Abstract
Background
Vision-based speed of processing (VSOP) training can result in broad cognitive improvements in older adults with amnestic mild cognitive impairment (aMCI). What remains unknown, however, is what neurophysiological mechanisms account for the observed training effect. Much of the work in this area has focused on the central nervous system, neglecting the fact that the peripheral system can contributes to changes of the central nervous system and vice versa.
Objective
We examined the prospective relationship between an adaptive parasympathetic nervous system response to cognitive stimuli and VSOP training-induced plasticity.
Method
Twenty-one participants with aMCI (10 for VSOP training, and 11 for mental leisure activities (MLA) control) were enrolled. We assessed high-frequency heart rate variability (HF-HRV) during training sessions, and striatum-related neural networks and cognition at baseline and post-training.
Results
Compared to MLA, the VSOP group showed a significant U-shaped pattern of HF-HRV response during training, as well as decreases in connectivity strength between bilateral striatal and prefrontal regions. These two effects were associated with training-induced improvements in both the trained (attention and processing speed) and transferred (working memory) cognitive domains.
Conclusion
This work provides novel support for interactions between the central and the peripheral nervous systems in relation to cognitive training, and motivates further studies to elucidate the causality of the observed link.
Keywords: Parasympathetic nervous system, amnestic mild cognitive impairment, cognitive training, striatum, processing speed, working memory
Introduction
Growing use of cognitive training interventions [1-3] urges the understanding of the physiological mechanisms that account for the observed training effects. Work addressing this question has been largely limited to the central nervous system [3-5], neglecting the important role the peripheral nervous system plays in supporting cognitive function [6]. Here, we examine the potential predictive role of the parasympathetic nervous system (PNS) in training-induced plasticity for older adults with amnestic mild cognitive impairment (aMCI). aMCI, a symptomatic pre-Alzheimer's disease phase, constitutes a key target for interventions aimed at preventing or slowing cognitive decline [7]. Adults with aMCI have ample room for cognitive improvements, which also makes this population suitable for studying the mechanisms of cognitive training. We recently showed that vision-based speed of processing (VSOP) training, a cognitive training paradigm addressing processing speed and attention widely used in healthy older adults [8], can result in improvements across a broad range of cognitive domains in aMCI [3]. Here, working with the same cohort of subjects, we aimed to investigate the role of PNS in cognitive and neural improvements resulting from VSOP training [3].
A core set of brain regions, most prominently the striatum, is involved in links between cognitive and peripheral physiological regulation [6]. Flexible adaptation to environmental stimuli is supported by cognitive and peripheral functions (e.g., heart rate variability) that are regulated by PNS [6]. Cognitive functions and PNS are also bi-directionally linked. Direct PNS stimulation can alone be sufficient for cognitive improvements through the release of cholinergic transmitters [9]. Conversely, PNS response to cognitive stimuli can lead to large-scale brain changes, strengthening the neural efficiency of information processing by regulating the dopamine production in the striatum [10].
Considering these lines of evidence together, we conjectured that PNS response to cognitive stimuli might underpin VSOP training-induced plasticity. Given the link between cognitive and peripheral functions [6], individuals who are more attentive to the cognitive training may have more dynamic PNS responses in terms of adjustment to the cognitive stimuli, followed by adaptation. As an initial step in elucidating this hypothesized link, we examine the prospective relationship between an adaptive PNS response to cognitive stimuli and VSOP training-induced plasticity. First, we focused on high-frequency heart rate variability (HF-HRV), a common index for PNS [6], comparing HF-HRV in response to stimuli during VSOP training vs. control training (mental leisure activities, MLA) [3]. We hypothesized that VSOP training would be associated with a more adaptive HF-HRV pattern during the training. The intuition is that VSOP training, compared to MLA, affords a more varying and stimulating environment. As such, VSOP training requires greater adjustment and adaptation efforts, including speed of processing and attention demands, resulting in greater engagement by PNS [11; 12]. Moreover, given that above described evidence that PNS activation can predict cognitive improvement, we hypothesize that individual differences in HF-HRV dynamics would correlate with improvements in trained and untrained cognitive domains. Second, emerging literature suggests that, in addition to its links with PNS, the striatum plays a critical role in inducing broad cognitive training effects [13]. Hence, we also examined the role of the striatum, hypothesizing that VSOP training would lead to more beneficial changes in striatum connectivity compared to control, and greater striatum connectivity changes would relate to larger improvements in trained and untrained cognitive domains. Specifically, we tested the hypothesis that more flexible adjustment and adaptation of HF-HRV would be associated with greater changes in striatum connectivity.
Materials and Methods
Design
Participants with aMCI were recruited from the university affiliated memory clinics using the clinical diagnosis of “mild cognitive impairment due to Alzheimer's disease” [7]. All participants had deficits in memory and executive function based on a comprehensive neuropsychological battery but intact basic activities of daily living, and absence of dementia using NINCDS-ADRDA criteria per assessments. If an individual was on Alzheimer's disease medication (i.e., memantine or cholinesterase inhibitors), it was required to have no changes in dosage in the 3 months prior to recruitment. Participants needed to have capacity to give consent based on clinician assessment and adequate visual acuity for testing, as well as be ≥60 years of age, English-speaking, and community-dwelling. We excluded individuals who had active participation in another cognitive intervention study or active treatment with antidepressants or anxiolytics. Twenty-four participants were enrolled and randomly assigned to the VSOP or MLA group after informed consent and baseline assessment, and three participants withdrew from the study due to reasons irrelevant to the present study. A total of 10 participants from VSOP group (age: 72 ± 8, 5 male, Montreal Cognitive Assessment (MOCA): 24.44 ± 2.60) and 11 from MLA group (age: 73 ± 10, 6 male, MOCA: 25.63 ± 1.63) completed the study. The study was approved by the university's Research Subject Review Board.
VSOP training included five computerized attention tasks with visual stimuli (Eye for Detail, Peripheral Challenge, Visual Sweeps, Double Decision, and Target Tracker) [4]. In the Eye for Detail task, a series of stimuli (e.g., butterflies) were briefly presented at the same time. Participants needed to identify a number of stimuli that were identical to each other. As the difficulty level increased, the stimuli became more similar to each other. In the Peripheral Challenge task, a number of birds were briefly presented in peripheral vision, including a target bird that was different from other distracter birds. The participants were asked to point out the location of the target bird. As the difficulty level increased, the target and distractor birds became more similar. In the Visual Sweeps task, two sweep patterns were presented simultaneously, and the participants indicated whether the sweeps were moving IN or OUT. In the Double Decision task, a target vehicle was presented in the center of the screen and a road sign was presented in the periphery. Participants needed to determine both the type of vehicle and the location of the road sign. With increases in difficulty level, the vehicles became more similar, and distracters were added. In the Target Tracker task, a number of target jewels were presented on the screen first, and then a number of identical distracter jewels were presented. All of the target and distracter jewels then moved in a Brownian motion fashion for a short period. Upon the pause of the movement, participants needed to pick the target jewels. As task difficulty increased, participants were required to simultaneously track more target jewels and the distracters would become more similar to the targets. Across tasks, participants identified the object and/or location of the object on the screen. To ensure the participants to always operate near their optimal capacity, the training would automatically adjust the task difficulty and speed, and switch the tasks based on the participant's performance. MLA control included three computerized vision-based activities (crossword, Sudoku, and solitaire) to control for computer experience and amount of time, and to stimulate participants' everyday mental activities. Training in each group lasted for 6 weeks.
Cognitive testing and resting-state imaging data were assessed at baseline and post-training at week 7. Cognitive testing included measurements of the Useful Field of View (UFOV) (trained effect) and working memory (transferred effect). We focused on these two domains because these were the tasks for which we observed significant VSOP training-induced improvements [3]. Examining group (VSOP vs. MLA) by time (baseline vs. 7 week) interaction, VSOP group improved significantly in UFOV (F1,19 = 6.61, partial η2 = 0.26, p = .02) and working memory (F1,19 = 7.33, partial η2 = 0.28, p = .01) compared to MLA group. Here, we asked if these training-induced improvements could be linked with HF-HRV. UFOV is a measure for processing speed and attention (Visual Awareness Research Group, Inc.), which are the primary domains targeted in the VSOP training, consisting of three subtests to detect, identify, and localize briefly presented targets in the center (subtest 1), in both the center and periphery (subtest 2), and in both the center and periphery with embedded distractors (subtest 3). UFOV is conceptually similar to the Double Decision Task in the VSOP training but uses different tasks and stimuli from the training paradigms. Working memory was assessed using two tasks – dot counting (requires participants to count a series of slides with various numbers of dots and remember the sequence of the number) and 1-back tasks (participants need to determine if the location of the object matches the previously shown location) from the EXAMINER package [14]. Different stimuli were used for the two tasks between baseline and week 7 to avoid practice effect. Changes in UFOV and working memory from baseline to 7 weeks were calculated in the analysis with higher change values indicating more positive changes.
Resting-state neuroimaging data was collected by acquiring two 5-minute BOLD functional scan with a gradient echo-planar imaging sequence (TR = 2000ms, TE = 30ms, 4mm3 resolution, 30 axial slices). A 2D axial fast Gradient-Recalled Echo pulse sequence was used to generate field maps, which was used to correct for field inhomogeneity distortions in echo-planar imaging sequences. Two 5-min BOLD functional scans were acquired for each assessment period, using a gradient echo-planar imaging sequence (TR = 2s, TE = 30ms, 4mm3 resolution, 30 axial slices). Participants were instructed to relax with their eyes open without falling asleep. Resting-state neuroimaging data preprocessing consisted of motion correction, slice-timing correction, non-brain signal removal and Gaussian spatial smoothing (5mm FWHM). Nuisance parameters (global, white matter and cerebrospinal fluid signals, motion) were removed through linear regression. Non-neuronal contributions were reduced with temporal filtering (0.01–0.08 Hz). Striatum-related networks analysis: Due to the eligibility of MRI safety and participants' willingness, only seven VSOP and four MLA participants completed all scans. The striatum-related network analysis was conducted in the following steps: first, bilateral striata were chosen as seeds according to Automated Anatomical Labeling template to calculate connectivity with voxels of the whole brain at baseline and post-training, respectively. Second, one sample t-test was applied to show the functional connectivity map of striatum in baseline with P < 0.05 (False discovery rate, FDR-correction) and voxel size > 50. In relation to the left striatum, three prefrontal regions were identified, including left inferior frontal gyrus (-30, 42, 0), right middle frontal lobe (30, 30, 24), and left superior frontal gyrus (-3, 30, 54). In relation to the right striatum, three regions were found, including right inferior frontal gyrus (33, 39, -6), right superior frontal gyrus (9, 30, 54), and left superior frontal gyrus (-21, 33, 27). Training-induced changes in functional connectivity were examined using paired t-tests with a threshold of individual P < 0.01, cluster size > 1755 mm3, corresponding to corrected P < 0.05. The correction was performed within the whole brain grey matter mask and determined with Monte Carlo simulations using the AFNI AlphaSim program. The analysis generated bilateral striatum-prefrontal networks. Previous studies consistently identified abnormally increased connectivity strength between striatum and prefrontal regions in the early stage of neurodegenerative process that are possibly due to the dopamine transmission deficiency [15; 16]; therefore, positive striatal changes in the present study refer to training-induced reductions in the striatum-prefrontal network.
Electrocardiography (ECG) was assessed at two in-lab training sessions during the 2nd and 3rd week of the training. The time point was chosen to balance the adequate understanding of the training procedures and novelty of the training content. That is, we aimed to capture HRV after subjects became reasonably familiar with the training tasks, but before task-specific expertise begins to accumulate. We consider this a useful point for understanding the role of PNS in predicting cognitive and neural gains from the training. ECG data were collected continuously, using a standard lead-II electrode configuration, at 1000 Hz with a BioNex Mainframe with an ECG module (MindWare®, LTD). HF-HRV was derived by spectral analysis of the interbeat interval collected from the ECG using Mindware software (MindWare®, LTD), obtaining total variance within the respiratory range (0.15–0.5 Hz). HF-HRV values were derived over twenty second sampling intervals, and the average HF-HRV was aggregated over the last minute of baseline and across each minute of each 60-minute cognitive training session. Finally, the results from the two training sessions were averaged. These aggregate, minute-by-minute HF-HRV values were log-transformed and used for analysis.
To examine the group difference in HF-HRV, the mixed-effects model was used to capture the quadric time structure: YHF-HRV = aXTime2 + bXTime + c + group(aXTime2 + bXTime + c) + ε where parameters (a, b, c) are modeled as random-effects at individual (i.e., within-time) and group levels. The quadratic term (a) represents how fast HF-HRV raised or dropped; the linear term (b) represents the minimum HF-HRV can reach; and the constant (c) represents the initial level of HF-HRV. A quadratic model is appropriate because it can capture an effective brain-regulated HF-HRV process that is indexed by flexible and dynamic withdrawal and followed by restoration of parasympathetic control with changing environmental demands [11; 12].
Results
HF-HRV Responses
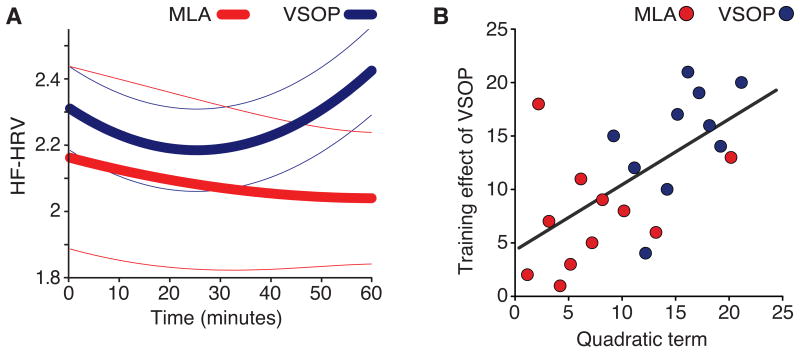
In terms of model fit, the results for the VSOP group revealed a dynamic U-shaped HF-HRV response pattern, which was well fitted with a quadratic model (all model terms significant; P ≤ .019). In contrast, for the MLA group, only the constant was significant (P < .001; Fig. 1A). In terms of group difference, the quadratic term, representing how “U-shaped” HF-HRV is, significantly differed between VSOP and MLA groups (t12 = 3.11, FDR-corrected P = .027; no difference in the linear term, P = .08, nor the constant, P = .63, all tests two-tailed; Fig. 1B). Additionally, to further examine differences related to the U-shaped HF-HRV response, we compared the raw HF-HRV data between groups for two time points: in the middle (30th minute:) and the end (60th minute). A significant group difference was only observed at the end point, indicating a lack of the HF-HRV rebound for the MLA group (bootstrap resampling with replacement (n=1000) to modify the variance; 30th minute, mean difference = 0.29, 95%CI: -0.24, 0.78; 60th minute, mean difference = 0.62, 95%CI: 0.17, 1.05).
Figure 1.
Effects of VSOP training on HF-HRV. (A) Modeling HF-HRV over training session using the quadratic model: YHF-HRV=aXtime2 + bXtime + c. Bold lines show the group average. Thin lines are SEM. (B) A comparison of model terms (a, b & c). The key group difference was in the quadratic term. ** p< .01; § p< .06).
In our earlier publication [3], we showed that VSOP training yielded improvements in both trained (attention and processing speed, as measured by UFOV) and transferred (working memory) cognitive domains. Here, we aimed to determine if the observed improvements are related to HF-HRV. Supporting the importance of the U-shaped HF-HRV response pattern, individual variation in the quadratic term was correlated with training-induced cognitive improvement, both in the trained domain (UFOV) and the transfer domain (working memory). Across all participants, individual variation in the quadratic term correlated with changes in UFOV (r = .39, 95%CI: 01, .70) and working memory (r = .33, 95%CI: .06, .64). When only considering data from the VSOP group, the correlation with UFOV remained significant (r = .61, 95%CI: .10, .94), while the link with improvement in working memory was not significant (r = .06, 95%CI: -.72, -.53). No significant correlations were found for the MLA control group (all |r| < 0.04). Taken together, our results reveal a consistent link between HF-HRV and training-induced improvements in UFOV—a task that has similar task demands as the VSOP intervention. The link between HF-HRV and working memory, however, is only present when considering the entire sample.
Striatum-prefrontal Network
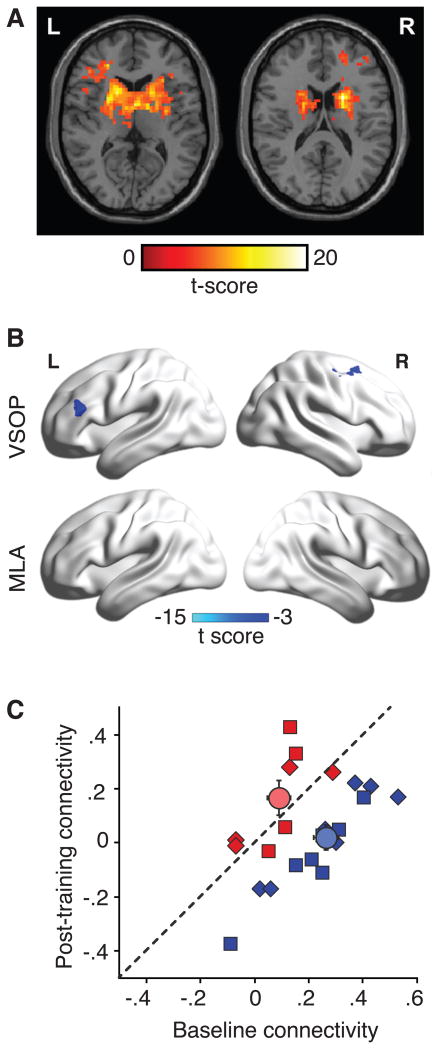
Seed-based analysis generated bilateral striatum-prefrontal networks (Fig. 2A, no group differences in baseline connectivity, P > .10). These striatum-prefrontal networks were differentially affected by VSOP and MLA training (Fig. 2B and C; time (baseline and post-training) and group (VSOP and MLA) interaction: F1,20 = 67.19, P < .001, Partial η2 = 0.77). This was also the case when each striatum-prefrontal network was analyzed separately (both left and right stratum: P < .001; Fig. 2C). Namely, we found decreased striatum-prefrontal connectivity as a result of VSOP training.
Figure 2.
Effects of VSOP training on bilateral striatum-prefrontal networks. (A) Bilateral striatum-prefrontal networks at baseline: L = left, R = right. (B) Training-induced changes in striatum-prefrontal networks for the VSOP group: L, left striatum-left inferior prefrontal gyrus (-45, 27, 21); R, right striatum-right middle frontal gyrus (42, 6, 60). No changes were found for the MLA group. (C) Changes in bilateral stratum-prefrontal connectivity from baseline to post-training. Squares show left striatum-prefrontal network. Triangles show right striatum-prefrontal network. Circles show group averages. Error bars are SEM.
We calculated changes in striatum-prefrontal connectivity from baseline to 7 week such that higher values indicated greater improvement after training. Changes in bilateral striatum-prefrontal networks were both significantly correlated to the changes in UFOV (Left: r = .35, 5%CI: .05, .64; Right: r = .55, 95%CI: .10, .88) and changes in working memory (Left: r = .41, 95%CI: .22, .93; Right: r = .55, 95%CI: .40, .92). Finally, changes in the striatum-prefrontal networks were correlated with the quadratic term of HF-HRV responses (Left: r = .41, 95%CI: .19, .91; Right: r = .55, 95%CI: .39, .93). Because of a smaller sample size for the neuroimaging analysis, we were not able to analyze data for each group separately.
Discussion
Our results provide neurophysiological evidence that flexible PNS adaptation to cognitive training stimuli is associated with cognitive and neural improvements following VSOP training. This includes significant group differences in both HF-HRV and striatum-prefrontal connectivity, as well as significant correlational links between HF-HRV and both training-induced changes in UFOV and striatum-prefrontal connectivity. Elucidating neurophysiological changes induced by VSOP training and its functional consequences is critical for a full understanding of the mechanisms that account for the observed training effects.
Why do we observe a link between HF-HRV and the effects of cognitive training? One possibility is that the nature of VSOP training, namely its attentional and processing demands, is well suited for stimulating PNS [11; 12]. This can explain both the group difference in HF-HRV and the correlation between HF-HRV and both the direct and transfer cognitive effects of training. For the correlations, the assumption is that those who are more attentive to the training would have both more dynamic HF-HRV responses and better training outcomes. This is further supported by a relatively strong correlation between HF-HRV and the direct training effect in VSOP group. In our opinion, it is very likely that the inherently engaging nature of VSOP training partially accounts for the observed results. Even if this fully accounts for the data reported here, identifying easily measured, peripheral markers, like HF-HRV, that can index effective cognitive training is itself a worthwhile endeavor.
Existing evidence also supports the rationale for the PNS as a mechanistic contributor to effects of VSOP training. In response to environmental stimuli such as those seen in VSOP tasks, the PNS carries information about viscerosensory states to the brain; concurrently, the PNS promotes ongoing adaptation, indexed by flexible withdrawal and restoration of PNS. Here, we showed that PNS response to VSOP training was characterized by a U-shaped HF-HRV response pattern that can be divided into two phases: phasic HRV suppression and enhancement. During the first suppression phase, a flexible withdrawal of HF-HRV occurred in response to the training stimuli. Such suppression is often seen in performing difficult cognitive tasks [17]. The second enhancement phase occurred with adaptation to the task, evident as a rebound of HF-HRV. Importantly, such a U-shaped HF-HRV response was also associated with greater trained cognitive effects (as measured by UFOV) and stronger transferred effects (as measured by changes in working memory). Our speculation is that this link might be explained by the fact that the PNS can directly lead to improvements in cognitive processes by PNS-activated release of neurohormones (e.g., noradrenalin, 5-hydroxytryptamine) [9]. The results of this study are consistent with an established link between PNS function and striatum, both at rest and in response to tasks [6] and add PNS to the examination of the known relationship between striatum and training effect on working memory [13]. The relationships observed here among HF-HRV, striatum connectivity, and cognitive change suggest that cognitive training studies scrutinizing the coordination between PNS and striatum-prefrontal network would be worthwhile.
Several limitations of this study should be acknowledged. The primary limitation is the small sample size. While we did find consistent converging evidence that points toward a link between HF-HRV and cognitive training, our sample size limits our ability to examine more specific aspects of that link. Notably, it is uncertain whether this link is limited to direct target domains of cognitive training or whether it generalizes to untrained transferred domains, such as working memory. As discussed above, PNS may primarily respond to attentionally demanding stimuli that are present in both VSOP training and the UFOV test, while its involvement in other cognitive domains, such as working memory, may be indirect and weaker, or even not present at all. If this is indeed the case, the detection of PNS' role in those cognitive domains will require a larger sample size. Moreover, to further elucidate PNS' relationships with different cognitive domains, it will be helpful to include training groups focusing on other aspects of cognition (e.g., working memory training). This may help clarify whether the role of PNS in cognitive training is specific to attentionally demanding training paradigms and tasks (e.g., VSOP training and UFOV) or whether PNS' predictive response depends on the type of cognitive stimuli being involved in training. Finally, we note that we only measured PNS' performance at the beginning of the training. Thus, we do not know whether and how PNS response changes over the duration of training. Whether training can causally modify PNS responses is an interesting future research direction that might help determine the exact role of PNS in cognitive training.
Conclusion
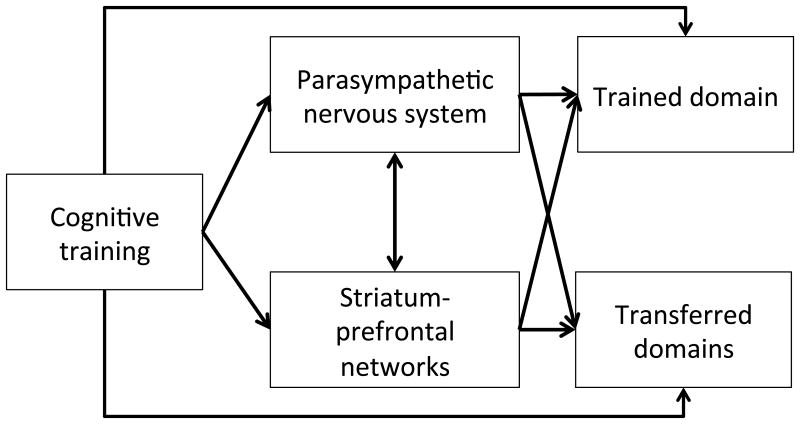
Taken together, our work suggests that attending to PNS function may shed light on the influence of cognitive training on both the central and peripheral nervous systems and provide new ways of evaluating the effectiveness of a cognitive training. As a potential starting point for new research, we provide a schematic diagram summarizing both established and hypothesized interactions among cognitive training and relevant brain systems, as discussed above (Fig. 3). A validation study with large sample size will be needed for further elucidate the precise role of PNS function in both healthy subjects and aMCI. Namely, a key question will be to determine whether and to what extent PNS is as a mechanism or a correlate of neurobiological effects of cognitive training.
Figure 3.
Schematic diagram of proposed relationships among cognitive training, trained/transferred domains, PNS and striatum related neural networks. Our results are consistent with a hypothesis that the effects of cognitive training, especially involving the trained domain, are enhanced through recruitment of PNS mechanisms and striatum-prefrontal networks. Whether and to what degree this generalizes to transferred domains is a question for future research.
Acknowledgments
The study was supported by the University of Rochester CTSA award No. KL2 TR000095 from the National Center for Advancing Translational Sciences of the National Institutes of Health to F.L. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The manuscript preparation was also funded by the Alzheimer's Association New Investigator Grant (NIRG-14-317353) and NIH R01 grant (NR015452) to F.L.
Footnotes
Conflict of interest: The authors report no declarations of interest. The authors claim no relationship with BrainHQ inc. which is the software developer of the VSOP training.
References
- 1.Anguera JA, Boccanfuso J, Rintoul JL, Al-Hashimi O, Faraji F, Janowich J, et al. Video game training enhances cognitive control in older adults. Nature. 2013;501(7465):97–101. doi: 10.1038/nature12486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bavelier D, Green CS, Pouget A, Schrater P. Brain plasticity through the life span: learning to learn and action video games. Annual review of neuroscience. 2012;35:391–416. doi: 10.1146/annurev-neuro-060909-152832. [DOI] [PubMed] [Google Scholar]
- 3.Lin F, Heffner KL, Ren P, Tivarus ME, Brasch J, Chen DG, et al. Cognitive and Neural Effects of Vision-Based Speed-of-Processing Training in Older Adults with Amnestic Mild Cognitive Impairment: A Pilot Study. J Am Geriatr Soc. 2016;64(6):1293–8. doi: 10.1111/jgs.14132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.O'Brien JL, Edwards JD, Maxfield ND, Peronto CL, Williams VA, Lister JJ. Cognitive training and selective attention in the aging brain: an electrophysiological study. Clin Neurophysiol. 2013;124(11):2198–208. doi: 10.1016/j.clinph.2013.05.012. [DOI] [PubMed] [Google Scholar]
- 5.Belleville S, Clement F, Mellah S, Gilbert B, Fontaine F, Gauthier S. Training-related brain plasticity in subjects at risk of developing Alzheimer's disease. Brain. 2011;134(Pt 6):1623–34. doi: 10.1093/brain/awr037. [DOI] [PubMed] [Google Scholar]
- 6.Thayer JF, Ahs F, Fredrikson M, Sollers JJ, 3rd, Wager TD. A meta-analysis of heart rate variability and neuroimaging studies: implications for heart rate variability as a marker of stress and health. Neurosci Biobehav Rev. 2012;36(2):747–56. doi: 10.1016/j.neubiorev.2011.11.009. [DOI] [PubMed] [Google Scholar]
- 7.Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, et al. The diagnosis of mild cognitive impairment due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7(3):270–9. doi: 10.1016/j.jalz.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rebok GW, Ball K, Guey LT, Jones RN, Kim HY, King JW, et al. Ten-Year Effects of the Advanced Cognitive Training for Independent and Vital Elderly Cognitive Training Trial on Cognition and Everyday Functioning in Older Adults. J Am Geriatr Soc. 2014 doi: 10.1111/jgs.12607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clark KB, Naritoku DK, Smith DC, Browning RA, Jensen RA. Enhanced recognition memory following vagus nerve stimulation in human subjects. Nat Neurosci. 1999;2(1):94–8. doi: 10.1038/4600. [DOI] [PubMed] [Google Scholar]
- 10.Surowka AD, Krygowska-Wajs A, Ziomber A, Thor P, Chrobak AA, Szczerbowska-Boruchowska M. Peripheral vagus nerve stimulation significantly affects lipid composition and protein secondary structure within dopamine-related brain regions in rats. Neuromolecular Med. 2015;17(2):178–91. doi: 10.1007/s12017-015-8349-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Porges SW. Orienting in a defensive world: Mammalian modifications of our evolutionary heritage: A Polyvagal Theory. Psychophysiology. 1995;32(4):301–18. doi: 10.1111/j.1469-8986.1995.tb01213.x. [DOI] [PubMed] [Google Scholar]
- 12.Porges SW. The Polyvagal Theory: Phylogenetic contributions to social behavior. Physiol Behav. 2003;79(3):503–13. doi: 10.1016/s0031-9384(03)00156-2. [DOI] [PubMed] [Google Scholar]
- 13.Dahlin E, Neely AS, Larsson A, Backman L, Nyberg L. Transfer of learning after updating training mediated by the striatum. Science. 2008;320(5882):1510–2. doi: 10.1126/science.1155466. [DOI] [PubMed] [Google Scholar]
- 14.Possin KL, Feigenbaum D, Rankin KP, Smith GE, Boxer AL, Wood K, et al. Dissociable executive functions in behavioral variant frontotemporal and Alzheimer dementias. Neurology. 2013 doi: 10.1212/WNL.0b013e318296e940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Supekar K, Menon V, Rubin D, Musen M, Greicius MD. Network analysis of intrinsic functional brain connectivity in Alzheimer's disease. PLoS computational biology. 2008;4(6):e1000100. doi: 10.1371/journal.pcbi.1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sanz-Arigita EJ, Schoonheim MM, Damoiseaux JS, Rombouts SA, Maris E, Barkhof F, et al. Loss of ‘small-world’ networks in Alzheimer's disease: graph analysis of FMRI resting-state functional connectivity. PLoS One. 2010;5(11):e13788. doi: 10.1371/journal.pone.0013788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weber CS, Thayer JF, Rudat M, Wirtz PH, Zimmermann-Viehoff F, Thomas A, et al. Low vagal tone is associated with impaired post stress recovery of cardiovascular, endocrine, and immune markers. European journal of applied physiology. 2010;109(2):201–11. doi: 10.1007/s00421-009-1341-x. [DOI] [PubMed] [Google Scholar]