Abstract
Metaplastic carcinoma of the breast (MBC) is a rare and heterogeneous type of neoplasm. Knowledge about its clinical characteristics, prognostic significance and optimal treatment modalities is fragmentary and controversial. The present retrospective study aimed to investigate the prognostic value of the clinicopathological features and different therapeutic strategies in MBC. For this purpose, the medical records of 69 MBC patients subjected to surgical resection for MBC at the Tianjin Medical University Cancer Institute and Hospital (Tianjin, China) were reviewed. A total of 69 MBC cases were followed up for 9–139 months. The 5-year disease-free survival (DFS) rate was 52.2% and the overall survival (OS) rate was 60.2%. Survival analysis revealed that large tumor size and lymph node (LN) metastases were correlated with shortened 5-year DFS and OS rates. In addition, chemotherapy significantly improved the prognosis of patients with LN metastasis, while radiation therapy (RT) significantly improved the 5-year OS and DFS rates of MBC patients with tumors ≥5 cm or with >4 metastatic LNs. In conclusion, MBC is a clinically aggressive subtype of breast cancer associated with a large tumor size. Chemotherapy may be recommended for certain subtypes of MBC with LN positivity, and RT may be a component of multimodality therapy for some MBC patients.
Keywords: metaplastic breast carcinoma, prognosis, therapy, characteristics
Introduction
Metaplastic breast carcinoma (MBC) is a rare and heterogeneous type of neoplasm characterized by the histological presence of ≥2 cellular types, commonly a mixture of epithelial and mesenchymal components (1). The World Health Organization (WHO) recognized this subtype of breast cancer as a unique pathological entity in 2000, and its incidence is <1% of all breast malignancies (2,3). The key concept in the pathogenesis and development of MBC may be that epithelial to mesenchymal transition (EMT)-associated genes are differentially upregulated (4) and enriched in tumor-initiating cells (5).
Only a few large studies on MBC have been published to date (6,7); however, MBC has long been recognized as a distinct histological type of breast cancer (8). Due to its low incidence rate and pathological variability, the clinical characteristics, prognostic significance and optimal treatment modalities are unclear and controversial. Certain reports have suggested that the prognosis of MBC is favorable, with survival similar to that of adenocarcinoma of a comparable stage (9,10), whereas others consider that MBC may exhibit an aggressive course, with worse outcomes than those of triple-negative invasive ductal carcinoma (11,12). MBCs usually present as large tumors that are negative for hormone receptors and rarely benefit from conventional chemotherapy or hormonal therapy (13). Although the incidence of axillary lymph node (LN) involvement is low, these tumors exhibit an increased risk of developing distant organ metastasis compared with infiltrating ductal carcinoma (IDC) (14). In clinical practice, MBC is usually treated based on the guidelines developed for IDC (15). The ideal treatment paradigm for MBC is unknown; thus, potential predictors of treatment efficacy must be explored.
The present study reviewed the clinical, pathological and biological characteristics of 69 patients with MBC who were diagnosed and treated at Tianjin Medical University Cancer Institute and Hospital (Tianjin, China), and evaluated the clinicopathological features, different therapeutic strategies and prognostic factors of survival.
Patients and methods
Patients
Among 30,053 patients who underwent surgery for breast cancer at Tianjin Medical University Cancer Institute and Hospital (Tianjin, China) from January 1, 2002 to January 1, 2015, 69 (0.23%) patients were identified as MBC. All patients were females and their ages ranged from 28 to 89 years, with a median age of 53 years. Details concerning clinicopathological characteristics, surgical treatment, chemotherapy and radiation therapy (RT) were also gathered from the medical records.
In total, 53 cases received modified radical mastectomy (MRM), 9 cases received radical mastectomy (RM), 2 cases received segmental mastectomy and axillary dissection, and 5 cases received segmental mastectomy only. A total of 50 cases had received chemotherapy, 14 cases had received radiotherapy, and 8 cases with positive estrogen receptor (ER) or progesterone receptor (PR) expression had received adjuvant hormonal therapy. The pathological diagnosis was performed in accordance with the histological classification of tumors developed by the WHO (16) and clinical staging was based on the tumor-node-metastasis staging of breast cancer developed by the American Joint Committee on Cancer (AJCC, 7th edition) (17).
The diagnosis of MBC was conducted in the absence of an associated primary metaplastic cell type in a secondary site and in the absence of skin involvement. The pathologic material for each case of MBC was retrieved from the archive of the Department of Breast Cancer at the Tianjin Medical University Cancer Institute and Hospital (Tianjin, China) and reviewed.
Immunohistochemistry (IHC) and fluorescent in situ hybridization (FISH)
Formalin-fixed paraffin embedded tissue sections were employed in each case using a standard protocol. IHC staining and FISH analysis were performed on tissue sections (3–4 µm) and tissue microarray slides. IHC staining (Benchmark XT; Ventana Medical Systems, Tucson, AZ, USA) was performed on tumor sections from 69 patients using the avidin-biotin-immunoperoxidase technique for ER (cat no. NCL-L-PGR-312; dilution, 1:100), PR (cat no. NCL-L-ER-6F11; dilution, 1:80) [both from Novocastra, Leica Biosystems (Newcastle) Ltd., Newcastle Upon Tyne, UK] and human epidermal growth factor receptor 2 (HER-2; cat no. 800-2996; dilution 1:300; Ventana Medical Systems). An iView DAB detection kit (Ventana Medical Systems) was used for secondary antibody. The FISH test was performed according to the Abbott/Vysis PathVysion HER2 DNA Probe kit (cat no. 30161060/02J01-030; Abbott Molecular Inc., Des Plaines, IL, USA) manufacturers protocol. The SpectrumOrange fluorophore-labeled DNA probe for the HER-2/neu gene locus and SpectrumGreen fluorophore-labeled α-satellite DNA probe for chromosome 17 from this kit were used. In total 2 separate fields of ≥20 cells were counted and an average of the results from the preselected tumor areas were used to create mean average gene and chromosomal counts, which were used to calculate the HER2:CEP17 signal ratio. Tumor cells from matching sites of IHC were scored for the number of red (HER2) and green (chromosome 17) signals. The slides were evaluated using an Olympus BX51 microscope (Olympus, Tokyo, Japan) with an oil-immersion objective lens and an appropriate filter set at magnification, ×100. The immunoreaction was evaluated independently by ≥2 pathologists.
The threshold used for positive ER and PR expression was 1% of nuclear staining in the total number of tumor cells stained. HER-2 immunoreactivity was evaluated on a standardized scale from 0–3 based on the intensity of staining of the cell membrane and the proportion of invasive tumor cells stained. Strong complete staining of the membrane in >10% of tumor cells (score, 3+) was considered positive. Intensity patterns with scores 0–1+ were considered negative, and samples scored as 2+ were further assessed by FISH test where HER2/CEP17 ratio of >2.0 was considered positive for HER2 gene amplification.
Follow-up
Overall survival (OS) was defined as the time from the date of first surgery to the date of mortality or last follow-up. Disease-free survival (DFS) was defined as the duration of time between the date of first surgery and the date of first local recurrence or distant metastasis or last follow-up. For all patients, follow-up started from the date of operation. The patients were followed up in the Outpatients Department of Tianjin Medical University Cancer Institute and Hospital at 3 months intervals for the first year, at 6 months intervals for the following 2 years and then annually. All patients were followed up until mortality or the cut-off date of January 1, 2015. A total of 3 cases were lost to follow-up, and the median follow-up time was 37 months (range, 9–139 months). OS data were obtained from medical records or by telephone calls or letter communication. This study was approved by the Institutional Review Board of the Tianjin Medical University Cancer Institute and Hospital and written consent was obtained from all participants.
Statistical analysis
All statistical analyses were carried out using SPSS 17.0 (SPSS, Inc., Chicago, IL, USA) for Windows. Cumulative survival analysis of the patients was used to evaluate possible associations between survival and patient covariates. The Kaplan-Meier method and the log-rank test were used for univariate analysis, while the Cox proportional hazard model was applied for multivariate analysis. All tests were two sided. P<0.05 or a 95% confidence interval (CI) that did not include 1 was considered to indicate a statistically significant difference.
Results
Clinicopathological characteristics
The clinical characteristics of MBC patients are summarized in Table I. Among the patients, squamous and sarcomatoid were the most common histological subtype, followed by adenosquamous, spindle cell and osseous/chondroid carcinoma. A total of 11 patients had a family history of cancer. The most common clinical presentation was a firm lump, which was noted in 66 patients, among which, 3 patients had a history of blood nipple discharge and other 3 patients had a history of blood nipple discharge with pain. Tumor sizes were determined by gross pathological examination, and ranged from 0.8 to 10.0 cm (mean, 3.7 cm; median, 3.5 cm). LN involvement was reported in 29.0% of the 69 MBC patients. Among all the MBC cases, 78.2% had triple-negative breast cancer (TNBC) tumors and 69.6% had basal-like type breast carcinomas.
Table I.
Clinicopathological features of patients with metaplastic carcinoma of the breast.
| Patients | ||
|---|---|---|
| Variables | No. | % |
| Age, years | ||
| <50 | 22 | 31.9 |
| ≥50 | 47 | 68.1 |
| Menopausal status | ||
| Premenopausal | 26 | 37.7 |
| Postmenopausal | 43 | 63.3 |
| Tumor stage | ||
| T1 | 10 | 14.5 |
| T2 | 42 | 60.9 |
| T3 | 12 | 17.4 |
| T4 | 5 | 7.2 |
| Lymph node stage | ||
| N0 | 44 | 63.8 |
| N1 | 14 | 20.3 |
| N2 | 5 | 7.2 |
| N3 | 1 | 1.4 |
| Unknown ER status | 5 | 7.2 |
| Negative | 59 | 85.5 |
| Positive | 10 | 14.5 |
| PR status | ||
| Negative | 63 | 91.3 |
| Positive | 6 | 8.70 |
| HER-2 status | ||
| Negative | 60 | 87.0 |
| Positive | 9 | 13.0 |
| Subtype | ||
| Squamous cell | 22 | 31.9 |
| Adenosquamous cell | 14 | 20.3 |
| Spindle cell | 9 | 13.0 |
| Chondroid | 2 | 2.9 |
| Sarcomatoid | 22 | 31.9 |
ER, estrogen receptor; PR, progesterone receptor; HER-2, human epidermal growth factor receptor 2.
Treatment modalities
All patients received surgical treatment. The most common surgical procedure was MRM, which was performed in 53 patients. If required, patients would receive this treatment in combination with chemotherapy, radiotherapy and/or hormonal therapy post-surgery (Table II). All chemotherapy regimens and their outcomes in 50 patients are summarized in Table III. The remaining 19 patients did not receive chemotherapy due to poor health or economic problems.
Table II.
Treatment modalities.
| Patients | ||
|---|---|---|
| Treatment modalities | No. | % |
| Breast surgery | ||
| Modified-radical mastectomy | 53 | 76.8 |
| Radical mastectomy | 9 | 13.0 |
| Breast-conserving surgery with | 2 | 2.9 |
| Axillary lymph nodes dissection | ||
| Segmental mastectomy | 5 | 7.2 |
| Chemotherapy | ||
| Yes | 50 | 72.5 |
| No | 19 | 27.5 |
| Radiation therapy | ||
| Yes | 14 | 20.3 |
| No | 55 | 79.7 |
| Adjuvant hormonal therapy | ||
| Yes | 8 | 11.6 |
| No | 61 | 88.4 |
| Adjuvant trastuzumab | ||
| Yes | 2 | 2.9 |
| No | 67 | 97.1 |
Table III.
Adjuvant chemotherapy regimens and their outcomes.
| Chemotherapy regimens | Patients, no. | 5-year DFS, % | MST |
|---|---|---|---|
| CMF | 9 | 62.2 | 6 relapse free at 122, 89, 73, 34, 27 and 22 months, respectively; 3 relapsed |
| AT/ET/TAC/TEC | 31 | 52.3 | 14 relapse free at 133, 99, 89, 76, 67, 66, 63, 62, 61, 60, 46, 41, 26 and 20 months, respectively; 17 relapsed |
| NP/CP/TP | 10 | 70.0 | 6 relapse free at 139, 105, 79, 76, 60 and 24 months, respectively; 4 relapsed |
DFS, disease-free survival; MST, median survival time; CMF cyclophosphamide, methotrexate and 5-fluorouracil; AT, docetaxel and adriamycin; ET, epirubicin and docetaxel; TAC, docetaxel, adriamycin and cyclophosphamide; TEC, docetaxel, epirubicin and cyclophosphamide; NP, vinorelbine and cisplatin; CP, cyclophosphamide and cisplatin; TP, docetaxel and cisplatin.
Outcome, recurrence and prognosis
Up to the cut-off date for the analysis, 3 patients were lost to follow-up, 35 patients were still alive without recurrence and 28 patients succumbed to disease progression. In total, 20 patients experienced locoregional recurrence during the follow-up subsequent to surgery. Of the 20 patients with locoregional relapse, 13 relapses occurred in the chest wall, 4 relapses occurred in the ipsilateral breast and 3 relapses occurred in the ipsilateral axilla. Among 28 patients who developed distant metastasis during the follow-up, the most common organs involved were the lung (n=16), liver (n=9), bone (n=7), supraclavicular LNs (n=3) and brain (n=4). The 5-year DFS and OS rates were 52.2 and 60.2%, respectively.
Univariate and multivariate analysis of OS and DFS
The univariate and multivariate analyses for the association between 5-year DFS and OS rates and clinicopathological characteristics are shown in Table IV. Kaplan-Meier analysis and log-rank test revealed that T stage and LN status were significant predictors for DFS and OS in univariate analysis. Chemotherapy could significantly improve the 5-year OS rate in univariate analysis, whereas no significantly difference for the 5-year DFS rate was observed. The 5-year OS rate was 68.7% in the chemotherapy group and 37.2% in the non-chemotherapy group. When the above variables were analyzed by a Cox proportional hazard model, T stage and LN status remained significant independent predictors for DFS and OS. The hazard ratio for patients subjected to chemotherapy was 0.27 (95% CI, 0.11–0.67) for OS.
Table IV.
Analysis of the prognostic factors for DFS and OS.
| 5-year DFS | 5-year OS | |||||||
|---|---|---|---|---|---|---|---|---|
| Univariate analysis | Multivariate analysis | Univariate analysis | Multivariate analysis | |||||
| Variables | HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value |
| Age, years | 0.213 | 0.380 | ||||||
| <50 | 1 | 1 | ||||||
| ≥50 | 1.63 (0.76–3.51) | 1.45 (0.63–3.31) | ||||||
| Menopausal status | 0.153 | 0.165 | ||||||
| Premenopausal | 1 | 1 | ||||||
| Postmenopausal | 1.70 (0.82–3.51) | 1.76 (0.79–3.93) | ||||||
| T stage | 0.001 | 0.001 | 0.003 | 0.001 | ||||
| T1-2 | 1 | 1 | 1 | 1 | ||||
| T3-4 | 3.18 (1.57–6.42) | 4.09 (1.84–9.06) | 3.27 (1.51–7.07) | 3.90 (1.71–8.90) | ||||
| LN stage | 0.026 | 0.016 | 0.013 | 0.028 | ||||
| LN− | 1 | 1 | 1 | 1 | ||||
| LN+ | 2.21 (1.01–4.45) | 2.62 (1.20–5.72) | 2.68 (1.24–5.81) | 2.45 (1.10–5.44) | ||||
| Hormone receptor expression status | 0.289 | 0.250 | ||||||
| Negative | 1 | 1 | ||||||
| Positive | 0.57 (0.20–1.62) | 0.49 (0.25–1.65) | ||||||
| Chemotherapy | 0.096 | 0.010 | 0.005 | |||||
| No | 1 | 1 | 1 | |||||
| Yes | 0.52 (0.24–1.12) | 0.33 (0.14–0.77) | 0.27 (0.11–0.67) | |||||
| Radiotherapy | 0.539 | 0.582 | ||||||
| No | 1 | 1 | ||||||
| Yes | 1.27 (0.59–2.74) | 1.26 (0.55–2.89) | ||||||
| Hormonal therapy | 0.240 | 0.359 | ||||||
| No | 1 | 1 | ||||||
| Yes | 0.49 (0.15–1.61) | 0.57 (0.17–1.90) | ||||||
P<0.05 was considered to indicate a statistical significant value. OS, overall survival; DFS, disease-free survival; HR, hazard ratio; CI, confidence interval.
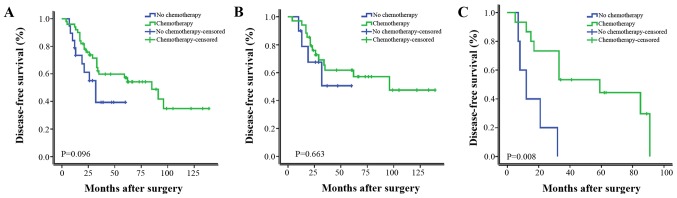
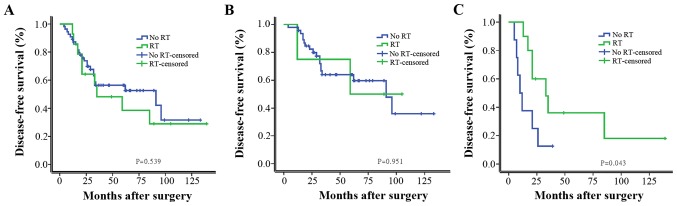
Furthermore, patients were stratified as LN positive (LN+) and LN negative (LN−). As shown in Fig. 1, chemotherapy significantly improved the DFS in univariate analysis. In a subgroup analysis of the patients with LN+ group and LN− group, chemotherapy significantly improved the DFS of LN+ patients, LN− patients did not gain a significant survival benefit from chemotherapy. As shown in Fig. 2, RT did not significantly improve the DFS in univariate analysis; however, it significantly improved the DFS of patients with tumors ≥5 cm or with >4 metastatic axillary LNs.
Figure 1.
Kaplan-Meier estimates of disease-free survival according to adjuvant chemotherapy for (A) all patients with metaplastic carcinoma of the breast (P=0.096), (B) LN− group (P=0.663) and (C) LN+ group (P=0.008). LN, lymph node.
Figure 2.
Kaplan-Meier estimates of disease-free survival according to RT for (A) all patients with metaplastic carcinoma of the breast (P=0.539), (B) patients with tumors <5 cm and with <4 metastatic LNs (P=0.951) and (C) patients with tumors ≥5 cm or with >4 metastatic LNs (P=0.043). RT, radiation therapy; LN, lymph node.
Discussion
MBC is an extremely rare malignancy and the current WHO 2012 classification distinguishes five subtypes of MBC (16). Previous studies reported that no significant difference was observed in terms of clinical outcome among patients with different MBC subtypes (18,19). In the present study, there was no significant difference in OS or DFS among squamous cell carcinoma, spindle cell carcinoma, adenosquamous carcinoma or carcinosarcoma. As previously reported, MBC tends to occur in older females, and usually presents as a palpable mass that grows rapidly, which may indicate that MBC is an aggressive disease (20). A previous study reported that tumor size was best correlated with prognosis (21), whereas this finding was not consistent with another study (22). In the current study, 24.6% of patients presented with a palpable tumor at stage T3-4. Among them, 14 experienced relapse. By contrast, of the 52 cases with a palpable tumor at stage T1-2, 25 relapsed, and had a significant difference in their 5-year DFS and OS rates in univariate analysis. The tumor size acted as an independent prognostic factor in multivariate analysis for MBC patients. Previous studies have demonstrated axillary LN metastases in 22–31% of patients with MBC (14,23). Despite this low rate, MBC patients with LN metastasis had a greater risk of developing metastatic disease and a poorer prognosis than IDC patients (24,25). This may also support the concept that MBC is an aggressive tumor with a high risk of recurrence following primary therapy. In the current study, 29.0% of patients had axillary LN metastases, which is consistent with the majority of previous reports (23). In univariate analysis, LN status was a significant prognostic factor, while in multivariate analysis, LN positivity remained an independent risk factor for the 5-year DFS and OS rates. MBC patients with a large tumor size and LN positivity had a poor survival outcome. Therefore, early diagnosis and treatment of this rare entity is critical to patient prognosis.
The majority of MBC cases have similar characteristics. MBCs have a basal-like immunophenotype, and rarely overexpress hormone receptors and HER-2 (26). In a study on 2,338 patients with MBC, 21.0% were hormone receptor positive, and hormone receptor positivity does not improve prognosis (27). According to the present study, 78.2% of patients had TNBC tumors and 69.6% had basal-like type breast carcinomas, which may help to explain the commonly observed higher grade and increased rapid growth of MBCs compared with IDCs. In addition, there was no significant difference in the 5-year DFS or OS rates between the TNBC group and the non-TNBC group in the present study.
As MBC patients typically present with large tumors, >70% of patients with MBC present with AJCC stage II (14) and a higher percentage of patients with MBC receive mastectomy rather than lumpectomy (3,9). However, a previous study observed no difference in OS or DFS in MBC patients treated with mastectomy compared with those treated with lumpectomy, even upon controlling for known prognostic factors (22). In the present study, the majority of cases were stage II and received MR/MRM. There was no significant difference between MRM/RM and other type of operation. Taking into account the large tumor size, and the somewhat refractory nature of the tumor to standard chemotherapy and hormonal therapy, MRM/RM was selected as an optimal surgical treatment; however, breast conservative surgery and segmental mastectomy cannot be precluded in certain eligible patients.
Several MBC cases have been shown to exhibit a good response to chemotherapy (28). Takuwa et al (29) reported that a patient had a good response to platinum combined with taxane or anthracycline therapy. The majority of previous studies, however, have revealed an ineffective response of MBC to chemotherapy (30). Compared with the response rates of stage-matched female patients with IDC, those with MBC receiving chemotherapy had lower response rates to the chemotherapy regimens (30,31). A single-institute retrospective study reported that tumor response to systemic chemotherapy remains generally poor, since only 17.6% of patients who received taxane-based chemotherapy exhibited a positive response (32). The reason for the ineffective response to chemotherapy may be that MBCs are part of the spectrum of basal-like breast carcinomas and display a myoepithelial and EMT-like molecular makeup (26). Basal-like tumors and breast cancer 1 (BRCA1)-associated breast cancer tumors were reported to be similar according to microarray and immunohistochemical analyses (33,34). In BRCA1-associated breast cancer and basal-like tumors, current standard anthracycline- and taxanes-containing chemotherapy regimens were prone to ineffectiveness (35). However, these regimens were able to sensitize tumors with homologous recombination DNA repair defects to platinum salts and poly ADP-ribose polymerase (PARP) inhibitors (36). The results from two randomized trials indicated that cyclophosphamide, methotrexate and 5-fluorouracil (CMF) chemotherapy significantly improved treatment outcome in patients with triple-negative, node-negative breast cancer (37). In the present study, MBC patients with LN− did not benefit from chemotherapy. By contrast in MBC patients with LN+, the 5-year OS and DFS rates significantly improved with chemotherapy. Among the different chemotherapy regimens, CMF and cisplatin-based regimens may be effective to certain subgroups of MBC patients, as suggested by their relatively long median 5-year DFS, although no significant survival benefit from different regimens was observed in these patients. These findings would suggest that a subset of MBC patients may potentially experience a curative benefit from systemic chemotherapy, but they also support the chemorefractory behavior of these tumors. Platinum salts combined with PARP inhibitors could be used for clinical treatment. The limitations of the present study include: i) The majority of patients who did not receive chemotherapy were elderly patients with a relative poor health; ii) a small sample was analyzed; and iii) the positive rate of LN was low. Therefore, additional clinical data and multicenter studies are required to confirm the present results.
Tseng and Martinez (38) described patients with MBC who had received RT and experienced a benefit in terms of OS and DFS, which suggests that patients undergoing breast conservation surgery and those with tumors ≥5 cm or with >4 metastatic axillary LNs undergoing mastectomy should receive RT. Another study suggested that RT, regardless of the type of surgery, should be considered as a part of the therapy for patients with MBC (15). In the present study, survival analysis did not reveal a significant survival benefit from RT, since no significant differences were observed in the risk of recurrence for those patients treated with adjuvant RT and not treated with RT. However, in MBC patients with tumors ≥5 cm or with >4 metastatic axillary LNs who received RT, the 5-year OS and DFS rates significantly improved. Genomic profiling of MBC tumors has shown downregulation of DNA repair pathways, including the BRCA1, phosphatase and tensin homolog and topoisomerase 2-α pathways, which may explain the lower incidence of LN spread and sensitivity towards RT of MBC (39). The present results demonstrated that RT should be considered as a component of multimodality therapy for MBC patients with tumors ≥5 cm or with >4 metastatic axillary LNs. However, since the present study is a retrospective analysis with a low LN metastasis rate and a small sample receiving RT, a larger multicenter study is required to draw definitive conclusions.
In MBC patients, the positive expression of hormone receptors has been reported to be low (40). However, hormone receptor positivity does not improve the prognosis of MBC patients (27), since in a retrospective study, the non-TNBC group of MBC patients had a poor prognosis compared with that of the TNBC group (41). MBC patients often show little or no response to adjuvant hormonal therapy or HER-2-targeted treatment (trastuzumab) (42). In the present study, only 8 patients had been prescribed tamoxifen/aromatase inhibitors therapy, as they had ER- and/or PR-positive tumors. Of these patients, 3 relapsed at 17, 28 and 62 months after surgery, respectively. The tumors in 9 patients were HER-2+; however, only 2 patients were treated with trastuzumab due to financial reasons. Of these 2 patients, 1 developed lung and brain metastases 33 months after surgery and succumbed to the disease 7 months later, while the other patient is still alive and free of recurrence at 43 months after surgery. Due to the high incidence of TNBC in MBC, hormonal therapy or HER-2-targeted treatment is unlikely to influence the survival of MBC patients.
In conclusion, MBC is a clinically aggressive subtype of breast cancer associated with large tumor size and a high proportion of TNBC and basal-like tumors. Hormonal therapy or HER-2-targeted treatment is unlikely to influence survival due to the high incidence of TNBC tumors. Chemotherapy may be recommended for certain subtype of MBC patients with LN positivity. Cisplatin-based regimens and CMF regimens may be effective in certain subgroups. MBC patients with tumors ≥5 cm or with >4 metastatic LNs could benefit from RT. Other therapeutic strategies, including mechanistic target of rapamycin, androgen receptor and transforming growth factor-β, should strongly be considered in clinical trials of innovative therapeutic regimens. However, the present study possess certain limitations: This is a retrospective analysis with a small sample size and not a prospective study; a multicenter study would be preferable in the future. There may have also been a lack of uniformity as the surgery was performed by different surgeons. To additionally clarify the characteristics and prognosis of MBC patients, and in order to improve treatment, the systematic study of a large number of cases with long-term follow-up will be necessary.
Acknowledgements
The present study was funded by the National Natural Science Foundation of China (grant no. 81472472).
References
- 1.Yigit S, Pehlivan FS, Evcim G, Etit D. Clinicopathologic features of the mixed epithelial and mesenchymal type metaplastic breast carcinoma with myoepithelial differentiation in a subset of six cases. Pathol Res Pract. 2012;208:147–150. doi: 10.1016/j.prp.2011.12.014. [DOI] [PubMed] [Google Scholar]
- 2.Velasco M, Santamaría G, Ganau S, Farrús B, Zanón G, Romagosa C, Fernández PL. MRI of metaplastic carcinoma of the breast. AJR Am J Roentgenol. 2005;184:1274–1278. doi: 10.2214/ajr.184.4.01841274. [DOI] [PubMed] [Google Scholar]
- 3.Al Sayed AD, El Weshi AN, Tulbah AM, Rahal MM, Ezzat AA. Metaplastic carcinoma of the breast clinical presentation, treatment results and prognostic factors. Acta Oncol. 2006;45:188–195. doi: 10.1080/02841860500513235. [DOI] [PubMed] [Google Scholar]
- 4.Lien HC, Hsiao YH, Lin YS, Yao YT, Juan HF, Kuo WH, Hung MC, Chang KJ, Hsieh FJ. Molecular signatures of metaplastic carcinoma of the breast by large-scale transcriptional profiling: Identification of genes potentially related to epithelial-mesenchymal transition. Oncogene. 2007;26:7859–7871. doi: 10.1038/sj.onc.1210593. [DOI] [PubMed] [Google Scholar]
- 5.Zhang Y, Toy KA, Kleer CG. Metaplastic breast carcinomas are enriched in markers of tumor-initiating cells and epithelial to mesenchymal transition. Mod Pathol. 2012;25:178–184. doi: 10.1038/modpathol.2011.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Langlands F, Cornford E, Rakha E, Dall B, Gutteridge E, Dodwell D, Shaaban AM, Sharma N. Imaging overview of metaplastic carcinomas of the breast: A large study of 71 cases. Br J Radiol. 2016 Jun 21; doi: 10.1259/bjr.20140644. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sherwell-Cabello S, Maffuz-Aziz A, Hernández-Hernández B, Bautista-Piña V, Labastida-Almendaro S, Rodríguez-Cuevas S. Metaplastic carcinoma of the breast and the impact of the p63 and cytokeratin 5/6: Experience of 40 patients. Ginecol Obstet Mex. 2016;84:127–135. (In Spanish) [PubMed] [Google Scholar]
- 8.Grechi G, Pagnini P. Study of mammary gland neoplasms with an osteocartilaginous component. I. Cartilaginous metaplastic epiphenomena in the course of connective tissue malignancy. Arch De Vecchi Anat Patol. 1965;46:277–303. (In Italian) [PubMed] [Google Scholar]
- 9.Gibson GR, Qian D, Ku JK, Lai LL. Metaplastic breast cancer: Clinical features and outcomes. Am Surg. 2005;71:725–730. [PubMed] [Google Scholar]
- 10.Chao TC, Wang CS, Chen SC, Chen MF. Metaplastic carcinomas of the breast. J Surg Oncol. 1999;71:220–225. doi: 10.1002/(SICI)1096-9098(199908)71:4<220::AID-JSO3>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 11.Jung SY, Kim HY, Nam BH, Min SY, Lee SJ, Park C, Kwon Y, Kim EA, Ko KL, Shin KH, et al. Worse prognosis of metaplastic breast cancer patients than other patients with triple-negative breast cancer. Breast Cancer Res Treat. 2010;120:627–637. doi: 10.1007/s10549-010-0780-8. [DOI] [PubMed] [Google Scholar]
- 12.Nelson RA, Guye ML, Luu T, Lai LL. Survival outcomes of metaplastic breast cancer patients: Results from a US population-based analysis. Ann Surg Oncol. 2015;22:24–31. doi: 10.1245/s10434-014-3890-4. [DOI] [PubMed] [Google Scholar]
- 13.Beatty JD, Atwood M, Tickman R, Reiner M. Metaplastic breast cancer: Clinical significance. Am J Surg. 2006;191:657–664. doi: 10.1016/j.amjsurg.2006.01.038. [DOI] [PubMed] [Google Scholar]
- 14.Pezzi CM, Patel-Parekh L, Cole K, Franko J, Klimberg VS, Bland K. Characteristics and treatment of metaplastic breast cancer: Analysis of 892 cases from the National Cancer Data Base. Ann Surg Oncol. 2007;14:166–173. doi: 10.1245/s10434-006-9124-7. [DOI] [PubMed] [Google Scholar]
- 15.Shah DR, Tseng WH, Martinez SR. Treatment options for metaplastic breast cancer. ISRN Oncol. 2012;2012:706162. doi: 10.5402/2012/706162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Frank GA, Danilova NV, Andreeva Iulu, Nefedova NA. WHO classification of tumors of the breast, 2012. Arkh Patol. 2013;75:53–63. (In Russian) [PubMed] [Google Scholar]
- 17.Sinn HP, Helmchen B, Wittekind CH. TNM classification of breast cancer: Changes and comments on the 7th edition. Pathologe. 2010;31:361–366. doi: 10.1007/s00292-010-1307-0. (In German) [DOI] [PubMed] [Google Scholar]
- 18.Okada N, Hasebe T, Iwasaki M, Tamura N, Akashi-Tanaka S, Hojo T, Shibata T, Sasajima Y, Kanai Y, Kinoshita T. Metaplastic carcinoma of the breast. Hum Pathol. 2010;41:960–970. doi: 10.1016/j.humpath.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 19.Yamaguchi R, Horii R, Maeda I, Suga S, Makita M, Iwase T, Oguchi M, Ito Y, Akiyama F. Clinicopathologic study of 53 metaplastic breast carcinomas: Their elements and prognostic implications. Hum Pathol. 2010;41:679–685. doi: 10.1016/j.humpath.2009.10.009. [DOI] [PubMed] [Google Scholar]
- 20.Choi BB, Shu KS. Metaplastic carcinoma of the breast: Multimodality imaging and histopathologic assessment. Acta Radiol. 2012;53:5–11. doi: 10.1258/ar.2011.110341. [DOI] [PubMed] [Google Scholar]
- 21.Oberman HA. Metaplastic carcinoma of the breast. A clinicopathologic study of 29 patients. Am J Surg Pathol. 1987;11:918–929. doi: 10.1097/00000478-198712000-00002. [DOI] [PubMed] [Google Scholar]
- 22.Dave G, Cosmatos H, Do T, Lodin K, Varshney D. Metaplastic carcinoma of the breast: A retrospective review. Int J Radiat Oncol Biol Phys. 2006;64:771–775. doi: 10.1016/j.ijrobp.2005.08.024. [DOI] [PubMed] [Google Scholar]
- 23.Park HS, Park S, Kim JH, Lee JH, Choi SY, Park BW, Lee KS. Clinicopathologic features and outcomes of metaplastic breast carcinoma: Comparison with invasive ductal carcinoma of the breast. Yonsei Med J. 2010;51:864–869. doi: 10.3349/ymj.2010.51.6.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bae SY, Lee SK, Koo MY, Hur SM, Choi MY, Cho DH, Kim S, Choe JH, Lee JE, Kim JH, et al. The prognoses of metaplastic breast cancer patients compared to those of triple-negative breast cancer patients. Breast Cancer Res Treat. 2011;126:471–478. doi: 10.1007/s10549-011-1359-8. [DOI] [PubMed] [Google Scholar]
- 25.Lee H, Jung SY, Ro JY, Kwon Y, Sohn JH, Park IH, Lee KS, Lee S, Kim SW, Kang HS, et al. Metaplastic breast cancer: Clinicopathological features and its prognosis. J Clin Pathol. 2012;65:441–446. doi: 10.1136/jclinpath-2011-200586. [DOI] [PubMed] [Google Scholar]
- 26.Cakir A, Gönül II, Uluoğlu O. Metaplastic breast carcinomas and their relationship with basal-like phenotype. Turk Patoloji Derg. 2012;28:134–141. doi: 10.5146/tjpath.2012.01112. [DOI] [PubMed] [Google Scholar]
- 27.Wright G Paul, Davis AT, Koehler TJ, Melnik MK, Chung MH. Hormone receptor status does not affect prognosis in metaplastic breast cancer: A population-based analysis with comparison to infiltrating ductal and lobular carcinomas. Ann Surg Oncol. 2014;21:3497–3503. doi: 10.1245/s10434-014-3782-7. [DOI] [PubMed] [Google Scholar]
- 28.Hennessy BT, Giordano S, Broglio K, Duan Z, Trent J, Buchholz TA, Babiera G, Hortobagyi GN, Valero V. Biphasic metaplastic sarcomatoid carcinoma of the breast. Ann Oncol. 2006;17:605–613. doi: 10.1093/annonc/mdl006. [DOI] [PubMed] [Google Scholar]
- 29.Takuwa H, Ueno T, Ishiguro H, Mikami Y, Kanao S, Takada M, Sugie T, Toi M. A case of metaplastic breast cancer that showed a good response to platinum-based preoperative chemotherapy. Breast Cancer. 2014;21:504–507. doi: 10.1007/s12282-011-0269-2. [DOI] [PubMed] [Google Scholar]
- 30.Rayson D, Adjei AA, Suman VJ, Wold LE, Ingle JN. Metaplastic breast cancer: Prognosis and response to systemic therapy. Ann Oncol. 1999;10:413–419. doi: 10.1023/A:1008329910362. [DOI] [PubMed] [Google Scholar]
- 31.Moulder S, Moroney J, Helgason T, Wheler J, Booser D, Albarracin C, Morrow PK, Koenig K, Kurzrock R. Responses to liposomal doxorubicin, bevacizumab, and temsirolimus in metaplastic carcinoma of the breast: Biologic rationale and implications for stem-cell research in breast cancer. J Clin Oncol. 2011;29:e572–575. doi: 10.1200/JCO.2010.34.0604. [DOI] [PubMed] [Google Scholar]
- 32.Chen IC, Lin CH, Huang CS, Lien HC, Hsu C, Kuo WH, Lu YS, Cheng AL. Lack of efficacy to systemic chemotherapy for treatment of metaplastic carcinoma of the breast in the modern era. Breast Cancer Res Treat. 2011;130:345–351. doi: 10.1007/s10549-011-1686-9. [DOI] [PubMed] [Google Scholar]
- 33.Lakhani SR, Reis-Filho JS, Fulford L, Penault-Llorca F, van der Vijver M, Parry S, Bishop T, Benitez J, Rivas C, Bignon YJ, et al. Prediction of BRCA1 status in patients with breast cancer using estrogen receptor and basal phenotype. Clin Cancer Res. 2005;11:5175–5180. doi: 10.1158/1078-0432.CCR-04-2424. [DOI] [PubMed] [Google Scholar]
- 34.Foulkes WD, Stefansson IM, Chappuis PO, Bégin LR, Goffin JR, Wong N, Trudel M, Akslen LA. Germline BRCA1 mutations and a basal epithelial phenotype in breast cancer. J Natl Cancer Inst. 2003;95:1482–1485. doi: 10.1093/jnci/djg050. [DOI] [PubMed] [Google Scholar]
- 35.Banerjee S, Reis-Filho JS, Ashley S, Steele D, Ashworth A, Lakhani SR, Smith IE. Basal-like breast carcinomas: Clinical outcome and response to chemotherapy. J Clin Pathol. 2006;59:729–735. doi: 10.1136/jcp.2005.033043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ashworth A. A synthetic lethal therapeutic approach: Poly(ADP) ribose polymerase inhibitors for the treatment of cancers deficient in DNA double-strand break repair. J Clin Oncol. 2008;26:3785–3790. doi: 10.1200/JCO.2008.16.0812. [DOI] [PubMed] [Google Scholar]
- 37.Colleoni M, Cole BF, Viale G, Regan MM, Price KN, Maiorano E, Mastropasqua MG, Crivellari D, Gelber RD, Goldhirsch A, et al. Classical cyclophosphamide, methotrexate, and fluorouracil chemotherapy is more effective in triple-negative, node-negative breast cancer: Results from two randomized trials of adjuvant chemoendocrine therapy for node-negative breast cancer. J Clin Oncol. 2010;28:2966–2973. doi: 10.1200/JCO.2009.25.9549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tseng WH, Martinez SR. Metaplastic breast cancer: To radiate or not to radiate? Ann Surg Oncol. 2011;18:94–103. doi: 10.1245/s10434-010-1198-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Weigelt B, Kreike B, Reis-Filho JS. Metaplastic breast carcinomas are basal-like breast cancers: A genomic profiling analysis. Breast Cancer Res Treat. 2009;117:273–280. doi: 10.1007/s10549-008-0197-9. [DOI] [PubMed] [Google Scholar]
- 40.Luini A, Aguilar M, Gatti G, Fasani R, Botteri E, Brito JA, Maisonneuve P, Vento AR, Viale G. Metaplastic carcinoma of the breast, an unusual disease with worse prognosis: The experience of the European institute of oncology and review of the literature. Breast Cancer Res Treat. 2007;101:349–353. doi: 10.1007/s10549-006-9301-1. [DOI] [PubMed] [Google Scholar]
- 41.Lim KH, Oh DY, Chie EK, Han W, Im SA, Kim TY, Park IA, Noh DY, Ha SW, Bang YJ. Metaplastic breast carcinoma: Clinicopathologic features and prognostic value of triple negativity. Jpn J Clin Oncol. 2010;40:112–118. doi: 10.1093/jjco/hyp139. [DOI] [PubMed] [Google Scholar]
- 42.Hu Q, Chen WX, Zhong SL, Li J, Luo Z, Tang JH, Zhao JH. Current progress in the treatment of metaplastic breast carcinoma. Asian Pac J Cancer Prev. 2013;14:6221–6225. doi: 10.7314/APJCP.2013.14.11.6221. [DOI] [PubMed] [Google Scholar]