Abstract
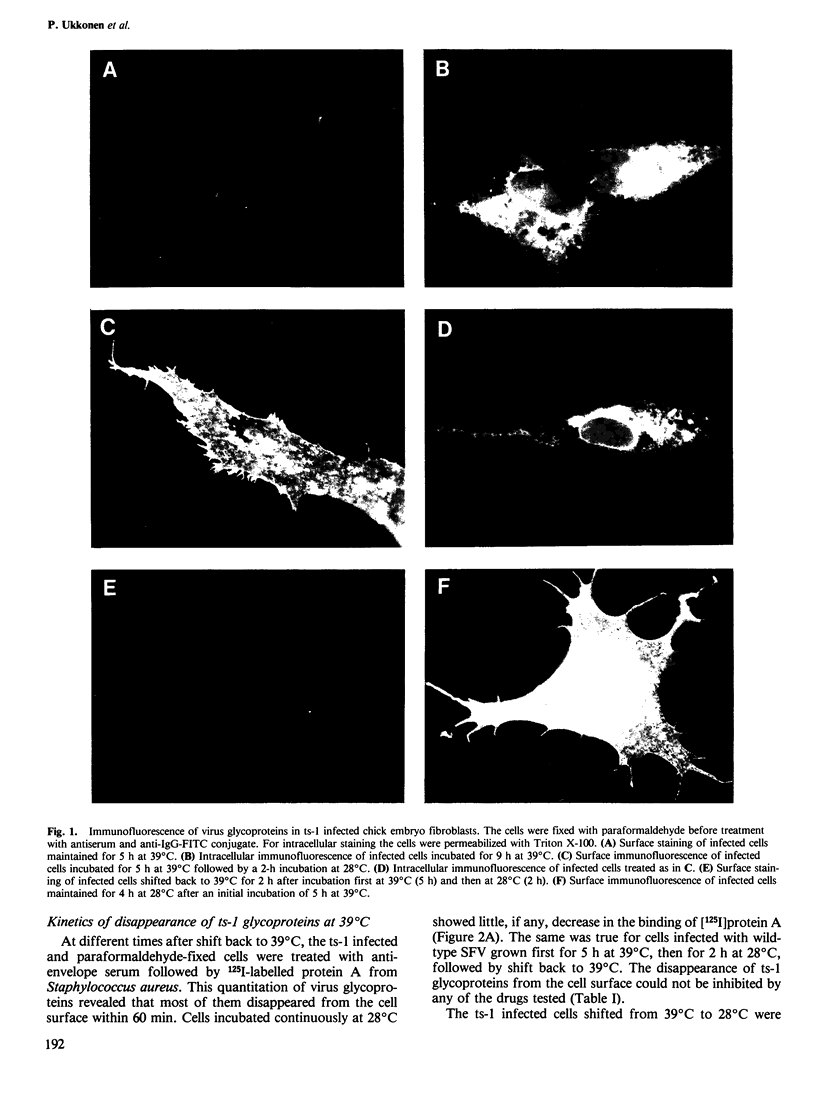
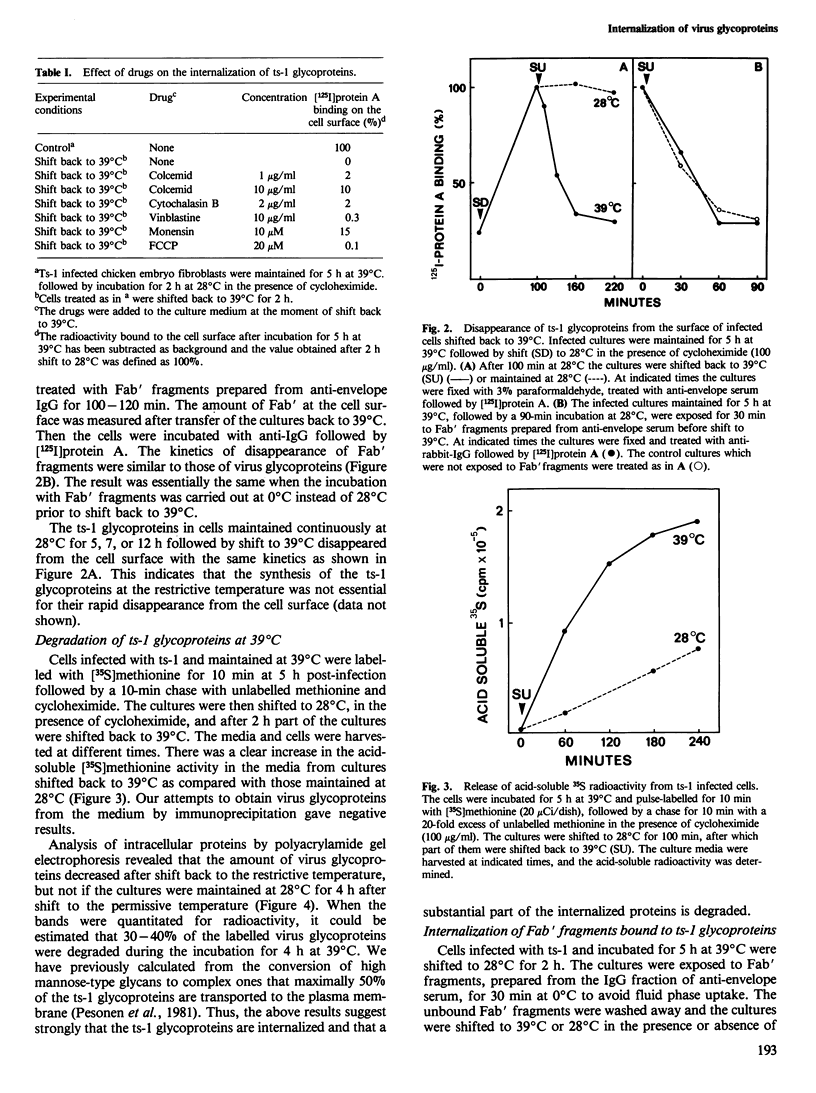
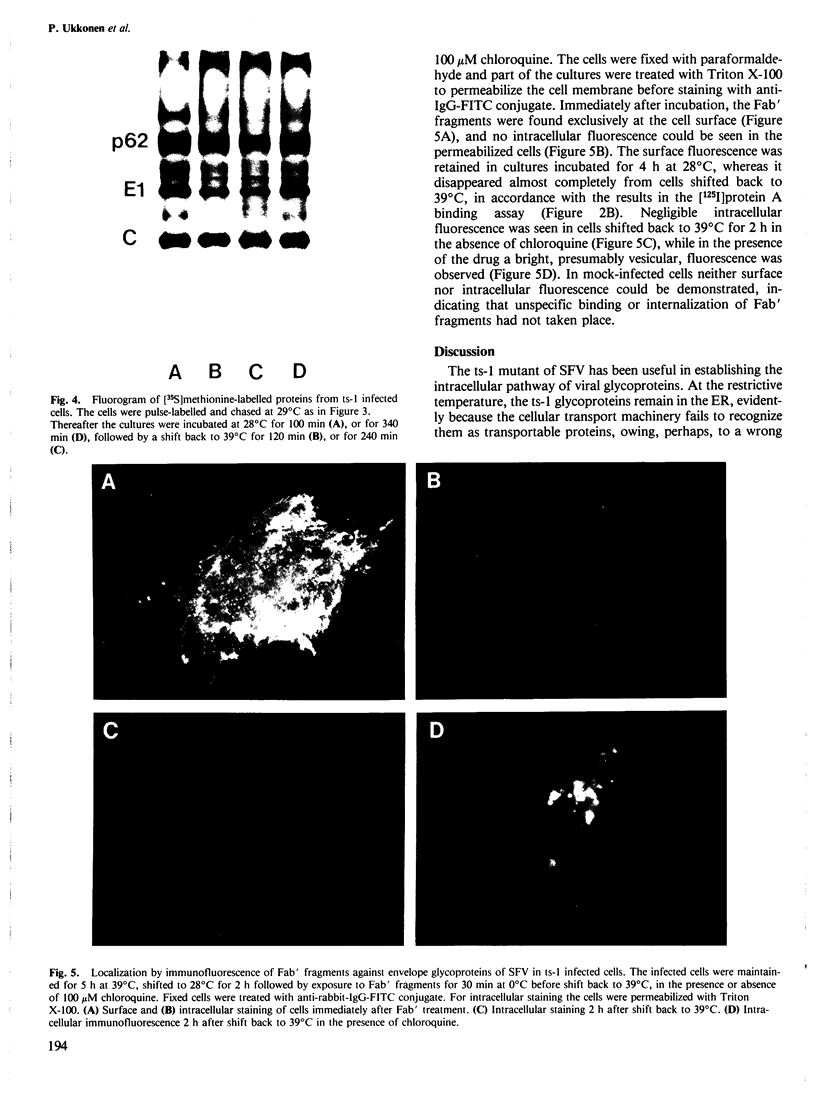
When the ts-1 mutant of Semliki Forest virus (SFV) was grown in chick embryo or BHK 21 cells at the restrictive temperature (39 degrees C), its membrane glycoproteins were arrested in the endoplasmic reticulum, but started to migrate to the cell surface once the cultures were shifted to the permissive temperature (28 degrees C). If the temperature of infected cells was raised back to 39 degrees C, ts-1 glycoproteins disappeared from the cell surface as evidenced by loss of surface immunofluorescence and by radioimmunoassay based on the binding of 125I-labeled protein A. This phenomenon was specific for ts-1 at 39 degrees C as it was observed neither in cells infected with wild-type SFV at 39 degrees C nor with ts-1 at 28 degrees C. The disappearance of the ts-1 glycoproteins was due to internalization. The internalized proteins were digested, as shown by specific decrease of virus glycoproteins labelled with [35S]methionine at 39 degrees C before shift to 28 degrees C, and by concomitant release of acid soluble 35S-activity into the culture medium. Ts-1 infected cells were treated before shift back to 39 degrees C with Fab' fragments, prepared from IgG against the viral membrane glycoproteins. After shift back to 39 degrees C, the Fab' fragments disappeared from the cell surface. In the presence of chloroquine, they could be visualized in vesicular structures, using an anti-IgG-fluorescein isothiocyanate conjugate. The internalization of ts-1 glycoproteins was not inhibited by carbonylcyanide p-trifluoromethoxy phenylhydrazone, chloroquine, cytochalasin B, vinblastine, colcemid, or monensin.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Garoff H., Frischauf A. M., Simons K., Lehrach H., Delius H. Nucleotide sequence of cdna coding for Semliki Forest virus membrane glycoproteins. Nature. 1980 Nov 20;288(5788):236–241. doi: 10.1038/288236a0. [DOI] [PubMed] [Google Scholar]
- Garoff H., Frischauf A. M., Simons K., Lehrach H., Delius H. The capsid protein of Semliki Forest virus has clusters of basic amino acids and prolines in its amino-terminal region. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6376–6380. doi: 10.1073/pnas.77.11.6376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garoff H., Simons K., Dobberstein B. Assembly of the Semliki Forest virus membrane glycoproteins in the membrane of the endoplasmic reticulum in vitro. J Mol Biol. 1978 Oct 5;124(4):587–600. doi: 10.1016/0022-2836(78)90173-0. [DOI] [PubMed] [Google Scholar]
- Goldstein J. L., Anderson R. G., Brown M. S. Coated pits, coated vesicles, and receptor-mediated endocytosis. Nature. 1979 Jun 21;279(5715):679–685. doi: 10.1038/279679a0. [DOI] [PubMed] [Google Scholar]
- Haigler H. T., McKanna J. A., Cohen S. Direct visualization of the binding and internalization of a ferritin conjugate of epidermal growth factor in human carcinoma cells A-431. J Cell Biol. 1979 May;81(2):382–395. doi: 10.1083/jcb.81.2.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasuga M., Kahn C. R., Hedo J. A., Van Obberghen E., Yamada K. M. Insulin-induced receptor loss in cultured human lymphocytes is due to accelerated receptor degradation. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6917–6921. doi: 10.1073/pnas.78.11.6917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keränen S., Käriäinen L. Isolation and basic characterization of temperature-sensitive mutants from Semliki Forest virus;. Acta Pathol Microbiol Scand B Microbiol Immunol. 1974 Dec;82(6):810–820. doi: 10.1111/j.1699-0463.1974.tb02378.x. [DOI] [PubMed] [Google Scholar]
- King A. C., Hernaez-Davis L., Cuatrecasas P. Lysomotropic amines cause intracellular accumulation of receptors for epidermal growth factor. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3283–3287. doi: 10.1073/pnas.77.6.3283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Käriäinen L., Hashimoto K., Saraste J., Virtanen I., Penttinen K. Monensin and FCCP inhibit the intracellular transport of alphavirus membrane glycoproteins. J Cell Biol. 1980 Dec;87(3 Pt 1):783–791. doi: 10.1083/jcb.87.3.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurila P., Wartiovaara J., Stenman S. Fibronectin expression is determined by the genotype of the transformed parental cells in heterokaryons between normal and transformed fibroblasts. J Cell Biol. 1979 Jan;80(1):118–127. doi: 10.1083/jcb.80.1.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mage M. G. Preparation of Fab fragments from IgGs of different animal species. Methods Enzymol. 1980;70(A):142–150. doi: 10.1016/s0076-6879(80)70045-9. [DOI] [PubMed] [Google Scholar]
- Pastan I. H., Willingham M. C. Journey to the center of the cell: role of the receptosome. Science. 1981 Oct 30;214(4520):504–509. doi: 10.1126/science.6170111. [DOI] [PubMed] [Google Scholar]
- Pastan I. H., Willingham M. C. Receptor-mediated endocytosis of hormones in cultured cells. Annu Rev Physiol. 1981;43:239–250. doi: 10.1146/annurev.ph.43.030181.001323. [DOI] [PubMed] [Google Scholar]
- Pesonen M., Saraste J., Hashimoto K., Käriäinen L. Reversible defect in the glycosylation of the membrane proteins of Semliki Forest virus ts-1 mutant. Virology. 1981 Feb;109(1):165–173. doi: 10.1016/0042-6822(81)90481-5. [DOI] [PubMed] [Google Scholar]
- Saraste J., von Bonsdorff C. H., Hashimoto K., Käriäinen L., Keränen S. Semliki forest virus mutants with temperature-sensitive transport defect of envelope proteins. Virology. 1980 Jan 30;100(2):229–245. doi: 10.1016/0042-6822(80)90516-4. [DOI] [PubMed] [Google Scholar]
- Schechter Y., Hernaez L., Schlessinger J., Cuatrecasas P. Local aggregation of hormone-receptor complexes is required for activation by epidermal growth factor. Nature. 1979 Apr 26;278(5707):835–838. doi: 10.1038/278835a0. [DOI] [PubMed] [Google Scholar]
- Schlessinger J., Shechter Y., Willingham M. C., Pastan I. Direct visualization of binding, aggregation, and internalization of insulin and epidermal growth factor on living fibroblastic cells. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2659–2663. doi: 10.1073/pnas.75.6.2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlessinger J., Van Obberghen E., Kahn C. R. Insulin and antibodies against insulin receptor cap on the membrane of cultured human lymphocytes. Nature. 1980 Aug 14;286(5774):729–731. doi: 10.1038/286729a0. [DOI] [PubMed] [Google Scholar]
- Seglen P. O., Grinde B., Solheim A. E. Inhibition of the lysosomal pathway of protein degradation in isolated rat hepatocytes by ammonia, methylamine, chloroquine and leupeptin. Eur J Biochem. 1979 Apr 2;95(2):215–225. doi: 10.1111/j.1432-1033.1979.tb12956.x. [DOI] [PubMed] [Google Scholar]
- Simons K., Garoff H. The budding mechanisms of enveloped animal viruses. J Gen Virol. 1980 Sep;50(1):1–21. doi: 10.1099/0022-1317-50-1-1. [DOI] [PubMed] [Google Scholar]
- Zidovetzki R., Yarden Y., Schlessinger J., Jovin T. M. Rotational diffusion of epidermal growth factor complexed to cell surface receptors reflects rapid microaggregation and endocytosis of occupied receptors. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6981–6985. doi: 10.1073/pnas.78.11.6981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziemiecki A., Garoff H., Simons K. Formation of the Semliki Forest virus membrane glycoprotein complexes in the infected cell. J Gen Virol. 1980 Sep;50(1):111–123. doi: 10.1099/0022-1317-50-1-111. [DOI] [PubMed] [Google Scholar]