Abstract
Aims
To investigate differential muscle atrophy during bed-rest, the impact of a high-intensity concentric-eccentric (flywheel) resistance exercise countermeasure and muscle recovery after bed-rest.
Methods
Twenty-five healthy male subjects underwent 90 dayshead-down tilt bed-rest. Volume of individual lower-limb muscles was measured via MRI before, twice during and four times up to 1 year after bed-rest. Subjects were either inactive (n=16) or performed flywheel exercise every third day of bed-rest (n=9). Functional performance was assessed via countermovement jump.
Results
On ‘intent-to-treat’ analysis, flywheel prevented atrophy in the vasti (p<0.001) and reduced atrophy in the hip adductor/extensor adductor magnus (p=0.001) and ankle dorsiflexors/toe flexors (soleus (p<0.001), gastrocnemius medialis (p<0.001), gastrocnemius lateralis (p=0.02), and tibialis posterior with flexor digitorum longus (p=0.04)). Flywheel exercise was not effective for the hamstrings, gracilis, sartorius, peroneals and anterior tibial muscles. Muscle atrophy in vasti, soleus, gastrocnemius medialis, gastrocnemius lateralis and adductor magnus correlated with losses in countermovement jump performance. Muscle volume recovered within 90 days after bed-rest, however long-term after bed-rest, the inactive subjects only showed significantly increased muscle volume versus prebed-rest in a number of muscles including soleus (+4.3%), gastrocnemius medialis (+3.9%) and semimembranosus (+4.3%). This was not associated with greater countermovement jump performance.
Conclusion
The exercise countermeasure was effective in preventing or reducing atrophy in the vasti, adductor magnus and ankle dorsiflexors/toe flexors but not the hamstrings, medial thigh muscles or peroneals and dorsiflexor muscles.
Trial registration number
NCT00311571; results.
Keywords: Muscle, Exercise, Exercise rehabilitation
What are the new findings?
In disuse, muscle atrophy occurs the fastest in the muscles of the lower-leg (especially soleus and gastrocnemius medialis) followed by those of the thigh (vasti, biceps femoris long head) and hip (quadratus femoris).
In ninety days of strict disuse, a fly-wheel exercise countermeasure reduced atrophy in the monoarticular knee, hip and ankle extensors, but did not impact the hamstrings or medial thigh muscles.
In the months after bed-rest, an overshoot of muscle volume recovery was observed in some muscles in the inactive control subjects with greater muscle size than before bed-rest.
Introduction
Optimising countermeasures against musculoskeletal deterioration is a priority for space agencies around the world. With a long-term view to missions to Mars or a moon base, it is important to maintain the musculoskeletal system to enable completion of mission tasks in the hypogravitational fields on Mars or moon, and also to safeguard the return to the Earth’s 1 g environment. Prolonged bed-rest is a methodology used to model the effects of spaceflight on the human body.1 Most of the musculoskeletal deterioration occurs in the lower quadrant of the body.
The literature to date2 points to the idea that muscle-specific higher-load resistance exercise appears to be more effective in maintaining muscle mass during prolonged bed-rest, with aerobic and/or low load type exercise being less effective. In microgravity, typical Earth-based implementations of resistance exercise are difficult. One approach to exercising in a gravity-independent manner is with the flywheel exercise device.3 During exercise with the flywheel device, resistance is determined by the effort applied to the device by the person during the concentric phase of movement. This energy is stored within the flywheels of the device and then returned as resistance during the eccentric phase. In this way, exercise is not dependent on force generation by, for example, a weight and pulley system. Flywheel exercise has been shown to be capable of generating greater eccentric muscle activation than with a standard gym weight system4 and feasible for implementation in a space station simulation.5 In another model of unloading, unilateral lower-limb suspension, flywheel exercise of the knee extensors was shown to prevent knee extensor, but not plantarflexor, muscle atrophy6 and in an 84 days bed-rest study, it countered some of the metabolic changes in the vastus lateralis muscle.7 With the implementation of leg press and calf press type exercises on the flywheel device, our primary hypothesis was that flywheel exercise will reduce atrophy in hip, knee and calf extensor muscles in microgravity simulation (prolonged bed-rest).
Prolonged bed-rest is also a model of clinical disuse8 and can be informative clinically on which musculature is most affected and hence how to target rehabilitation programmes. While a series of works have examined muscle atrophy in the lower limb in disuse,9–17 yet less is known about atrophy of the deep muscles of the hip and the lower leg. A secondary aim of the current study was to examine which muscles of the lower limb atrophy the most. Furthermore, the recovery of the human body after strict disuse has not been well studied. This is important for estimating time windows of increased injury risk and designing rehabilitation programs. Our tertiary aim was to examine the time course of muscle atrophy after bed-rest and our tertiary hypothesis was that muscle atrophy during disuse will return to prebed-rest levels in the months after bed-rest.
Methods
Study design, participants and study conduct
Twenty-five healthy male subjects underwent 90 days of 6° head-down tilt bed-rest (HDT) as part of the ‘Long Term Bed Rest’ (LTBR) Study at MEDES in Toulouse, France. The LTBR Study was supported by the European, French and Japanese space agencies (ESA, CNES and NASDA). The LTBR Study was approved by the Toulouse I ethics committee (CCPPRB Toulouse I) of the Rangueil University Hospital as well as the ethical committee of the Free University of Berlin. All subjects gave their informed written consent. Subjects were randomised to either a group performing high-intensity eccentric-concentric resistance exercise on the ‘fly-wheel’ exercise device (flywheel group; n=9; mean(SD) age, height and weight: 31.0 (5.5) years, 1.75 (0.05) m and 70.9 (5.4) kg) or a group that performed no exercise (inactive group; n=16; 32.5 (3.4) years, 1.74 (0.04) m, 70.3 (6.1) kg). Diet was strictly monitored and controlled during the in-house periods. Meals were prepared by the hospital kitchen to achieve energy intake with 30% fat, 15% protein and 55% carbohydrate and this was supervised by a dietician. Data on subjects’ physical activity levels showed that 90 days after bed-rest, subjects’ total physical activity scores were significantly less than before bed-rest.18
Countermeasure exercise
The flywheel exercise programme was designed to target muscle and bone in the lower limbs. The exercise device was designed to be gravity-independent. During the concentric contraction phase, the energy applied to the foot plate by the subject was transferred to the flywheels via a strap which was wound around the flywheels. Then, during the eccentric phase, the rotating flywheels provided resistance. Repetitions were performed as one continuous movement cycle, that is, movement was not ceased between each repetition. Prior to bed-rest all subjects performed two familiarisation sessions with the training apparatus on 2 days separated by 4–6 days. In the first session, subjects practised the manoeuvres on the flywheel device at low to moderate effort intensities and in the second session, subjects were progressed to putting in maximum effort. During bed-rest, the flywheel group performed supine leg press exercises (4 sets of 7 repetitions, 2 min between sets; targeting the hip and knee extensor groups) with then 5 min rest and then calf-raise exercises (4 sets of 14 repetitions, 2 min between sets; targeting plantar flexors) every third day from the fifth day of bed-rest onwards. At the start of each set of exercises, two submaximal manoeuvres of the action (ie, leg press or calf-raise) were performed and these were immediately followed by the prescribed number of eccentric-concentric repetitions at maximum contraction. The subject was encouraged to put maximum effort into each concentric-eccentric loading cycle. After maximal concentric contraction at the initiation of the movement, the subject was instructed to start eccentric contraction (deceleration) once half of the range of motion was passed with then maximal effort into an eccentric contraction towards the end of the range of motion. Electrogoniometers positioned at the knee and ankle provided instantaneous feedback on joint position via a screen positioned in the subject's field of view.
Magnetic resonance imaging
A 1.0T Siemens Somatom Impact (Erlangen, Germany) scanner was used to conduct MRI before bed-rest (Baseline), on day 27 (HDT27) and day 89 (HDT89) of bed-rest, and then 13 days (R+13), 90 days (R+90), 180 days (R+180) and 360 days (R+360) after bed-rest during the recovery phase. Both lower limbs, starting at the hip joint and extending to the feet, were scanned (see online supplementary figure 1). Images were then stored for offline analysis. To blind the operator (DLB) to group and study date, each data set was coded with a random number (obtained from www.random.org). The operator used ImageJ (http://rsb.info.nih.gov/ij/) to measure the area of muscles of the lower limb in every image on the right side of the body (see online supplementary figure 1). For the quadratus femoris and obturator internus muscles, based on our prior data19 it was deemed necessary to measure both left and right sides with the results averaged prior to further analysis.
bmjsem-2016-000196supp001.pdf (172.9KB, pdf)
Countermovement jump
For the comparison of changes in countermovement jump performance to muscle atrophy we used data from previously published work20 where detailed methodology of the countermovement jump test was reported. For the purpose of the correlation analyses, only data on percentage change between baseline and first testing session after bed-rest (day 3 after bed-rest; n=25) and the last testing session in the recovery phase (180 days postbed-rest; n=24) were used. We examined peak jump power; similar results are seen when assessing peak jump height or acceleration. The reproducibility of countermovement jump power in adult and elderly subjects is excellent21 22 with data from our own laboratory23 on 24 men aged 20–45 years tested 4 days apart showing a between-day correlation of 0.93 for countermovement jump power. Percentage change in peak jump power versus before bed-rest was used in correlation analyses.
Statistical analyses
Analysis was done on an ‘intent-to-treat’ (ITT) approach. For the primary analysis, data were converted to fractional change in muscle volume and compared with the prebed-rest value. To examine whether the rates of atrophy differed between groups, non-linear mixed-effects modelling was used to calculate exponential decay rates per week of bed-rest (ek*weeks; where k is the decay rate coefficient and weeks is the week of bed-rest). A factor of ‘group’, with subsequent analysis of variance (ANOVA), was examined to determine whether rate of muscle atrophy differed significantly between groups. To examine the recovery of the musculature after bed-rest, the data on whole muscle volume were used. A ‘study-date’ effect with a priori contrasts comparing each time point to baseline was evaluated. Linear mixed-effects models were implemented. For correlation analyses, Pearson’s correlation coefficient was calculated between percentage change in muscle volume and percentage change in countermovement jump power. An α level of 0.05 was taken for statistical significance. To guard against false positives, all p values were adjusted by the false discovery rate method. The ‘R’ statistical environment (V.3.0.2, www.r-project.org) was used for all analyses.
Results
One flywheel subject ceased the training protocol after 7 weeks bed-rest due to a previously unreported knee injury, but he completed the bed-rest study phase. This subject was treated as a flywheel subject in the ITT analysis. Data from one inactive subject were lost on HDT29. Two inactive subjects did not attend on R+90 and one of these also did not attend scanning on R+180. One flywheel subject did not return for the final scanning session on R+360.
Effects in the inactive group
In the inactive subjects, the soleus and gastrocnemius medialis showed the fastest rates of muscle atrophy (figure 1) both losing 28% of their volume by end of bed-rest (table 1). This was followed by the remaining muscles of the lower leg (with the exception of the anterior tibial muscles), vasti, biceps femoris long head and semimembranosus (figure 1). The other members of the hamstrings, semitendinosus and biceps femoris short head, while atrophying, did not show as rapid atrophy of other members of the hamstrings. Of the muscles localised at the hip joint, quadratus femoris atrophied the fastest and of the medial thigh muscles, the greatest atrophy was seen in adductor magnus.
Figure 1.
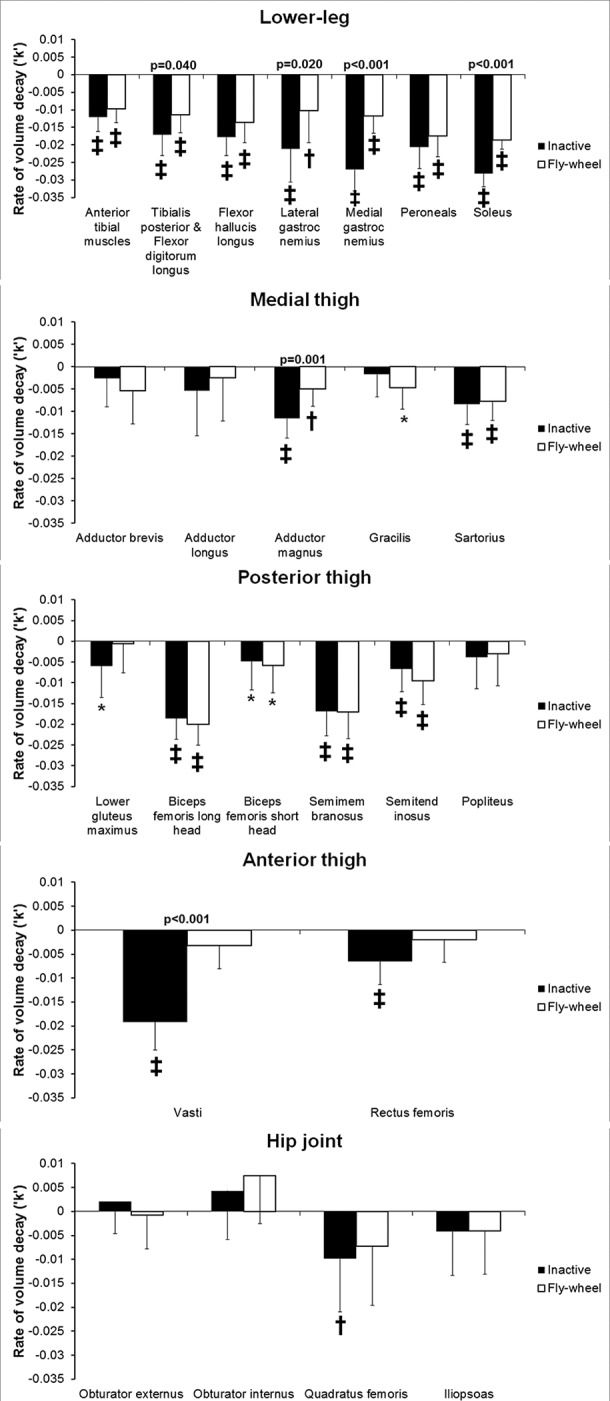
Atrophy and impact of exercise on muscle atrophy values are mean (SD) time constants (‘k’) of exponential decay of muscle volume per week of bed-rest. *: p<0.05; †:p<0.01; ‡: p<0.001 and indicate significance of difference of time constant to zero (ie, whether atrophy occurred). p Values above the columns show where the difference between groups was significant. All p values are adjusted for false positives via the ‘false discovery rate’ method.
Table 1.
Muscle volume changes in the lower leg, anterior thigh
| Group | Baseline | Bed-rest | Recovery | ||||
| 28 days (%) | 89 days (%) | 13 days (%) | 90 days (%) | 180 days (%) | 360d (%) | ||
| Anterior tibial muscles | |||||||
| Inactive | 252.1 (24.9) | −7.0 (4.0)‡ | −13.6 (4.9)‡ | −5.1 (3.9)‡ | −0.3 (3.9) | 0.1 (3.9) | 0.4 (4.7) |
| Flywheel | 246.9 (11.8) | −5.1 (4.0)‡ | −11.2 (4.0)‡ | −3.0 (4.6) | 0.5 (4.5) | 1.2 (4.2) | 0.4 (4.5) |
| Flexor digitorum with tibialis posterior | |||||||
| Inactive | 136.6 (25.3) | −11.4 (7.4)‡ | −19.0 (10.4)‡ | −7.3 (6.7)‡ | 1.8 (5.4) | 0.5 (4.2) | 0.9 (4.0) |
| Flywheel | 133.5 (28.5) | −7.1 (6.4)† | −13.0 (7.9)‡ | −6.0 (7.2)* | −1.2 (5.0) | −0.5 (4.6) | 1.0 (5.1) |
| Flexor hallucis longus | |||||||
| Inactive | 80.2 (13.4) | −9.5 (7.0)‡ | −19.6 (8.9)‡ | −14.7 (9.8)‡ | 0.0 (6.5) | 1.8 (7.5) | 2.1 (9.0) |
| Flywheel | 78.3 (8.7) | −8.8 (9.8)* | −15.5 (8.4)‡ | −10.1 (8.4)‡ | −4.8 (7.1) | −2.9 (8.6) | −2.2 (9.3) |
| Lateral gastrocnemius | |||||||
| Inactive | 132.6 (20.7) | −10.7 (10.3)‡ | −23.3 (13.0)‡ | −11.8 (11.6)‡ | 2.6 (9.6) | 1.1 (10.1) | 3.0 (10.5) |
| Flywheel | 140.2 (15.2) | −4.0 (8.4)) | −12.8 (10.7)‡ | −7.5 (9.8)* | 2.3 (8.4) | −0.8 (7.0) | −1.4 (8.2) |
| Medial gastrocnemius | |||||||
| Inactive | 222.1 (40.6) | −15.6 (7.6)‡ | −28.0 (12.3)‡ | −7.6 (8.4)‡ | 2.7 (6.4) | 2.5 (4.8) | 3.9 (6.0)* |
| Flywheel | 235.2 (30.6) | −5.6 (5.2)† | −13.3 (7.3)‡ | −5.1 (6.6)* | 1.7 (4.0) | 0.9 (3.2) | 0.0 (3.0) |
| Peroneals | |||||||
| Inactive | 136.9 (29.0) | −12.1 (6.4)‡ | −22.5 (11.8)‡ | −12.8 (7.7)‡ | −0.1 (4.6) | 0.7 (4.2) | 1.6 (4.9) |
| Flywheel | 140.9 (29.2) | −10.3 (4.7)‡ | −18.4 (6.3)‡ | −9.0 (6.0)‡ | 0.1 (6.7) | −2.0 (8.1) | −0.5 (7.9) |
| Soleus | |||||||
| Inactive | 449.4 (61.0) | −15.6 (6.1)‡ | −28.6 (8.5)‡ | −5.5 (6.1)‡ | 2.4 (4.6) | 3.9 (3.2)‡ | 4.3 (5.0)† |
| Fly-wheel | 496.4 (64.5) | −10.6 (3.0)‡ | −19.9 (4.5)‡ | −5.5 (4.7)† | 1.3 (3.8) | 0.7 (4.5) | 1.2 (3.5) |
| Vasti | |||||||
| Inactive | 1688.8 (203.7) | −12.1 (6.0)‡ | −20.3 (7.3)‡ | −10.1 (6.7)‡ | 0.9 (7.5) | 1.5 (6.8) | 3.1 (8.5) |
| Flywheel | 1670.2 (115.0) | −1.5 (4.3) | −3.8 (5.8) | −0.5 (6.1) | 1.6 (2.1) | 1.0 (3.2) | −0.5 (2.4) |
| Rectus femoris | |||||||
| Inactive | 259.3 (33.0) | −3.6 (5.3)* | −7.9 (6.2)‡ | −4.2 (6.2)* | −0.8 (6.0) | −0.1 (5.4) | 1.0 (6.9) |
| Flywheel | 285.2 (37.3) | −0.2 (3.5) | −2.7 (3.7) | 0.0 (3.9) | 0.0 (3.8) | 2.8 (10.4) | 0.7 (5.4) |
At baseline, values are mean (SD) muscle volume in cm³. During and after bed-rest, values are mean (SD) percentage change in muscle volume compared with baseline. *: p<0.05; †:p<0.01; ‡: p<0.001 and indicate significance of difference to baseline. All p values are adjusted for false positives via the ‘false discovery rate’ method.
Impact of flywheel exercise
ANOVA showed that flywheel exercise significantly reduced the rate of atrophy of the posterior calf muscles soleus (p<0.001; figure 1), gastrocnemius medialis (p<0.001), gastrocnemius lateralis (p=0.02) and tibialis posterior with flexor digitorum longus (p=0.04). At the thigh, flywheel exercise reduced atrophy of the vasti (p<0.001) and adductor magnus muscles (p<0.001). Lower gluteus maximus and rectus femoris atrophied significantly in the inactive group and not in the flywheel group, but the differences between the groups were not statistically significant on ANOVA (p>0.07). Similarly, gracilis atrophied significantly in the flywheel group but not in the inactive group, however the difference between groups in the rates of atrophy was not significant (p=0.28). The flywheel exercise had no impact on atrophy of the hamstrings or other muscles of the thigh and hip.
Long-term recovery of muscle volume after 90 days bed-rest study
Of the muscles that atrophied during bed-rest, significant atrophy was still present 13 days after bed-rest (tables 1 and 2). By 90 days after 90 days bed-rest, all muscles returned to their prebed-rest volumes. In the inactive subjects only, some muscles showed hypertrophy in the months after bed-rest. Specifically, at the hip, adductor brevis was increased in volume on 180 days (+6.0%, p=0.008) and 360 days (+4.9%, p=0.040) of recovery and obturator externus also showed greater volume in recovery (90 days: +8.6%, p=0.004; 180 days: +7.6%, p=0.007; 360 days: +6.4%, p=0.009). In the thigh, semimembranosus (180 days: +2.7%, p=0.019; 360 days: +4.3%, p=0.020) and sartorius (180 days: 3.8%, p=0.023; 360 days: +5.0%, p=0.010) were hypertrophied in the long term after bed-rest. In the calf, soleus (180 days: +3.9%, p<0.001; 360 days: +4.3%, p=0.004) and medial gastrocnemius (360 days: +3.9%, p=0.040) also showed increased volume late in recovery. This effect was not seen in the flywheel group (table 1,2; figure 2).
Table 2.
Muscle volume changes in the medial thigh, posterior thigh and hip
| Group | Baseline | Bed-rest | Recovery | ||||
| 28d (%) | 89d (%) | 13d (%) | 90d (%) | 180d (%) | 360d (%) | ||
| Adductor brevis | |||||||
| Inactive | 164.1 (24.1) | −1.0 (6.0) | −2.5 (7.3) | −0.8 (8.5) | 5.6 (8.9) | 6.0 (7.1)† | 4.9 (7.5)* |
| Flywheel | 186.7 (20.2) | −8.2 (7.6)† | −4.8 (6.8) | −0.4 (6.0) | −0.6 (5.2) | 0.5 (6.5) | −2.4 (7.1) |
| Adductor longus | |||||||
| Inactive | 159.0 (32.5) | −0.7 (11.3) | −7.1 (12.6)* | −0.9 (14.0) | 2.9 (9.3) | 4.5 (10.7) | 5.8 (12.7) |
| Flywheel | 175.8 (20.7) | −1.4 (7.8) | −2.9 (9.1) | −0.9 (9.7) | 3.2 (11.1) | −0.6 (11.5) | −1.3 (9.9) |
| Adductor magnus | |||||||
| Inactive | 585.8 (78.2) | −7.8 (5.4)‡ | −13.0 (6.2)‡ | −4.7 (4.9)‡ | 1.6 (5.6) | 1.4 (5.2) | 3.0 (5.6) |
| Flywheel | 586.6 (62.5) | −3.0 (4.1) | −6.1 (3.8)‡ | −1.2 (3.9) | 2.2 (2.3)* | 1.3 (4.3) | −0.2 (3.8) |
| Gracilis | |||||||
| Inactive | 101.6 (20.3) | 0.2 (5.4) | −2.6 (5.8) | 1.0 (7.0) | 3.5 (6.4) | 3.3 (5.9) | 6.2 (9.9) |
| Flywheel | 115.2 (27.1) | −2.5 (5.0) | −6.8 (8.5)* | 1.6 (7.4) | 3.9 (6.8) | 2.5 (6.1) | 0.2 (5.5) |
| Sartorius | |||||||
| Inactive | 162.3 (24.2) | −5.9 (4.9)‡ | −9.4 (5.9)‡ | −3.8 (5.8)* | 2.3 (6.5) | 3.8 (5.3)* | 5.0 (6.3)* |
| Flywheel | 172.8 (11.4) | −4.3 (3.3)‡ | −9.1 (4.9)‡ | −1.7 (5.4) | 0.8 (4.0) | 4.9 (7.0) | 0.5 (6.7) |
| Biceps femoris long head | |||||||
| Inactive | 190.2 (24.0) | −10.5 (6.4)‡ | −20.0 (7.8)‡ | −10.5 (7.0)‡ | 2.7 (6.9) | 1.7 (4.9) | 2.2 (6.0) |
| Flywheel | 234.1 (31.3) | −11.8 (3.8)‡ | −21.1 (5.2)‡ | −11.7 (5.9)‡ | 0.9 (6.6) | 0.9 (7.2) | 0.5 (7.3) |
| Biceps femoris short head | |||||||
| Inactive | 97.4 (25.0) | −2.8 (7.5) | −6.3 (8.6)† | −0.2 (7.1) | 4.5 (8.5) | 3.0 (7.8) | 3.1 (8.4) |
| Flywheel | 104.7 (14.9) | −4.4 (3.1)‡ | −6.8 (7.6)* | −0.1 (5.9) | −1.1 (5.8) | −0.7 (3.9) | −1.5 (5.1) |
| Semimembranosus | |||||||
| Inactive | 252.6 (43.9) | −10.4 (4.1)‡ | −18.0 (6.3)‡ | −8.4 (6.5)‡ | 2.4 (4.5) | 2.7 (3.7)* | 4.3 (6.0)* |
| Flywheel | 300.5 (50.0) | −10.7 (6.8)‡ | −18.0 (9.0)‡ | −9.3 (7.5)‡ | 1.2 (6.2) | −0.6 (6.5) | 1.4 (4.7) |
| Semitendinosus | |||||||
| Inactive | 182.3 (19.3) | −4.1 (5.6)* | −7.3 (6.9)‡ | −3.6 (6.0)* | 2.2 (5.8) | 2.2 (6.4) | 2.0 (7.6) |
| Flywheel | 189.5 (11.6) | −6.5 (4.5)‡ | −10.6 (3.2)‡ | −7.1 (4.0)‡ | 0.7 (6.3) | −1.1 (5.4) | −2.7 (6.5) |
| Popliteus | |||||||
| Inactive | 21.0 (4.3) | −1.6 (6.3) | −5.1 (10.2) | −0.4 (8.2) | 2.4 (7.4) | −0.7 (6.2) | 1.8 (8.3) |
| Flywheel | 22.1 (3.7) | −2.6 (7.5) | −4.0 (7.8) | 2.6 (6.0) | −3.4 (8.5) | 0.3 (7.5) | −3.6 (5.9) |
| Lower gluteus maximus | |||||||
| Inactive | 535.6 (96.6) | −4.1 (7.4) | −6.9 (8.5)† | −4.5 (6.4)* | 1.6 (7.2) | 0.8 (8.1) | 5.5 (10.9) |
| Flywheel | 590.4 (80.7) | −0.4 (6.1) | −0.5 (6.6) | −0.3 (7.1) | −2.4 (6.6) | −0.8 (5.5) | −3.5 (7.4) |
| Obturator externus | |||||||
| Inactive | 72.6 (10.2) | 3.5 (6.6) | 1.9 (7.8) | 3.5 (8.3) | 8.6 (8.9)† | 7.6 (8.7)† | 6.4 (7.8)† |
| Flywheel | 73.8 (8.2) | −2.8 (7.2) | −0.7 (9.6) | 3.9 (5.7) | 2.4 (8.4) | 0.2 (3.9) | 3.6 (8.0) |
| Obturator internus | |||||||
| Inactive | 57.8 (8.5) | 5.6 (7.8)* | 3.6 (9.4) | 3.3 (9.2) | 3.1 (9.0) | 2.2 (8.9) | −1.0 (9.4) |
| Flywheel | 60.4 (7.1) | 6.1 (6.6)* | 9.4 (5.0)‡ | 9.2 (6.8)† | 3.0 (6.2) | 0.2 (8.6) | 0.3 (7.8) |
| Quadratus femoris | |||||||
| Inactive | 36.1 (9.5) | −8.8 (13.3)* | −13.5 (12.2)‡ | −7.8 (11.5)* | −4.5 (8.2) | 3.9 (9.5) | 4.7 (10.5) |
| Flywheel | 40.0 (6.7) | −7.7 (10.0)* | −9.8 (11.0)* | −1.0 (10.3) | 2.0 (6.4) | 3.7 (8.0) | −2.8 (9.4) |
| Iliopsoas | |||||||
| Inactive | 71.8 (13.1) | −1.0 (11.3) | −5.9 (11.0) | 0.5 (9.5) | −1.3 (9.0) | 0.4 (10.0) | 1.7 (9.7) |
| Flywheel | 74.0 (16.8) | −5.0 (9.3) | −4.3 (9.8) | −2.1 (12.1) | 0.8 (11.8) | 4.6 (11.1) | −1.7 (10.2) |
At baseline, values are mean (SD) muscle volume in cm³. During and after bed-rest, values are mean (SD) percentage change in muscle volume compared with baseline. *: p<0.05; †:p<0.01; ‡: p<0.001 and indicate significance of difference to baseline. All p values are adjusted for false positives via the ‘false discovery rate’ method.
Figure 2.
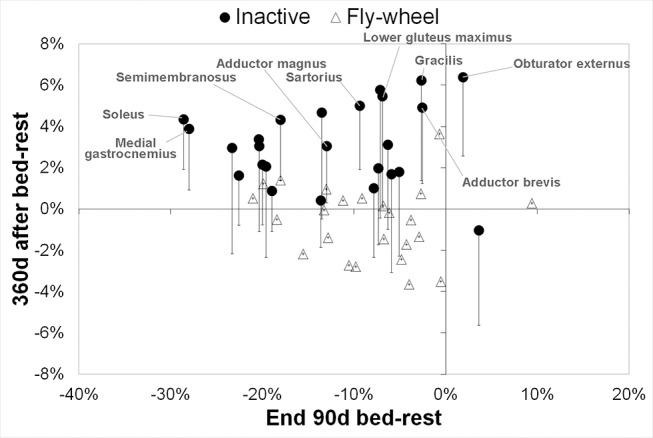
Increases muscle volume 1 year after bed-rest in inactive subjects only: muscle volume change at end bed-rest versus muscle volume 1 year after bed-rest. Values are mean percentage difference to baseline at end of bed-rest (x-axis) versus 360 days after bed-rest (y-axis). For the inactive group only, the error bars indicate the unadjusted 95% CI of the mean percentage change 360 days after bed-rest. The muscles where the unadjusted 95% CI 360 days after bed-rest does not cross zero are labelled. Note that the effect (A) was isolated to the inactive group and (B) did not appear to be related to the extent of muscle loss during bed-rest.
Relationship of muscle atrophy to peak lower limb performance
At the end of bed-rest significant associations were seen between loss of muscle volume in the vasti, soleus, gastrocnemius medialis, gastrocnemius lateralis and adductor magnus and losses of countermovement jump performance (table 3). There were no positive correlations between muscle volume differences versus baseline and countermovement jump performance 180 days after bed-rest.
Table 3.
Relationship between muscle atrophy and countermovement jump performance at the end of bed-rest and after 180 days of recovery
| Muscle | End of bed-rest | 180 days after bed-rest |
| Anterior tibial muscles | 0.47a | −0.08 |
| Flexor digitorum with tibialis posterior | 0.53a | −0.38 |
| Flexor hallucis longus | 0.50a | −0.54b,* |
| Lateral gastrocnemius | 0.64b,* | −0.01 |
| Medial gastrocnemius | 0.72c,** | −0.03 |
| Peroneals | 0.22 | −0.16 |
| Soleus | 0.71c,** | 0.15 |
| Vasti | 0.77c,*** | −0.09 |
| Rectus femoris | 0.33 | −0.42a |
| Adductor brevis | −0.04 | −0.17 |
| Adductor longus | 0.28 | −0.38 |
| Adductor magnus | 0.57b,* | 0.14 |
| Gracilis | −0.04 | 0.23 |
| Sartorius | 0.00 | −0.38 |
| Lower glut max | −0.05 | 0.23 |
| Biceps femoris long head | 0.03 | −0.13 |
| Biceps femoris short head | −0.22 | −0.39 |
| Semimembranosus | −0.10 | −0.10 |
| Semitendinosus | 0.32 | −0.27 |
| Popliteus | 0.29 | 0.17 |
| Obturator externus | −0.06 | −0.35 |
| Obturator internus | −0.07 | −0.02 |
| Quadratus femoris | 0.26 | −0.27 |
| Iliopsoas | 0.02 | −0.23 |
Values are Pearson’s correlation coefficient. *: p<0.05; **:p<0.01; ***: p<0.001 and indicate significance of correlation with p value adjusted for false positives via the ‘false discovery rate’ method. a: p<0.05; b: p<0.01; c: p<0.001 indicate significance of correlation with raw p values. Percentage change in muscle volume versus baseline was correlated with percentage change in countermovement jump power versus baseline. A positive (or negative) correlation implies greater (or less) muscle atrophy is associated with greater (or less) loss of countermovement jump power. Strong positive correlations between calf and thigh muscle atrophy were seen at the end of bed-rest, implying a dependence of jump performance loss on atrophy in these muscles. Since no positive correlations were seen 180 days after bed-rest, this implies that increased muscle volume seen in the inactive control group long-term after bed-rest (figure 2; tables 1 and 2) was not associated with functional benefits.
Discussion
The current study permits some important insights into countermeasure exercise prescription. The flywheel exercise countermeasure was effective, compared with control, in preventing or reducing atrophy in the monoarticular knee extensors (vasti), mono articular hip extensors (adductor magnus) and ankle dorsiflexors/toe flexors. The actual exercise time was 7–9 min ‘time under tension’ with 20 min total training time including rest breaks. Importantly, these findings underscore19 that a short duration, high load, resistance exercise programme can be very effective in reducing muscle atrophy in bed-rest. Previous studies that have implemented low load, non-specific muscle exercises, were unsuccessful at reducing muscle atrophy: whole body vibration in standing in a squat position without exercise did not impact lower limb muscle atrophy,17 nor did whole body vibration implemented in supine position prevent spinal muscle atrophy.24 Furthermore, exercise protocols involving aerobic-based cycling25 and lower body negative pressure26 were largely ineffective at reducing lower limb muscle atrophy. An ergometer-based cycling exercise27 can be effective in reducing lower limb suspension induced muscle atrophy when higher intensities, and hence need for higher force muscle contraction, are implemented.
The countermeasure programme did not have an impact on atrophy in the hamstrings, which are important for ambulation. Of the hamstrings, semimembranosus and biceps femoris long head are primarily hip extensors28 with semitendinosus playing more of a role in control of knee flexion.29 30 In contrast, another research group11 reported complete prevention of hamstring muscle atrophy due to their resistance exercise protocol in 20 days bed-rest when the leg press exercise was performed up to 110° of hip flexion. This underscores the need for greater specificity of exercise prescription for the hamstrings.
Maintenance of the calf musculature in disuse bed-rest via exercise has, in a number of studies, proven difficult. In the current study the atrophy of the calf muscles was significantly reduced, but muscle atrophy still occurred: 20% of soleus muscle volume was lost in the flywheel group compared with 29% loss in the inactive group at the end of bed-rest. Prior work does suggest that higher volumes of calf muscle exercise do appear to have a greater protective effect: when resistive vibration exercise including calf press exercises was performed three times a week,19 15% loss in soleus muscle volume after 56 days of bed-rest was observed, compared with 7% loss in soleus muscle volume after 56 days of bed-rest in another study2 where resistive vibration exercise was performed 11 times a week. However, for the calf muscles, there is evidence in the literature31 that attaining hypertrophy in ambulant exercise studies is more difficult with standard hypertrophy protocols than it is for the knee extensors. This is in line with the notion that muscles that are typically load-bearing in daily life are more difficult to hypertrophy than non-load-bearing muscles.32 Investigation of other exercise modes, such as treadmill running or jumping exercises, is needed to improve exercise prescription for preventing disuse atrophy of the calf musculature.
Recovery of muscle volume occurred in all muscles by 90 days after prolonged bed-rest. We observed, however, increase of muscle volume over and beyond the prebed-rest baseline volume in the inactive subjects in the long term after bed-rest. Importantly, this effect was observed in major muscle groups and not just in minor and/or small muscle groups. The time subjects spent lying supine prior to MR-scanning was strictly controlled in this study. Also, this effect was seen only in the inactive subjects, not in the flywheel exercise subjects (figure 2). We can therefore rule out a systematic, confounding, effect particular to the current study. In further support of our interpretation, peripheral quantitative CT examinations were performed in the same collective33 and these investigations also showed significant increases in lower leg muscle area 90 days and beyond after bed-rest. After another 56 days bed-rest study performed at a different facility,34 the authors observed a statistical trend towards an overshoot of calf musculature area recovery after bed-rest, again specific to the inactive group, but not the exercise group, of that study. While we cannot be certain that this muscle hypertrophy involved true muscle fibre hypertrophy, we did not see any association between these muscle volume increases in the long term after bed-rest with improvements in countermovement jump performance.
Finally, of the muscles active in the hip joint, the quadratus femoris showed the fastest rate and extent, of atrophy. When we observed this in a prior bed-rest study,35 we were not sure if this was a chance finding. In a recent cadaver study, one group concluded that quadratus femoris is a primary extensor of the hip when it is in a flexed position.36 In line with this, recent fine-wire electromyography findings37 found that quadratus femoris shows a peak of activity in walking and running during the first part of stance (ie, when the flexed hip begins to bear load) and also in late swing phase in running, presumably to decelerate the flexing hip during late swing. Overall, the recent finding shows that quadratus femoris is important for load-bearing and control of extension force at the hip joint, particularly in a flexed position. This helps to understand why quadratus femoris in particular is affected in disuse.
It is important to mention limitations of the current work. As is typical of bed-rest studies in Europe, only one gender was included in the study in an effort to reduce intersubject variability. As such, we cannot be certain that our results are applicable to female bed-rest participants. The number of subjects was limited due to logistical and financial restraints. Due to the limited number of subjects, some non-significant results for the effect of the exercise protocol may represent false negatives. For some smaller muscles, such as adductor brevis and obturator externus, reproducibility of the measurements is not as high as for larger muscle groups and non-significant findings for these muscles might be false negatives. Even though the current study provides important evidence for exercise prescription in astronauts, application of the current protocols to spaceflight could not occur on a 1:1 basis.
In conclusion, the current study investigated the impact of 90 days bed-rest on atrophy of the muscles of the lower limb, the impact of a flywheel exercise countermeasure, and the recovery of the musculature after bed-rest. The greatest rates and extent of atrophy was seen in the soleus and gastrocnemius medialis, followed by the gastrocnemius lateralis, peroneals, vasti, biceps femoris long head, other posterior calf musculature, semimembranosus, anterior tibial muscles, adductor magnus and quadratus femoris. The flywheel exercise countermeasure was effective, compared with control, in preventing or reducing atrophy in the vasti, adductor magnus and ankle dorsiflexors/toe flexors. Thus, a short-duration high-intensity resistance exercise programme performed every third day can be effective in preventing muscle atrophy in disuse. The countermeasure was, however, not effective in preventing atrophy of the hamstrings, medial thigh muscles, ankle evertors and dorsiflexors. Finally, in the long term after bed-rest we saw that inactive subjects exhibited an overshoot of muscle volume recovery in some muscle groups compared with before bed-rest.
Footnotes
Acknowledgements: The authors thank the subjects for their participation in the study. Centre National d’Etudes Spatiales (CNES) was the ‘Promoteur’ of the study according to French law and the LTBR study has been performed by MEDES, Institute for Space Physiology and Medicine. The results of the present study do not constitute endorsement by ACSM.
Contributors: DLB: analysis of MRI data, statistical analyses, drafting of manuscript.
HO: conception and design of the experiments, secured funding, approved final version of the manuscript.
JR: conception and design of the experiments, organised data acquisition, secured funding, interpretation of results, revision of manuscript.
DF: conception and design of the experiments, secured funding, revision of manuscript.
Funding: The participation of Rittweger/Felsenberg in the LTBR study was supported by the German Aerospace Center with grant number 50 WB 0156. The analyses of the image data were supported by grant number 50 WB 1220 from the German Aerospace Center (DLR). The LTBR study was sponsored by the European Space Agency (ESA), the Japan Aerospace Exploration Agency (JAXA) and the Centre National d’Etudes Spatiales (CNES).
Competing interests: None declared.
Ethics approval: Toulouse I ethics committee (CCPPRB Toulouse I) of the Rangueil University Hospital as well as the ethical committee of the Free University of Berlin.
Provenance and peer review: Not commissioned; externally peer reviewed.
Correction notice: This paper has been amended since it was published Online First. Owing to a scripting error, some of the publisher names in the references were replaced with 'BMJ Publishing Group'. This only affected the full text version, not the PDF. We have since corrected these errors and the correct publishers have been inserted into the references.
References
- 1.Nicogossian AE, Dietlein LF. Microgravity Simulation and Analogues Nicogossian AE, Space physiology and Medicine. Philadelphia: Lea & Febiger, 1982:240–8. [Google Scholar]
- 2.Belavý DL, Miokovic T, Armbrecht G, et al. Resistive vibration exercise reduces lower limb muscle atrophy during 56-day bed-rest. J Musculoskelet Neuronal Interact 2009;9:225–35. [PubMed] [Google Scholar]
- 3.Berg HE, Tesch A. A gravity-independent ergometer to be used for resistance training in space. Aviat Space Environ Med 1994;65:752–6. [PubMed] [Google Scholar]
- 4.Norrbrand L, Pozzo M, Tesch PA. Flywheel resistance training calls for greater eccentric muscle activation than weight training. Eur J Appl Physiol 2010;110:997–1005. 10.1007/s00421-010-1575-7 [DOI] [PubMed] [Google Scholar]
- 5.Alkner BA, Berg HE, Kozlovskaya I, et al. Effects of strength training, using a gravity-independent exercise system, performed during 110 days of simulated space station confinement. Eur J Appl Physiol 2003;90:44–9. 10.1007/s00421-003-0850-2 [DOI] [PubMed] [Google Scholar]
- 6.Tesch PA, Trieschmann JT, Ekberg A. Hypertrophy of chronically unloaded muscle subjected to resistance exercise. J Appl Physiol 2004;96:1451–8. 10.1152/japplphysiol.01051.2003 [DOI] [PubMed] [Google Scholar]
- 7.Irimia JM, Guerrero M, Rodriguez-Miguelez P, et al. Metabolic adaptations in skeletal muscle after 84 days of bed rest with and without concurrent flywheel resistance exercise. J Appl Physiol 2017;122:96–103. 10.1152/japplphysiol.00521.2016 [DOI] [PubMed] [Google Scholar]
- 8.Booth FW, Gollnick PD. Effects of disuse on the structure and function of skeletal muscle. Med Sci Sports Exerc 1983;15:415–20. 10.1249/00005768-198315050-00013 [DOI] [PubMed] [Google Scholar]
- 9.Akima H, Kubo K, Imai M, et al. Inactivity and muscle: effect of resistance training during bed rest on muscle size in the lower limb. Acta Physiol Scand 2001;172:269–78. 10.1046/j.1365-201x.2001.00869.x [DOI] [PubMed] [Google Scholar]
- 10.Akima H, Kubo K, Kanehisa H, et al. Leg-press resistance training during 20 days of 6 degrees head-down-tilt bed rest prevents muscle deconditioning. Eur J Appl Physiol 2000;82:30–8. 10.1007/s004210050648 [DOI] [PubMed] [Google Scholar]
- 11.Akima H, Ushiyama J-ichi, Kubo J, et al. Effect of unloading on muscle volume with and without resistance training. Acta Astronaut 2007;60:728–36. 10.1016/j.actaastro.2006.10.006 [DOI] [Google Scholar]
- 12.Belavý DL, Miokovic T, Armbrecht G, et al. Differential atrophy of the lower-limb musculature during prolonged bed-rest. Eur J Appl Physiol 2009;107:489–99. 10.1007/s00421-009-1136-0 [DOI] [PubMed] [Google Scholar]
- 13.Kouzaki M, Masani K, Akima H, et al. Effects of 20-day bed rest with and without strength training on postural sway during quiet standing. Acta Physiol 2007;189:279–92. 10.1111/j.1748-1716.2006.01642.x [DOI] [PubMed] [Google Scholar]
- 14.LeBlanc AD, Schneider VS, Evans HJ, et al. Regional changes in muscle mass following 17 weeks of bed rest. J Appl Physiol 1992;73:2172–8. [DOI] [PubMed] [Google Scholar]
- 15.Miokovic T, Armbrecht G, Felsenberg D, et al. Heterogeneous atrophy occurs within individual lower limb muscles during 60 days of bed rest. J Appl Physiol 2012;113:1545–59. 10.1152/japplphysiol.00611.2012 [DOI] [PubMed] [Google Scholar]
- 16.Shackelford LC, LeBlanc AD, Driscoll TB, et al. Resistance exercise as a countermeasure to disuse-induced bone loss. J Appl Physiol 2004;97:119–29. 10.1152/japplphysiol.00741.2003 [DOI] [PubMed] [Google Scholar]
- 17.Zange J, Mester J, Heer M, et al. 20-Hz whole body vibration training fails to counteract the decrease in leg muscle volume caused by 14 days of 6 degrees head down tilt bed rest. Eur J Appl Physiol 2009;105:271–7. 10.1007/s00421-008-0899-z [DOI] [PubMed] [Google Scholar]
- 18.Rittweger J, Frost HM, Schiessl H, et al. Muscle atrophy and bone loss after 90 days' bed rest and the effects of flywheel resistive exercise and pamidronate: results from the LTBR study. Bone 2005;36:1019–29. 10.1016/j.bone.2004.11.014 [DOI] [PubMed] [Google Scholar]
- 19.Miokovic T, Armbrecht G, Gast U, et al. Muscle atrophy, pain, and damage in bed rest reduced by resistive (vibration) exercise. Med Sci Sports Exerc 2014;46:1506–16. 10.1249/MSS.0000000000000279 [DOI] [PubMed] [Google Scholar]
- 20.Rittweger J, Felsenberg D, Maganaris C, et al. Vertical jump performance after 90 days bed rest with and without flywheel resistive exercise, including a 180 days follow-up. Eur J Appl Physiol 2007;100:427–36. 10.1007/s00421-007-0443-6 [DOI] [PubMed] [Google Scholar]
- 21.Matheson LA, Duffy S, Maroof A, et al. Intra- and inter-rater reliability of jumping mechanography muscle function assessments. J Musculoskelet Neuronal Interact 2013;13:480–6. [PubMed] [Google Scholar]
- 22.Rittweger J, Schiessl H, Felsenberg D, et al. Reproducibility of the jumping mechanography as a test of mechanical power output in physically competent adult and elderly subjects. J Am Geriatr Soc 2004;52:128–31. 10.1111/j.1532-5415.2004.52022.x [DOI] [PubMed] [Google Scholar]
- 23.Gast U, John S, Runge M, et al. Short-duration resistive exercise sustains neuromuscular function after bed rest. Med Sci Sports Exerc 2012;44:1764–72. 10.1249/MSS.0b013e318256b53b [DOI] [PubMed] [Google Scholar]
- 24.Holguin N, Muir J, Rubin C, et al. Short applications of very low-magnitude vibrations attenuate expansion of the intervertebral disc during extended bed rest. Spine J 2009;9:470–7. 10.1016/j.spinee.2009.02.009 [DOI] [PubMed] [Google Scholar]
- 25.Suzuki Y, Kashihara H, Takenaka K, et al. Effects of daily mild supine exercise on physical performance after 20 days bed rest in young persons. Acta Astronaut 1994;33:101–11. 10.1016/0094-5765(94)90114-7 [DOI] [PubMed] [Google Scholar]
- 26.Berry P, Berry I, Manelfe C. Magnetic resonance imaging evaluation of lower limb muscles during bed rest--a microgravity simulation model. Aviat Space Environ Med 1993;64:212–8. [PubMed] [Google Scholar]
- 27.Akima H, Hotta N, Sato K, et al. Cycle ergometer exercise to counteract muscle atrophy during unilateral lower limb suspension. Aviat Space Environ Med 2009;80:652–6. 10.3357/ASEM.2399.2009 [DOI] [PubMed] [Google Scholar]
- 28.Ono T, Higashihara A, Fukubayashi T. Hamstring functions during hip-extension exercise assessed with electromyography and magnetic resonance imaging. Res Sports Med 2011;19:42–52. 10.1080/15438627.2011.535769 [DOI] [PubMed] [Google Scholar]
- 29.Mendiguchia J, Garrues MA, Cronin JB, et al. Nonuniform changes in MRI measurements of the thigh muscles after two hamstring strengthening exercises. J Strength Cond Res 2013;27:574–81. 10.1519/JSC.0b013e31825c2f38 [DOI] [PubMed] [Google Scholar]
- 30.Ono T, Okuwaki T, Fukubayashi T. Differences in activation patterns of knee flexor muscles during concentric and eccentric exercises. Res Sports Med 2010;18:188–98. 10.1080/15438627.2010.490185 [DOI] [PubMed] [Google Scholar]
- 31.Ferri A, Scaglioni G, Pousson M, et al. Strength and power changes of the human plantar flexors and knee extensors in response to resistance training in old age. Acta Physiol Scand 2003;177:69–78. 10.1046/j.1365-201X.2003.01050.x [DOI] [PubMed] [Google Scholar]
- 32.Welle S, Totterman S, Thornton C. Effect of age on muscle hypertrophy induced by resistance training. J Gerontol A Biol Sci Med Sci 1996;51:M270–M275. 10.1093/gerona/51A.6.M270 [DOI] [PubMed] [Google Scholar]
- 33.Rittweger J, Felsenberg D. Recovery of muscle atrophy and bone loss from 90 days bed rest: results from a one-year follow-up. Bone 2009;44:214–24. 10.1016/j.bone.2008.10.044 [DOI] [PubMed] [Google Scholar]
- 34.Rittweger J, Beller G, Armbrecht G, et al. Prevention of bone loss during 56 days of strict bed rest by side-alternating resistive vibration exercise. Bone 2010;46:137–47. 10.1016/j.bone.2009.08.051 [DOI] [PubMed] [Google Scholar]
- 35.Miokovic T, Armbrecht G, Felsenberg D, et al. Differential atrophy of the postero-lateral hip musculature during prolonged bedrest and the influence of exercise countermeasures. J Appl Physiol 2011;110:926–34. 10.1152/japplphysiol.01105.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vaarbakken K, Steen H, Samuelsen G, et al. Primary functions of the quadratus femoris and obturator externus muscles indicated from lengths and moment arms measured in mobilized cadavers. Clin Biomech 2015;30:231–7. 10.1016/j.clinbiomech.2015.02.004 [DOI] [PubMed] [Google Scholar]
- 37.Semciw AI, Freeman M, Kunstler BE, et al. Quadratus femoris: an EMG investigation during walking and running. J Biomech 2015;48:3433–9. 10.1016/j.jbiomech.2015.05.029 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjsem-2016-000196supp001.pdf (172.9KB, pdf)


