Abstract
Microparticles (MPs) are small membrane vesicles that are classified into subcategories based on their origin, such as platelet-derived MPs (PMPs), endothelial MPs (EMPs), red blood cell MPs (RMPs) and tissue factor MPs (TF + MPs). Philadelphia chromosome-negative myeloproliferative neoplasms (Ph−MPN) are disorders characterized by abnormal haematopoiesis, thrombosis and the JAK2V617F mutation. MPs are biomarkers for procoagulant state in cancer patients, but their relevance in patients with Ph−MPN was unclear. The present study aimed to measure MP variation in MPN patients and evaluate association with the JAK2V617F mutation and with thrombosis and splenomegaly. In total, 92 patients with MPN were enrolled in the present study, including 60 with essential thrombocythaemia (ET), 20 with polycythaemia vera (PV), and 12 with primary myelofibrosis (PMF). RMPs, PMPs, TF + MPs and EMPs were measured by flow cytometry. The levels of RMPs, PMPs, EMPs and TF + MPs in patients with Ph−MPN were all found to be significantly increased compared with controls (P<0.05). Additionally, the levels of all four types of MPs in the PMF group were significantly increased compared with the PV group (P<0.05), and the level of RMPs in the PMF group was significantly increased compared with the ET group (P<0.05). MP levels were increased in the Ph−MPN patients with thrombosis compared with patients without thrombosis (P<0.05). MP levels were increased in Ph−MPN patients with splenomegaly compared with patients without splenomegaly (P<0.05). The level of PMPs in patients with the JAK2V617F mutation was increased compared with patients without the mutation (P<0.05). In conclusion, the present study showed that MPs are associated with Ph−MPN pathogenesis, and may promote thrombosis.
Keywords: microparticles, myeloproliferative neoplasms, thrombosis, splenomegaly, JAK2V617F mutation
Introduction
Myeloproliferative neoplasms (MPNs) are cancers that originate from hematopoietic stem cells and are characterized by proliferation of myeloid cells. The classic Philadelphia chromosome-negative MPNs (Ph−MPNs) include polycythaemia vera (PV), essential thrombocythaemia (ET) and primary myelofibrosis (PMF) (1). MPN patients usually exhibit elevated blood cells and splenomegaly. The JAK2V617F mutation is a diagnostic marker for MPN and it also plays an important role in patient treatment since inhibition of JAK2-associated proliferative pathways has the potential to inhibit cell proliferation in MPNs (2). This mutation is present in ~95% of PV patients and 50% of ET or PMF patients (3,4). Thrombosis and haemostasis are major complications that affect the life expectancy of patients with MPN. The incidence of thrombosis ranges between 12 and 39% in PV patients, ranges between 11 and 25% in ET patients (5), and is ~9.5% in PMF patients (6). Several studies have shown that JAK2V617F+ patients experience a significantly increased frequency of thrombosis complications compared with JAK2V617F− patients, and that the thrombosis complications are associated with the JAK2V617F+ mutant allele burden (7,8).
Circulating microparticles (MPs) are small membrane vesicles that are shed from activated and/or apoptotic cells, such as platelets, endothelial cells and red blood cells. MPs express specific antigens that are present on the surface of their mother cells. MPs are considered to be biomarkers indicating the procoagulant state associated with a host of clinical diseases, including cancer, cardiovascular disease, sepsis and diabetes. Numerous studies have shown that the levels of circulating MPs are elevated in cancer patients, and that MPs contribute to the development of thrombosis-associated complications (9–11). MPs trigger blood coagulation and are expressed by numerous types of cancer cells (8). It has been suggested that the majority of MPs in cancer patients are derived from tumour cells (12,13). At present, the effect of the Ph− state on MPs has not yet been fully elucidated. Therefore, the levels of MPs originating from platelets (PMPs), red blood cell (RMPs), endothelial cells (EMPs) and tissue factor-positive MPs (TF + MPs) were measured in patients with PV, ET and PMF.
Materials and methods
Patients
Characteristics of the MPN patients included in the present study are listed in Table I. A total of 92 patients with MPN who were treated at the First Affiliated Hospital of Soochow University were enrolled. Patients with Ph−MPN were diagnosed according to the 2008 World Health Organization (WHO) criteria. The present study included 60 patients with ET, 20 with PV and 12 with PMF. The MPN patients were divided into two groups, a JAK2V617F mutation-positive group (n=55) and a JAK2V617F mutation-negative group (n=37). Healthy volunteers (n=30), with no history of thrombosis or cancer and no drug use over the previous 2 weeks, were used as age- and sex-matched controls. The present study was approved by the First Affiliated Hospital of Soochow University Ethical Committee (Suzhou, China) and patient consent was also obtained.
Table I.
Clinical characteristics of patients with myeloprolife-rative neoplasms.
| Characteristics | ET | PV | PMF |
|---|---|---|---|
| Cases, n | 60 | 20 | 12 |
| Sex, n | |||
| Male | 26 | 8 | 6 |
| Female | 34 | 12 | 6 |
| Median age, years | 50 | 61 | 58 |
| Median WBC, ×109/1 | 10 | 12 | 12 |
| Median Hb, g/l | 128 | 186 | 74 |
| Median PLT, ×109/1 | 667 | 358 | 116 |
| Thrombosis, n | |||
| Yes | 16 | 5 | 2 |
| No | 44 | 15 | 10 |
| Splenomegaly, n | |||
| Yes | 30 | 10 | 10 |
| No | 30 | 10 | 2 |
| JAK2V617F mutation, n | |||
| Yes | 32 | 17 | 6 |
| No | 28 | 3 | 6 |
WBC, white blood cell count; Hb, haemoglobin; PLT, platelet count; ET, essential thrombocythaemia; PV, polycythaemia vera; PMF, primary myelofibrosis.
Materials
Monoclonal CD235a-phycoerythrin (PE) (cat. no. A07792), CD61-PE (cat. no. IM3605), CD142-PE (cat. no. 550312) and CD62E-PE (cat. no. IM1243 U) antibodies (all dilutions, 1:100; Beckman Coulter, Inc., Brea, CA, USA) were used for the detection of RMPs, PMPs, EMPs, and TF + MPs. Flow-Count™ fluorescent microspheres (diameter, 10 µm; density, 992/µl; Beckman Coulter) were used for the calibration of the flow cytometry instrument, and the Flow Cytometer FC500 instrument was obtained from Beckman Coulter.
Sample collection and preparation
Samples of peripheral venous blood (3 ml) were collected from patients with MPN and healthy control individuals into 3.2% sodium citrate tubes (containing 300 nM PGE1). Platelet-poor plasma (PPP) was obtained by centrifugation at 1,900 × g twice for 15 min and then stored at −80°C until use. Samples were processed within 2 h of collection.
Detection of MPs by flow cytometry
After thawing, PPP was diluted in PBS (dilution, 1:10), with 5 µl of the aforementioned monoclonal CD235a-PE, CD61-PE, CD142-PE and CD62E-PE antibodies. Samples were incubated with the antibodies for 30 min in the dark at room temperature. Subsequently, 10 µl Flow-Count fluorescent microspheres was added to all tubes as a calibration for the calculation of the absolute concentration of MPs in PPP. MPs were resuspended and mixed in PBS prior to loading for flow cytometry. Rat anti-human IgG-PE (dilution, 1:100; cat. no. PA129628; Thermo Fisher Scientific, Inc., Waltham, MA, USA) was used as a negative control. Forward scatter was calibrated using fluorescent microspheres of 0.8 µm. Standard fluorescent microbeads (0–0.8 µm) in diameter were used to set the MP gate. The flow cytometer analysed 20,000 particles, and the number of MPs and fluorescent microspheres were presented in the upper right quadrant of the flow cytometric graph. All flow cytometry data were analysed using BD CellQuest Pro 5.1 software (BD Biosciences, San Jose, CA, USA). MP analyses were performed using the flow cytometer as previously described (14). The absolute number of MPs was calculated using the following formula: (992x number of MPs)/(4x number of Flow-Count fluorescent microspheres).
JAK2V617F mutation detection
Total genomic DNA was extracted from EDTA-anticoagulated peripheral blood samples using the UNlQ-10 Column Clinical Sample DNA Isolation kit (cat. no. B511341; Sangon Biotech Co., Ltd., Shanghai, China) according to the manufacturer's protocol. The JAK2V617F mutation was detected using allele-specific PCR, as previously described (15). A sample of 80 ng of each patient's DNA was PCR amplified in the ABI 9600 machine (Applied Biosystems, Foster City, CA) using the specific forward primer (0.5 µmol/l), 5′AGCATTTGGTTTTAAATTATGGAGTATATT 3′, the internal control primer (0.5 µmol/l), 5′ATCTATAGTCATGCTGAAAGTAGGAGAAAG3′ and the reverse primer (1 µmol/l) 5′CTGAATAGTCCTACAGTGTTTTCAGTTTCA3′. The thermocycler parameters were 36 cycles of 30 sec at 94°C, 58°C for 30 sec and 72°C for 45 sec Subsequently, LDR products were analyzed with a DNA sequencer (model 377; Applied Biosystems; Thermo Fisher Scientific, Inc.). All assays were conducted without the knowledge of case or control status.
Statistical analysis
Statistical analysis was performed using SPSS 17.0 software (SPSS, Inc., Chicago, IL, USA). Data were expressed as the mean ± standard deviation. Two-tailed unpaired Student's t-tests were used for comparison between two groups. One-way analysis of variance with Dunnett's multiple comparisons test was used to compare the differences amongst groups (n≥3). Spearman's rank correlation coefficient was used for correlation analysis. P<0.05 was considered to indicate a statistically significant difference.
Results
Alteration of MP level in patients with MPN
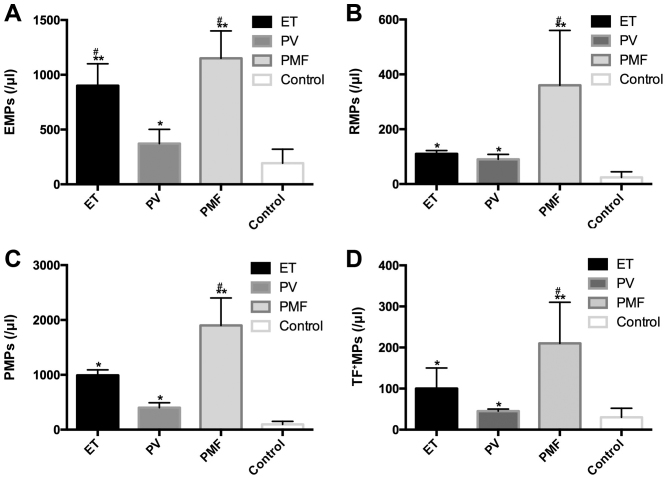
The abundance of the four types of MPs was increased in patients with Ph−MPN compared with healthy control individuals [RMPs (24.4±20.2/µl), PMPs (95.2±55.8/µl) and EMPs (193.1±127.1/µl), all P<0.01; TF + MPs (193.1±127.1/µl), P<0.05]. It was also found that the abundance of the four types of MPs in patients with PMF was increased compared with patients with PV (P<0.05). No evident difference was identified between the MP levels in PV and ET groups, with the exception of EMPs (Fig. 1).
Figure 1.
Comparison of MP abundance in patients with different subtypes of Ph−MPN. The level of all four types of MPs was increased in patients with MPN compared with the control group. (A) EMP abundance in patients with ET and PMF was increased compared with patients with PV. (B) RMP abundance in patients with PMF was increased compared with patients with PV. (C) PMP abundance in patients with PMF was increased compared with patients with PV. (D) TF + MP abundance in patients with PMF increased compared with patients with PV. *P<0.05, **P<0.01 vs. Control. #P<0.05 vs. PV. Ph−MPN, Philadelphia chromosome-negative myeloproliferative neoplasms; ET, essential thrombocythaemia; PV, polycythaemia vera; PMF, primary myelofibrosis; MPs, microparticles; EMPs, endothelial MPs; PMPs, platelet-derived MPs; RMPs, red blood cell MPs; TF+MPs, tissue factor-positive MPs.
MPs and MPN-associated thrombotic complications and splenomegaly
In the present sample, 50 patients were diagnosed with splenomegaly and 42 patients were not diagnosed with splenomegaly. The mean concentration of RMPs, PMPs, EMPs and TF + MPs in the splenomegaly group was 189.9+370.9, 1,447.5+1,873.1, 1,092.1+1,518.9 and 157.9+403.4/µl, respectively, and these values were significantly increased compared with those in the non-splenomegaly group, which were 69.9±127.2, 381.1±656.8, 471.6±682.3 and 37.9±45.3/µl (P<0.05), respectively, as shown in Fig. 2A.
Figure 2.
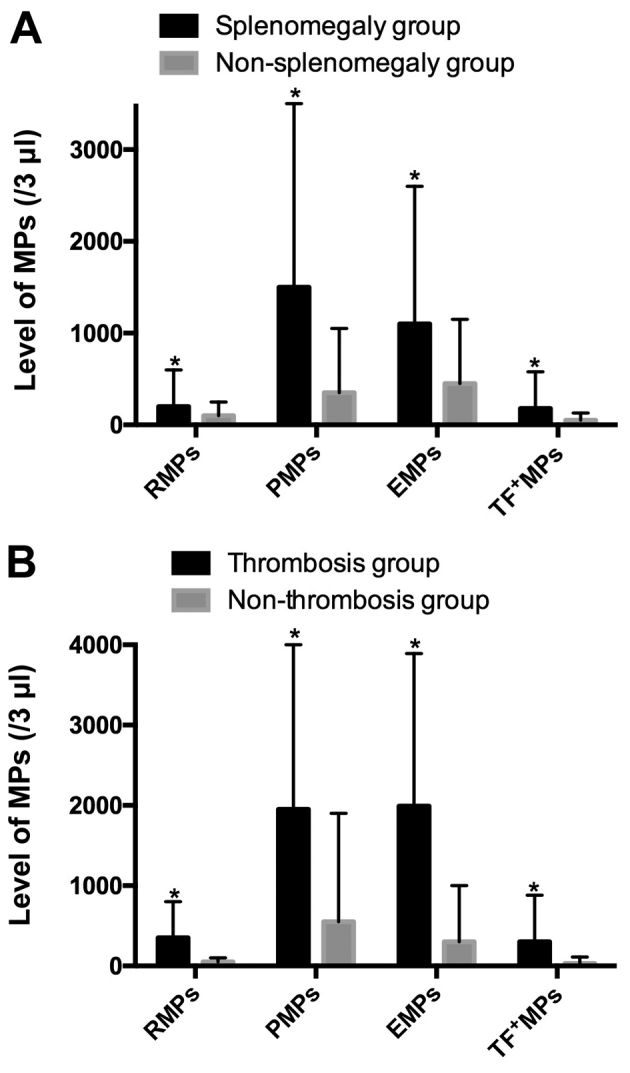
Comparison of MP levels of patients with Philadelphia chromosome-negative myeloproliferative neoplasms, with and without (A) splenomegaly or (B) thrombosis. *P<0.05. MPs, microparticles; EMPs, endothelial MPs; PMPs, platelet-derived MPs; RMPs, red blood cell MPs; TF+MPs, tissue factor-positive MPs.
Among the 92 MPN patients, there were 23 patients with thrombosis complications and 69 without thrombosis. The mean concentration of RMPs, PMPs, EMPs, and TF + MPs in thrombosis group was 375.9+504.5, 1989.7+2,023.7, 2000.5±1,851.7 and 268.0+566.0/µl, respectively. These counts were significantly increased compared with the non-thrombosis group, which were 54.9+72.6, 617.7+1,169.5, 411.6+568.2 and 48.2+86.5/µl (P<0.05), as shown in Fig. 2B.
Association between MPs and the JAK2V617F mutation
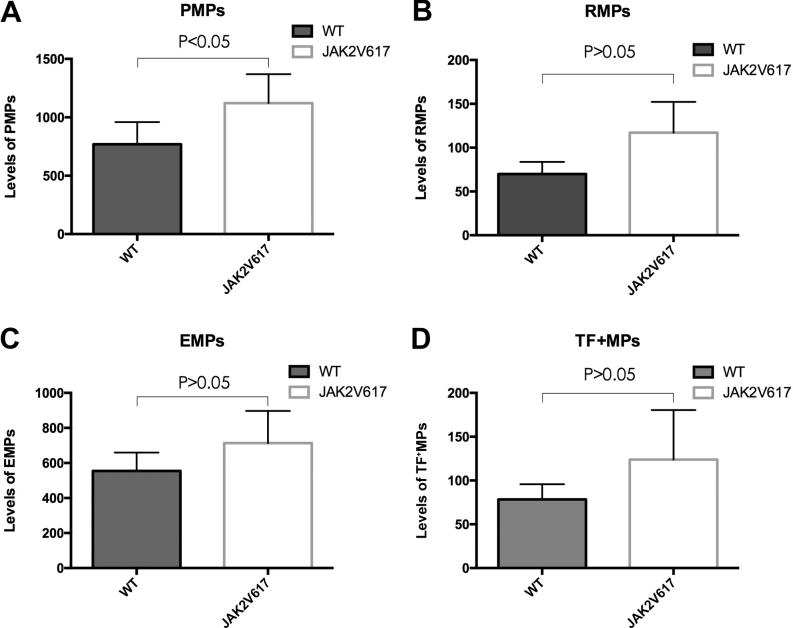
The MPN patients were divided into JAK2V617F+ (n=50) and JAK2V617F− (n=42) groups. PMP levels in the JAK2V617F+ group were found to be increased compared with the JAK2V617F− group (Fig. 3A). No statistically significant differe-nces were observed between the levels of the three remaining MPs in the two groups (Fig. 3B-D).
Figure 3.
(A-D) Comparison of MP levels between JAK2V617F+ and JAK2V617F− patients with Philadelphia chromosome-negative myeloproliferative neoplasms. MPs, microparticles; EMPs, endothelial MPs; PMPs, platelet-derived MPs; RMPs, red blood cell MPs; TF+MPs, tissue factor-positive MPs; WT, wild-type.
Discussion
MPs are small membrane vesicles that are secreted by almost all types of cells during activation, apoptosis or injury. Increased levels of procoagulant MPs in the blood may result in thrombogenesis (16). Several animal studies have investigated and confirmed the function of MPs in cancer models. Yu et al (17) showed that mice with colorectal tumours showed increased levels of tumour-derived MPs, and the levels of circulating MPs were associated with tumour progression. Circulating MPs were also shown by Davila et al (18) to be associated with the activation of coagulation in mice with pancreatic tumours. A study by Thomas et al (19) showed that MPs enhance the development of thrombosis in mice.
MPs are potentially procoagulant and they have a crucial role in thrombosis and haemostasis. Scott syndrome is a bleeding disorder that is characterised by the diminished excretion of phosphatidylserine to the cell surface of activated platelets (20). A large number of studies have detected elevated circulating MPs in cancer patients, and observed that the composition of MPs in cancer patients was different from that of healthy controls. Hron et al (21) found that patients with advanced colorectal cancer exhibited increased levels of MPs, which were almost exclusively derived from platelets (21). A previous study found increased levels of MPs in patients with pancreatic and metastatic breast cancer compared to controls (22). The MPs that are found in patients with acute promyelocytic leukaemia are mainly TF + MPs that are derived from promyelocytic cells, with low levels of PMPs, which may be the result of decreased platelet counts, as reported by Ma et al (23). In addition, patients with venous thromboembolism (VTE) exhibit an increased level of MPs compared with patients without VTE (24). At present, there has been little research into the role of MPs in Ph−MPN patients. Trappenburg et al (25) reported that ET patients have elevated levels of PMPs, EMPs and TF + MPs, with the exception of RMPs. However, to the best of our knowledge, no previous study has considered the variation in level of MPs in PV and PMF patients. As Trappenburg et al (25) described, ET patients have been observed to show increased von Willebrand factor, which is synthesized by endothelial cells and has an important role in platelet thrombus formation, accompanied by elevated MPs.
The plasma levels of four types of MPs (RMPs, PMPs, EMPs and TF + MPs) were evaluated in 92 Ph−MPN patients by flow cytometry. The present study reveals an increased number of MPs in MPN patients compared with controls. Additionally, it was found that RMPs were elevated in Ph−MPN patients and that MPs are elevated in patients with PV or PMF. No correlation between PMPs and platelet counts was identified, which may be due to the frequently abnormal platelet function of MPN patients. The increasing number of EMPs that was observed is indicative of endothelial cell activation.
The ET-associated result is consistent with the previous findings of Trappenburg et al (25). Increased numbers of MPs were observed in PMF patients compared with ET and PV patients, and the prognosis of PMF patients is less favourable than that of other MPN patients. Due to the limitation of a small number of patients in the present study, the impact of MPs on different phenotypes requires additional study.
Thrombosis and splenomegaly are common complications in MPN patients, and the former varies from mild microcirculatory disturbances to severe and potentially fatal complications, such as ischemic stroke and acute myocardial infarction. A previous study by Duchemin et al (26) documented increased thrombin generation in MPN patients. This result is compatible with previous observations in patients with other cancers (27). Splenomegaly, resulting from extramedullary haematopoiesis, is present in almost all PMF patients and a subset of ET and PV patients at diagnosis. It is associated with systemic symptoms that have a negative impact on the quality of life and life expectancy (28). Increased MPs may promote thrombosis in MPN patients with splenomegaly.
Among the patients in the present study, 23 patients showed various thrombotic complications and 69 cases did not show evidence of thrombosis, while 50 showed splenomegaly and 42 did not. The present results show that the levels of MPs in the group of patients with thrombosis were increased compared with those in the non-thrombosis group. The levels of MPs in the patients with splenomegaly were increased compared with those in the patients without splenomegaly. To the best of our knowledge, the present study is the first to describe the association between MPs, thrombosis and splenomegaly complications in Chinese patients with MPN.
The JAK2V617F mutation often occurs in patients with Ph−MPN. It has been reported that the JAK2V617F mutation is associated with the increased occurrence of thrombosis in MPN patients (29–31). Marchetti et al (32) showed that ET patients with the JAK2V617F mutation were more procoagulant than patients without the mutation, and that JAK2V617F was the major determinant that contributes to increased thrombin generation. However, to the best of our knowledge, no previous study has addressed the association between the JAK2V617F mutation, MPs and thrombosis.
To evaluate the potential association between the JAK2V617F mutation and MPs, JAK2V617F mutation screening was performed by allele-specific polymerase chain reaction. A total of 17 PV patients (85.0%), 32 ET patients (53.3%) and 6 PMF patients (50.0%) were positive for the JAK2V617F mutation. The present study found that the concentration of PMPs in the JAK2V617F+ group is increased compared with the group lacking the mutation. The increased level of PMPs observed in MPN patients with the JAK2V617F mutation indicates that the JAK2V617F mutation may enhance a hypercoagulable state.
In summary, the present study observed elevated levels of MPs (RMPs, PMPs, EMPs and TF + MPs) in patients with MPN. Increased values were present in patients with thrombosis or splenomegaly. In addition, increased MPs were also observed in JAK2V617F-positive patients, compared with JAK2V617F-negative patients. Therefore, MPs may be useful as a new biomarker for the development of thrombotic events in MPN patients. Additional prospective studies are required to better understand the relevance of MPs in different subtypes of MPN, and to explore their potential roles in the diagnosis and prognosis of MPN patients.
Acknowledgements
The authors thank San Francisco Edit for their assistance in editing this manuscript. This study was supported by grants from the Jiangsu Province of China (grant nos. BK20131167, RC2011105 and ZX201102), National Nature Science Foundation of China (grant nos. 81270591 and 81670132), National Key Basic Research Program of China (grant no. 2012CB526600), Jiangsu Provincial Special Program of Social Development (grant no. SBE2016740635), Jiangsu Provincial Special Program of Medical Science (grant no. BL2012005), and the Priority Academic Program Development of Jiangsu Higher Education Institutions.
References
- 1.Tefferi A, Vardiman JW. Classification and diagnosis of myeloproliferative neoplasms: The 2008 World Health Organization criteria and point-of-care diagnostic algorithms. Leukemia. 2008;22:14–22. doi: 10.1038/sj.leu.2404955. [DOI] [PubMed] [Google Scholar]
- 2.Barrio S, Gallardo M, Arenas A, Ayala R, Rapado I, Rueda D, Jimenez A, Albizua E, Burgaleta C, Gilsanz F, Martinez-Lopez J. Inhibition of related JAK/STAT pathways with molecular targeted drugs shows strong synergy with ruxolitinib in chronic myeloproliferative neoplasm. Br J Haematol. 2013;161:667–676. doi: 10.1111/bjh.12308. [DOI] [PubMed] [Google Scholar]
- 3.Levine RL, Wadleigh M, Cools J, Ebert BL, Wernig G, Huntly BJ, Boggon TJ, Wlodarska I, Clark JJ, Moore S, et al. Activating mutation in the tyrosine kinase JAK2 in polycythemia vera, essential thrombocythemia, and myeloid metaplasia with myelofibrosis. Cancer Cell. 2005;7:387–397. doi: 10.1016/j.ccr.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 4.Baxter EJ, Scott LM, Campbell PJ, East C, Fourouclas N, Swanton S, Vassiliou GS, Bench AJ, Boyd EM, Curtin N, et al. Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders. Lancet. 2005;365:1054–1061. doi: 10.1016/S0140-6736(05)74230-6. [DOI] [PubMed] [Google Scholar]
- 5.Tefferi A. Polycythemi a vera and essential thrombocythemia: 2012 update on diagnosis, risk stratification, and management. Am J Hematol. 2012;87:285–293. doi: 10.1002/ajh.23135. [DOI] [PubMed] [Google Scholar]
- 6.Barbui T, Carobbio A, Cervantes F, Vannucchi AM, Guglielmelli P, Antonioli E, Alvarez-Larrán A, Rambaldi A, Finazzi G, Barosi G. Thrombosis in primary myelofibrosis: Incidence and risk factors. Blood. 2010;115:778–782. doi: 10.1182/blood-2009-08-238956. [DOI] [PubMed] [Google Scholar]
- 7.Cheung B, Radia D, Pantelidis P, Yadegarfar G, Harrison C. The presence of the JAK2 V617F mutation is associated with a higher haemoglobin and increased risk of thrombosis in essential thrombocythemia. Br J Haematol. 2006;132:244–245. doi: 10.1111/j.1365-2141.2005.05858.x. [DOI] [PubMed] [Google Scholar]
- 8.Finazzi G, Rambaldi A, Guerini V, Carobbo A, Barbui T. Risk of thrombosis in patients with essential thrombocythemia and polycythemia vera according to JAK2 V617F mutation status. Haematologica. 2007;92:135–136. doi: 10.3324/haematol.10634. [DOI] [PubMed] [Google Scholar]
- 9.Willms A, Müller C, Julich H, Klein N, Schwab R, Güsgen C, Richardsen I, Schaaf S, Krawczyk M, Krawczyk M, et al. Tumour-associated circulating microparticles: A novel liquid biopsy tool for screening and therapy monitoring of colorectal carcinoma and other epithelial neoplasia. Oncotarget. 2016;7:30867–30875. doi: 10.18632/oncotarget.9018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tseng CC, Wang CC, Hsiao CC, Lu HI, Leu S, Chang HC, Huang KT, Fang WF, Chen YM, Liu SF, et al. Time courses and value of circulating microparticles in patients with operable stage non-small cell lung cancer undergoing surgical intervention. Tumour Biol. 2016;37:11873–11882. doi: 10.1007/s13277-016-5047-5. [DOI] [PubMed] [Google Scholar]
- 11.Campello E, Zanetto A, Spiezia L, Radu CM, Gavasso S, Ferrarese A, Farinati F, Senzolo M, Simioni P. Hypercoagulability detected by circulating microparticles in patients with hepatocellular carcinoma and cirrhosis. Thromb Res. 2016;143:118–121. doi: 10.1016/j.thromres.2016.05.021. [DOI] [PubMed] [Google Scholar]
- 12.Owens AP, III, Mackman N. Microparticles in hemostasis and thrombosis. Circ Res. 2011;108:1284–1297. doi: 10.1161/CIRCRESAHA.110.233056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zwicker JI, Liebman HA, Neuberg D, Lacroix R, Bauer KA, Furie BC, Furie B. Tumor-derived tissue factor-bearing microparticles are associated with venous thromboembolic events in malignancy. Clin Cancer Res. 2009;15:6830–6840. doi: 10.1158/1078-0432.CCR-09-0371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morel O, Luca F, Grunebaum L, Jesel L, Meyer N, Desprez D, Robert S, Dignat-George F, Toti F, Simon C, Goichot B. Short-term very low-calorie diet in obese females improves the haemostatic balance through the reduction of leptin levels, PAI-1 concentrations and a diminished release of platelet and leukocyte-derived microparticles. Int J Obes (Lond) 2011;35:1479–1486. doi: 10.1038/ijo.2011.19. [DOI] [PubMed] [Google Scholar]
- 15.Baxter EJ, Scott LM, Campbell PJ, East C, Fourouclas N, Swanton S, Vassiliou GS, Bench AJ, Boyd EM, Curtin N, et al. Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders. Lancet. 2005;365:1054–1061. doi: 10.1016/S0140-6736(05)74230-6. [DOI] [PubMed] [Google Scholar]
- 16.Boulanger CM, Amabile N, Tedgui A. Circulating microparticles: A potential prognostic marker for atherosclerotic vascular disease. Hypertension. 2006;48:180–186. doi: 10.1161/01.HYP.0000231507.00962.b5. [DOI] [PubMed] [Google Scholar]
- 17.Yu JL, May L, Lhotak V, Shahrzad S, Shirasawa S, Weitz JI, Coomber BL, Mackman N, Rak JW. Oncogenic events regulate tissue factor expression in colorectal cancer cells: Implications for tumor progression and angiogenesis. Blood. 2005;105:1734–1741. doi: 10.1182/blood-2004-05-2042. [DOI] [PubMed] [Google Scholar]
- 18.Davila M, Amirkhosravi A, Coll E, Desai H, Robles L, Colon J, Baker CH, Francis JL. Tissue factor-bearing microparticles derived from tumor cells: Impact on coagulation activation. J Thromb Haemost. 2008;6:1517–1524. doi: 10.1111/j.1538-7836.2008.02987.x. [DOI] [PubMed] [Google Scholar]
- 19.Thomas GM, Panicot-Dubois L, Lacroix R, Dignat-George F, Lombardo D, Dubois C. Cancer cell-derived microparticles bearing P-selectin glycoprotein ligand 1 accelerate thrombus formation in vivo. J Exp Med. 2009;206:1913–1927. doi: 10.1084/jem.20082297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weiss HJ. Scott syndrome: A disorder of platelet coagulant activity. Semin Hematol. 1994;31:312–319. [PubMed] [Google Scholar]
- 21.Hron G, Kollars M, Weber H, Sagaster V, Quehenberger P, Eichinger S, Kyrle PA, Weltermann A. Tissue factor-positive microparticles: Cellular origin and association with coagulation activation in patients with colorectal cancer. Thromb Haemost. 2007;97:119–123. [PubMed] [Google Scholar]
- 22.Rodriguez P Garcia, Eikenboom HC, Tesselaar ME, Huisman MV, Nijkeuter M, Osanto S, Bertina RM. Plasma levels of microparticle-associated tissue factor activity in patients with clinically suspected pulmonary embolism. Thromb Res. 2010;126:345–349. doi: 10.1016/j.thromres.2010.07.006. [DOI] [PubMed] [Google Scholar]
- 23.Ma G, Liu F, Lv L, Gao Y, Su Y. Increased promyelocytic-derived microparticles: A novel potential factor for coagulopathy in acute promyelocytic leukemia. Ann Hematol. 2013;92:645–652. doi: 10.1007/s00277-013-1676-6. [DOI] [PubMed] [Google Scholar]
- 24.Tesselaar ME, Romijn FP, van der Linden IK, Prins FA, Bertina RM, Osanto S. Microparticle-associated tissue factor activity: A link between cancer and thrombosis? J Thromb Haemost. 2007;5:520–527. doi: 10.1111/j.1538-7836.2007.02369.x. [DOI] [PubMed] [Google Scholar]
- 25.Trappenburg MC, van Schilfgaarde M, Marchetti M, Spronk HM, ten Cate H, Leyte A, Terpstra WE, Falanga A. Elevated procoagulant microparticles expressing endothelial and platelet markers in essential thrombocythemia. Haematologica. 2009;94:911–918. doi: 10.3324/haematol.13774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Duchemin J, Ugo V, Ianotto JC, Lecucq L, Mercier B, Abgrall JF. Increased circulating procoagulant activity and thrombin generation in patients with myeloproliferative neoplasms. Thromb Res. 2010;126:238–242. doi: 10.1016/j.thromres.2010.06.025. [DOI] [PubMed] [Google Scholar]
- 27.Campello E, Spiezia L, Radu CM, Bulato C, Castelli M, Gavasso S, Simioni P. Endothelial, platelet, and tissue factor-bearing microparticles in cancer patients with and without venous thromboembolism. Thromb Res. 2011;127:473–477. doi: 10.1016/j.thromres.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 28.Randhawa J, Ostojic A, Vrhovac R, Atallah E, Verstovsek S. Splenomegaly in myelofibrosis-new options for therapy and the therapeutic potential of Janus kinase 2 inhibitors. J Hematol Oncol. 2012;5:43. doi: 10.1186/1756-8722-5-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee HS, Park LC, Lee EM, Lee SJ, Shin SH, Im H, Do KM, Kim EJ, Ye BJ, Song MK, et al. Incidence rates and risk factors for vascular events in patients with essential thrombocythemia: A multicenter study from Korea. Clin Lymphoma Myeloma Leuk. 2012;12:70–75. doi: 10.1016/j.clml.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 30.Lussana F, Caberlon S, Pagani C, Kamphuisen PW, Büller HR, Cattaneo M. Association of V617F Jak2 mutation with the risk of thrombosis among patients with essential thrombocythaemia or idiopathic myelofibrosis: A systematic review. Thromb Res. 2009;124:409–417. doi: 10.1016/j.thromres.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 31.Antonioli E, Guglielmelli P, Poli G, Bogani C, Pancrazzi A, Longo G, Ponziani V, Tozzi L, Pieri L, Santini V. Influence of JAK2V617F allele burden on phenotype in essential thrombocythemia. Haematologica. 2008;93:41–48. doi: 10.3324/haematol.11653. [DOI] [PubMed] [Google Scholar]
- 32.Marchetti M, Tartari CJ, Russo L, Panova-Noeva M, Leuzzi A, Rambaldi A, Finazzi G, Woodhams B, Falanga A. Phospholipid-dependent procoagulant activity is highly expressed by circulating microparticles in patients with essential thrombocythemia. Am J Hematol. 2014;89:68–73. doi: 10.1002/ajh.23590. [DOI] [PMC free article] [PubMed] [Google Scholar]