMed] [Google Scholar]
- 7.Curtis JR, Singh JA. Use of biologics in rheumatoid arthritis: current and emerging paradigms of care. Clin Ther. 2011;33(6):679–707. doi: 10.1016/j.clinthera.2011.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Emery P, Sebba A, Huizinga TW. Biologic and oral disease-modifying antirheumatic drug monotherapy in rheumatoid arthritis. Ann Rheum Dis. 2013;72(12):1897–1904. doi: 10.1136/annrheumdis-2013-203485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Felson DT, Smolen JS, Wells G, et al. American College of Rheumatology/European League Against Rheumatism provisional definition of remission in rheumatoid arthritis for clinical trials. Arthritis Rheum. 2011;63(3):573–586. doi: 10.1002/art.30129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chikanza IC, Kozaci DL. Corticosteroid resistance in rheumatoid arthritis: molecular and cellular perspectives. Rheumatology (Oxford) 2004;43(11):1337–1345. doi: 10.1093/rheumatology/keh333. [DOI] [PubMed] [Google Scholar]
- 11.Ghaly NR, Kotb NA, Nagy HM, Rageh el SM. Toll-like receptor 9 in systemic lupus erythematosus, impact on glucocorticoid treatment. J Dermatolog Treat. 2013;24(6):411–417. doi: 10.3109/09546634.2012.697110. [DOI] [PubMed] [Google Scholar]
- 12.Seki M, Ushiyama C, Seta N, et al. Apoptosis of lymphocytes induced by glucocorticoids and relationship to therapeutic efficacy in patients with systemic lupus erythematosus. Arthritis Rheum. 1998;41(5):823–830. doi: 10.1002/1529-0131(199805)41:5<823::AID-ART8>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 13.Wallace DJ, Goldfinger D, Savage G, et al. Predictive value of clinical, laboratory, pathologic, and treatment variables in steroid/immunosup-pressive resistant lupus nephritis. J Clin Apher. 1988;4(1):30–34. doi: 10.1002/jca.2920040107. [DOI] [PubMed] [Google Scholar]
- 14.Fiehn C, Kessler S, Muller K. Morning stiffness of the joints is the sole predictor of short-term response to glucocorticoid treatment in active rheumatoid arthritis (RA) Rheumatol Int. 2012;32(12):4069–4070. doi: 10.1007/s00296-011-2075-8. [DOI] [PubMed] [Google Scholar]
- 15.Quax RA, Manenschijn L, Koper JW, et al. Glucocorticoid sensitivity in health and disease. Nat Rev Endocrinol. 2013;9(11):670–686. doi: 10.1038/nrendo.2013.183. [DOI] [PubMed] [Google Scholar]
- 16.Sliwinska-Stanczyk P, Pazdur J, Ziolkowska M, Jaworski J, Kaminska-Tchorzewska E, Lacki JK. The effect of methylprednisolone on proliferation of PBMCs obtained from steroid-sensitive and steroid-resistant rheumatoid arthritis patients. Scand J Rheumatol. 2007;36(3):167–171. doi: 10.1080/03009740601154111. [DOI] [PubMed] [Google Scholar]
- 17.van Schaardenburg D, Valkema R, Dijkmans BA, et al. Prednisone treatment of elderly-onset rheumatoid arthritis. Disease activity and bone mass in comparison with chloroquine treatment. Arthritis Rheum. 1995;38(3):334–342. doi: 10.1002/art.1780380307. [DOI] [PubMed] [Google Scholar]
- 18.Papagoras C, Voulgari PV, Drosos AA. Strategies after the failure of the first anti-tumor necrosis factor alpha agent in rheumatoid arthritis. Autoimmun Rev. 2010;9(8):574–582. doi: 10.1016/j.autrev.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 19.Rendas-Baum R, Wallenstein GV, Koncz T, et al. Evaluating the efficacy of sequential biologic therapies for rheumatoid arthritis patients with an inadequate response to tumor necrosis factor-alpha inhibitors. Arthritis Res Ther. 2011;13(1):R25. doi: 10.1186/ar3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Acthar HP. (R) Gel (repository corticotropin injection) [package insert] Hazelwood, MO: Mallinckrodt ARD Inc.; 2015. [Accessed November 19, 2015]. Available from: http://www.acthar.com/pdf/Acthar-PI.pdf. [Google Scholar]
- 21.Hench PS, Kendall EC, Slocumb CH, Polley HF. The effect of a hormone of the adrenal cortex (17-hydroxy-11-dehydrocorticosterone; compound E) and of pituitary adrenocorticotropic hormone on rheumatoid arthritis. Proc Staff Meet Mayo Clin. 1949;24(8):181–197. [PubMed] [Google Scholar]
- 22.Montero-Melendez T. ACTH: the forgotten therapy. Semin Immunol. 2015;27(3):216–226. doi: 10.1016/j.smim.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 23.Aletaha D, Neogi T, Silman AJ, et al. 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. 2010;62(9):2569–2581. doi: 10.1002/art.27584. [DOI] [PubMed] [Google Scholar]
- 24.Smolen JS, Aletaha D, Bijlsma JW, et al. Treating rheumatoid arthritis to target: recommendations of an international task force. Ann Rheum Dis. 2010;69(4):631–637. doi: 10.1136/ard.2009.123919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ailinger RL, Dear MR. Self-care agency in persons with rheumatoid arthritis. Arthritis Care Res. 1993;6(3):134–140. doi: 10.1002/art.1790060305. [DOI] [PubMed] [Google Scholar]
- 26.Hawker GA, Mian S, Kendzerska T, French M. Measures of adult pain: Visual Analog Scale for Pain (VAS Pain), Numeric Rating Scale for Pain (NRS Pain), McGill Pain Questionnaire (MPQ), Short-Form McGill Pain Questionnaire (SF-MPQ), Chronic Pain Grade Scale (CPGS), Short Form-36 Bodily Pain Scale (SF-36 BPS), and Measure of Intermittent and Constant Osteoarthritis Pain (ICOAP) Arthritis Care Res (Hoboken) 2011;63(S11):S240–S252. doi: 10.1002/acr.20543. [DOI] [PubMed] [Google Scholar]
- 27.Barton JL, Imboden J, Graf J, Glidden D, Yelin EH, Schillinger D. Patient-physician discordance in assessments of global disease severity in rheumatoid arthritis. Arthritis Care Res (Hoboken) 2010;62(6):857–864. doi: 10.1002/acr.20132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Keenan RT, Swearingen CJ, Yazici Y. Erythrocyte sedimentation rate and C-reactive protein levels are poorly correlated with clinical measures of disease activity in rheumatoid arthritis, systemic lupus erythematosus and osteoarthritis patients. Clin Exp Rheumatol. 2008;26(5):814–819. [PubMed] [Google Scholar]
- 29.Catania A, Gatti S, Colombo G, Lipton JM. Targeting melanocortin receptors as a novel strategy to control inflammation. Pharmacol Rev. 2004;56(1):1–29. doi: 10.1124/pr.56.1.1. [DOI] [PubMed] [Google Scholar]
- 30.Getting SJ. Targeting melanocortin receptors as potential novel therapeutics. Pharmacol Ther. 2006;111(1):1–15. doi: 10.1016/j.pharmthera.2005.06.022. [DOI] [PubMed] [Google Scholar]
- 31.Catania A. The melanocortin system in leukocyte biology. J Leukoc Biol. 2007;81(2):383–392. doi: 10.1189/jlb.0706426. [DOI] [PubMed] [Google Scholar]
- 32.Bohm M, Grassel S. Role of proopiomelanocortin-derived peptides and their receptors in the osteoarticular system: from basic to translational research. Endocr Rev. 2012;33(4):623–651. doi: 10.1210/er.2011-1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Patel HB, Bombardieri M, Sampaio AL, et al. Anti-inflammatory and antiosteoclastogenesis properties of endogenous melanocortin receptor type 3 in experimental arthritis. FASEB J. 2010;24(12):4835–4843. doi: 10.1096/fj.10-167759. [DOI] [PubMed] [Google Scholar]
- 34.Zhong Q, Sridhar S, Ruan L, et al. Multiple melanocortin receptors are expressed in bone cells. Bone. 2005;36(5):820–831. doi: 10.1016/j.bone.2005.01.020. [DOI] [PubMed] [Google Scholar]
- 35.Ericson MD, Lensing CJ, Fleming KA, Schlasner KN, Doering SR, Haskell-Luevano C. Bench-top to clinical therapies: a review of melanocortin ligands from 1954 to 2016. Biochim Biophys Acta. 2017 Mar 29; doi: 10.1016/j.bbadis.2017.03.020. Epub. [DOI] [PMC free article] [PubMed] [Google Scholar]