Abstract
The present study was planned to explore the correlation between the methylation of APC (adenomatous polyposis coli) and colon carcinogenesis. Colon cancer tissues and tumor-adjacent normal tissues of 60 colon cancer patients (who received surgical operation in our hospital from January 2012 to December 2014) were collected. SW1116 cells in human colon cancer tissues were selected for culturing. 5-aza-2c-deoxycytidine (5-aza-dC) was utilized as an inhibitor of the methylation for APC gene. Methylation specific PCR (MSP) was utilized for detection of APC methylation in SW1116 cells. The MTT and Transwell assays were performed to detect the effect of the methylation of APC gene on the proliferation and invasive abilities of SW1116 cells. The correlation between the methylation of APC gene and pathological parameters of colon cancer patients was analyzed. MSP results revealed that 41 cases (68.33%) showed methylation of APC gene in colon cancer tissues. No methylation of APC gene was found in tumor-adjacent normal tissues. 5-aza-dC was able to inhibit the methylation of APC gene in SW1116 cells. APC gene methylation was correlated with tumor size, differentiation degree, lymph node metastasis and Dukes staging. In conclusion, the levels of the methylation of APC in colon cancer tissues and SW1116 cells are relatively high. The methylation of APC promoted the proliferation and invasion abilities of SW1116 cells. Furthermore, methylation is correlated with a variety of clinicopathological features of colon cancer patients.
Keywords: adenomatous polyposis coli, colon cancer, SW1116 cells, methylation
Introduction
Colon cancer is a digestive tract cancer that ranks third among all cancers in incidence and mortality. Rapidly changing life styles along with diet structure are prime reasons for the rapid increase of this pathological state (1). The incidence and development of tumor includes abnormal changes in multiple cancer and tumor suppressor genes. The methylation of CpG island in the area of the tumor suppressor gene promoter is one of the most important factors responsible for cancer (2). APC (adenomatous polyposis coli) is a tumor suppressor gene of the length of 8535 bp and is located on the 5q21 chromosome. It plays an important role in Wnt signal transduction pathway (3). APC gene encodes APC protein, which has the ability to regulate cell differentiation, proliferation and migration (4). Research has found that the methylation of CpG island in the area of APC gene promoter could lead to deletion of APC gene. This in turn, disturbs the conduction of Wnt signal pathway leading to the incidence and development of tumors (5). Research has confirmed the hyper-methylation of APC gene and the phenomenon of deletion of gene transcription during multiple cancers including breast, gastric, esophagus, pancreatic and lung cancer (6). In addition, methylation of APC gene is closely correlated with the incidence, development, invasion and migration of malignant tumors (7,8).
The present study adopted the method of methylation specific PCR (MSP) to examine the state of methylation of APC gene in the tissues of colon cancer patients and SW1116 cells. 5-aza-dC exerted demethylation by inhibiting methylase (9). Thus, in the present study we inhibited the methylation of APC gene and then studied the effect of APC methylation on the proliferation and invasion of SW1116 cells. Clinico-pathological data of patients were statistically summarized. The correlation between the methylation of APC gene and clinicopathological features was analyzed. Finally, the correlation between the methylation of APC gene and colon cancer is discussed.
Materials and methods
Materials
Human colon cancer cells SW1116 were procured from the Chinese Academy of Sciences Shanghai Cell Bank (Shanghai, China); 5-aza-dC, MTT was obtained from Sigma (St. Louis, MO, USA). RPMI-1640 culture medium and fetal bovine serum (FBS) were procured from HyClone Laboratories, Inc. (Logan, UT, USA); Epi-Tect Bisulfite kits agent was provided by Qiagen (Nordrhein-Westfalen, Germany); DNA extraction kits were from Tiangen Biotech (Beijing) Co., Ltd., (Beijing, China); Taq DNA polymerase, dNTP mixture, DNA marker and primer synthesis were obtained from Takara Bio (Dalian) Co., Ltd. (Dalian, China) and Transwell cabinet was obtained from Corning, Inc. (New York, NY, USA).
Colon tissue samples of 60 colon cancer patients, who were given surgical treatment in our hospital from January 2012 to December 2014 were collected. Colon cancer tissues were taken from resected tumor samples. Normal tumor-adjacent tissues were also excised from the part 10 cm away from tumor margin (no infiltration of cancer cells were found under microscope). Tissue samples were immediately preserved in liquid nitrogen after collection. There were 32 male cases, 28 female cases and the average patient age was 54.7±14.3 years. All patients were confirmed with colon cancer by clinical and pathological diagnosis. The patients were operated for the first time and had no history of radiotherapy or chemotherapy. All participants or their families signed a written informed consent form. Furthernore, the Ethics Committee of Xuzhou Cental Hospital approved the present study.
Detection of the methylation of APC gene in patient tissue samples by MSP
DNA extraction kits were used to extract the total DNA in tissue samples. The ultraviolet spectrophotometer (U-3010; Hitachi, Ltd., Tokyo, Japan) was used to detect the DNA content and purity (A260/A280 >1.8 was considered qualified). The total DNA extracted was modified by bisulfite according to the instructions on the Epi-Tect Bisulfite kits. The modified DNA was respectively given MSP and non-MSP (Table I). The 2% agarose gel was used for electrophoresis and ethidium bromide was used for staining. Gel imaging system (UVP, LLC, Upland, CA, USA) was utilized for R result analyses.
Table I.
Primer sequences of APC gene methylation and non-methylation.
| Primer | Sequence | Product size (bp) |
|---|---|---|
| APC-MF | 5′-TATTGCGGAGTGCGGGTC-3′ | 98 |
| APC-MR | 5′-TCGACGAACTCCCGACGA-3′ | |
| APC-UF | 5′-GTGTTTTATTGTGGAGTGTGGGTT-3′ | 108 |
| APC-UR | 5′-CCAATCAACAAACTCCCAACAA-3′ |
APC, adenomatous polyposis coli.
Cell culture and group processing
SW1116 cells were cultured in RPMI-1640 culture medium (including 10% FBS) in the incubator at 37°C with 5% CO2. The medium was changed every 24 h. Digestion passage was conducted after cell fusion. The experiment was divided into the normal control group and the 5-aza-dC group. The control group was cultured in a standard manner. The 5-aza-dC group was given 5-aza-dC of 5 µmol/l terminal concentration to process SW1116 cells.
Detection of the methylation of APC gene in cells
SW1116 cells at the logarithmic growth phase were collected. Each well was added with 2 ml (2×105 cells/ml) SW1116 cell suspension. Cells were cultured in 6 well plates for 24 h, then processed as described above. Cells were collected after digestion by pancreatin for 24 h. DNA extraction kits were applied to extract total DNA. The state of methylation of APC gene in cells of each group was determined by MSP.
Detection of proliferation ability of SW1116 cells by MTT
SW1116 cells at the logarithmic growth phase were collected. The wells were added with 200 ml (1×104 cells/ml) SW1116 cell suspension. Cells were cultured in 96-well plates for 24 h, and processed as described above. Each group was set with 6 parallel wells and culture was continued for 24 h then, the culture medium was discarded. The plates were then washed 3 times, followed by addition of 100 µl MTT (5 mg/ml) in each well and the culture was continued for 4 h. The wells were then added with 100 µl DMSO and the plate shaken for 10 min in the dark. The absorbance value (OD value) was recorded at 570 nm. The cell proliferation rate was calculated according to the following formula: proliferation rate = OD value of the experiment group/OD value of the normal group × 100%.
Detection of invasion ability of SW1116 cells by Transwell
The upper chamber of Transwell was evenly added with 100 µl single cell suspension at 4×105/ml and 100 µl culture medium. The lower chamber was added with 500 µl culture medium with 30% FBS, 24 h later, 4% paraformaldehyde was added for fixation and crystal violet for staining. After 15 min, images were taken for analysis.
The correlation between the methylation of APC gene and the pathological parameters of colon cancer patients
Patients were divided into groups according to sex, age, tumor size, differentiation degree, lymph node metastasis and Dukes staging index. The correlation between parameters and the methylation of APC gene was analyzed.
Statistical analysis
The data were processed by SPSS17.0 (IBM Corp., Armonk, NY, USA). Measurement data was expressed by mean ± standard deviation and analyzed by single factor ANOVA. Comparison of numeration data among different groups was by χ2 analysis. P≤0.05 values confirmed statistical significance.
Results
APC gene methylation in the tissues of colon cancer patients
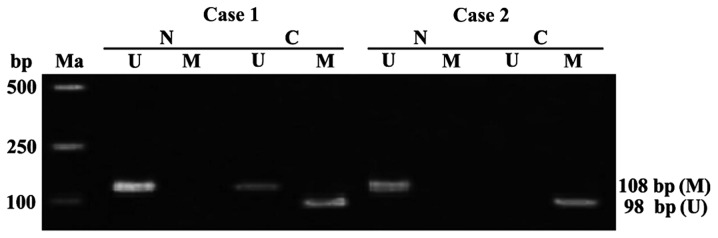
APC genes in the tumor-adjacent normal tissues of 60 colon cancer patients were free from methylation. The detection rate of APC gene methylation in colon cancer tissues was 68.33% (41/60), including 36 cases of complete methylation, 5 cases of partial methylation (Fig. 1). The state of APC gene methylation in the colon cancer was significantly different from that in the tumor-adjacent normal tissues (P<0.01).
Figure 1.
The condition of the APC gene methylation in the tissues of colon cancer patients and tumor-adjacent normal tissues detected by MSP. Ma, Takara DL2000 DNA marker; case 1-case 2, colon cancer patients; N, tumor-adjacent normal tissues; C, colon cancer tissues; U, results of non-MSP amplification; M, results of MSP amplification; APC, adenomatous polyposis coli; MSP, methylation specific PCR; Non-MSP, non-methylation specific PCR.
Effect of 5-aza-dC on APC gene methylation in SW1116 cells
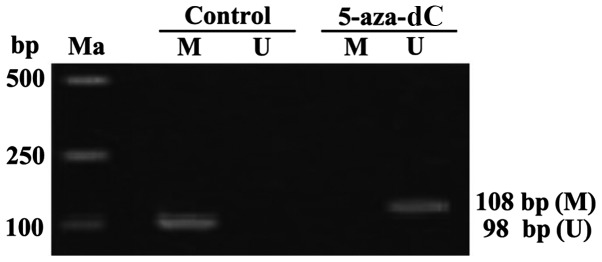
APC genes in the cells of the normal group showed complete methylation. Whereas, the APC genes in the cells of the 5-aza-dC group revealed no methylation. thus, intervention with 5-aza-dC for 24 h, inhibited the APC gene methylation in SW1116 cells (Fig. 2).
Figure 2.
The condition of the APC gene methylation in the SW1116 cells of colon cancer patients detected by MSP. Ma, Takara DL2000 DNA marker; Control, cells in the normal control group; 5-aza-dC, cells were processed by 5-aza-dC with the terminal concentration as 5 µmol/l; U, results of non-methylation specific PCR amplification; M, results of methylation specific PCR amplification; APC, adenomatous polyposis coli; MSP, methylation specific PCR; non-MSP, non-methylation specific PCR. 5-aza-dC, 5-aza-2c-deoxycytidine.
Effect of 5-aza-dC induces inhibition of APC gene methylation on the cell proliferation in SW1116 cells
The results clearly showed that the 5-aza-dC-induced inhibition of APC gene methylation significantly lowered the proliferation rate in SW1116 cells in comparison to the cells of normal group (P<0.01) (Fig. 3). This observation confirmed that the inhibition of APC gene methylation in the SW1116 cells reduced the proliferation of SW1116 cells.
Figure 3.
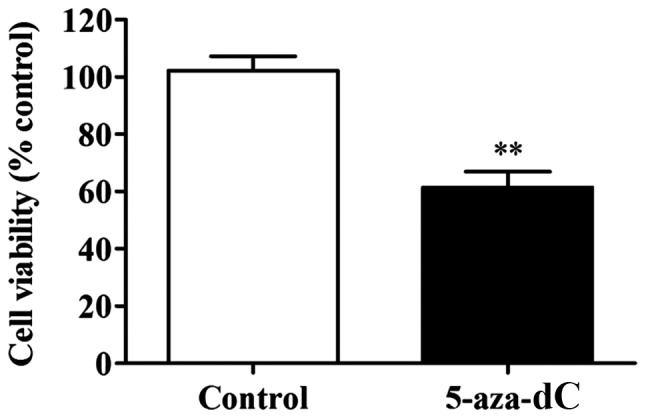
Effect of 5-aza-dC inhibition of APC gene methylation in the SW1116 cells on the cell proliferation. Compared with the normal control group, **P<0.01. APC, adenomatous polyposis coli; MSP, methylation specific PCR; Non-MSP, non-methylation specific PCR. 5-aza-dC, 5-aza-2c-deoxycytidine.
Effect of 5-aza-dC inhibition of APC gene methylation on cell invasion in the SW1116 cells
The number of invaded cells in the 5-aza-dC group was significantly lower than that in the normal group (P<0.01). This confirmed that the inhibition of APC gene methylation in the SW1116 cells has the ability to inhibit the invasion ability of SW1116 cells (Fig. 4).
Figure 4.
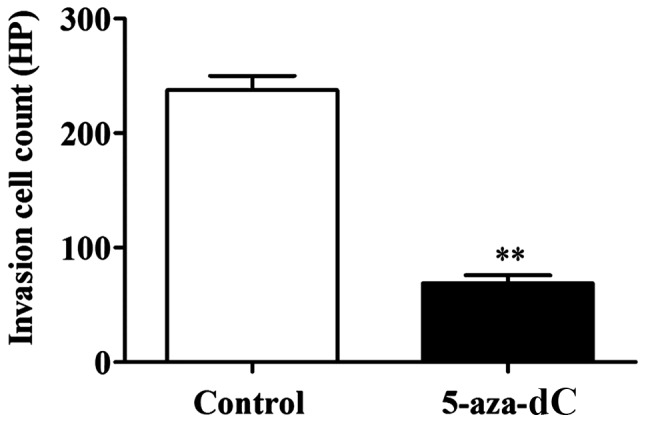
Effect of 5-aza-dC inhibition of APC gene methylation in the SW1116 cells on the cell invasion. Compared with the normal control group, **P<0.01. APC, adenomatous polyposis coli. 5-aza-dC, 5-aza-2c-deoxycytidine.
The correlation between APC gene methylation and clinicopathological parameters
Results of the correlation between clinicopathological parameters of colon cancer and APC gene methylation are shown in Table II. The correlation test indicated that APC gene methylation was correlated with tumor size, differentiation degree, lymph node metastasis and Dukes staging (P<0.05, P<0.01, P<0.05 and P<0.05), respectively. However, no correlation was observed with age or sex (P>0.05).
Table II.
The correlation between APC gene methylation and clinicopathological parameters.
| APC gene methylation | ||||
|---|---|---|---|---|
| Clinicopathological parameters | Case no. | Methylation (%) | Non-methylation (%) | P-value |
| Sex | >0.05 | |||
| Male | 32 | 21 (65.63) | 11 (34.37) | |
| Female | 28 | 20 (71.43) | 8 (28.57) | |
| Age | >0.05 | |||
| ≥54 years | 36 | 28 (77.78) | 8 (22.22) | |
| <54 years | 24 | 13 (54.17) | 11 (45.83) | |
| Tumor size | <0.05 | |||
| ≥5 cm | 33 | 27 (81.82) | 6 (18.18) | |
| <5 cm | 27 | 14 (51.85) | 13 (48.15) | |
| Differentiation degree | <0.01 | |||
| High | 37 | 30 (81.08) | 7 (18.92) | |
| Low | 23 | 11 (47.83) | 12 (52.17) | |
| Lymph node metastasis | <0.05 | |||
| Yes | 31 | 25 (80.65) | 6 (19.35) | |
| No | 29 | 16 (55.17) | 13 (44.83) | |
| Dukes staging | <0.01 | |||
| A+B | 27 | 13 (48.15) | 14 (51.85) | |
| C+D | 33 | 28 (84.85) | 5 (15.15) | |
APC, adenomatous polyposis coli.
Discussion
The incidence of colorectal cancer is a complex process that involves multiple factors. As the early clinical symptoms of colon cancer patients are not obvious, most patients are diagnosed at the advanced stage of colon cancer (10). The epigenetic changes also play a very important role in the incidence and development of colon cancer (11). DNA methylation is a common epigenetic modification that could cause transcriptional silencing of tumor suppressor genes (12). Research has found that the methylation of tumor suppressor genes is closely correlated with the differentiation and proliferation of tumor cells (13,14). DNA methylation is an important event occurring at the early stage of tumors. Therefore, detection of the methylation of tumor-related genes could provide reference for the diagnosis, therapy and prognosis of colorectal cancer (15). APC is a tumor suppressor gene which is important in the Wnt signal pathway. This protein plays an important role in cell proliferation, apoptosis and invasion (16). Hyper-methylation of APC gene has been found in malignant tumors such as gastric, liver and pancreatic cancer (17,18).
In the present study, the methylation rate of APC gene in colon cancer tissues was 68.33%. No methylation of APC gene was observed in tumor-adjacent tissues. To confirm the effect of APC gene methylation on colon cancer cells, it was perceived that the inhibition of APC gene methylation resulted in the reduction of proliferation and invasion of SW1116 cells. To further confirm the clinical significance of APC gene methylation, the present study analyzed the correlation between APC gene methylation and clinicopathological parameters. Results confirmed that APC gene methylation was correlated with tumor size, differentiation degree, lymph node metastasis and Dukes staging.
Arnold et al (19) found that APC gene showed high methylation rate in the tumor tissues of colon cancer patients. Deng et al (20) revealed that the abnormal APC gene methylation was correlated well with the deactivation of APC protein functions. In the present study, APC gene was found with high methylation in the tumor tissues of colon cancer patients. The APC gene methylation is not only correlated with the proliferation and invasion of colon cancer cells, but also with tumor size, differentiation degree, lymph node metastasis and Dukes staging of patients with colon cancer.
The present study concludes that the methylation of APC gene is closely correlated with colon cancer, especially with tumor size, differentiation degree, lymph node metastasis, and Dukes staging of patients. Therefore, the application of demethylation drugs to inhibit the methylation of APC gene is likely to be useful in treatment of colon cancer.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Grady WM, Willis J, Guilford PJ, Dunbier AK, Toro TT, Lynch H, Wiesner G, Ferguson K, Eng C, Park JG, et al. Methylation of the CDH1 promoter as the second genetic hit in hereditary diffuse gastric cancer. Nat Genet. 2000;26:16–17. doi: 10.1038/79120. [DOI] [PubMed] [Google Scholar]
- 3.Jarrett CR, Blancato J, Cao T, Bressette DS, Cepeda M, Young PE, King CR, Byers SW. Human APC2 localization and allelic imbalance. Cancer Res. 2001;61:7978–7984. [PubMed] [Google Scholar]
- 4.van Noort M, Meeldijk J, van der Zee R, Destree O, Clevers H. Wnt signaling controls the phosphorylation status of β-catenin. J Biol Chem. 2002;277:17901–17905. doi: 10.1074/jbc.M111635200. [DOI] [PubMed] [Google Scholar]
- 5.Zysman M, Saka A, Millar A, Knight J, Chapman W, Bapat B. Methylation of Αdenomatous polyposis coli in endometrial cancer occurs more frequently in tumors with microsatellite instability phenotype. Cancer Res. 2002;62:3663–3666. [PubMed] [Google Scholar]
- 6.Dong SM, Kim HS, Rha SH, Sidransky D. Promoter hypermethylation of multiple genes in carcinoma of the uterine cervix. Clin Cancer Res. 2001;7:1982–1986. [PubMed] [Google Scholar]
- 7.Tamura G, Maesawa C, Suzuki Y, Ogasawara S, Terashima M, Saito K, Satodate R. Primary gastric carcinoma cells frequently lose heterozygosity at the APC and MCC genetic loci. Jpn J Cancer Res. 1993;84:1015–1018. doi: 10.1111/j.1349-7006.1993.tb02794.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen J, Röcken C, Lofton-Day C, Schulz HU, Müller O, Kutzner N, Malfertheiner P, Ebert MP. Molecular analysis of APC promoter methylation and protein expression in colorectal cancer metastasis. Carcinogenesis. 2005;26:37–43. doi: 10.1093/carcin/bgh280. [DOI] [PubMed] [Google Scholar]
- 9.Bender CM, Pao MM, Jones PA. Inhibition of DNA methylation by 5-aza-2-deoxycytidine suppresses the growth of human tumor cell lines. Cancer Res. 1998;58:95–101. [PubMed] [Google Scholar]
- 10.Gordon MB, Nakhle S, Ludlam WH. Patients with acromegaly presenting with colon cancer: A case series. Case Rep Endocrinol. 2016;2016:1–4. doi: 10.1155/2016/5191903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lim S, Metzger E, Schüle R, Kirfel J, Buettner R. Epigenetic regulation of cancer growth by histone demethylases. Int J Cancer. 2010;127:1991–1998. doi: 10.1002/ijc.25538. [DOI] [PubMed] [Google Scholar]
- 12.Jubb AM, Bell SM, Quirke P. Methylation and colorectal cancer. J Pathol. 2001;195:111–134. doi: 10.1002/path.923. [DOI] [PubMed] [Google Scholar]
- 13.Guo Y, Guo W, Chen Z, Kuang G, Yang Z, Dong Z. Hypermethylation and aberrant expression of Wnt-antagonist family genes in gastric cardia adenocarcinoma. Neoplasma. 2011;58:110–117. doi: 10.4149/neo_2011_02_110. [DOI] [PubMed] [Google Scholar]
- 14.Wu D, Wong P, Li W, Vogel CF, Matsumura F. Suppression of WIF-1 through promoter hypermethylation causes accelerated proliferation of the aryl hydrocarbon receptor (AHR) overexpressing MCF10AT1 breast cancer cells. Toxicology. 2011;285:97–103. doi: 10.1016/j.tox.2011.04.008. [DOI] [PubMed] [Google Scholar]
- 15.Prabhu JS, Korlimarla A, Banerjee A, Wani SKP, Sahoo R. Gene-specific methylation: Potential markers for colorectal cancer. Int J Biol Markers. 2009;24:57–62. doi: 10.5301/JBM.2009.3486. [DOI] [PubMed] [Google Scholar]
- 16.Fearnhead NS, Britton MP, Bodmer WF. The ABC of APC. Hum Mol Genet. 2001;10:721–733. doi: 10.1093/hmg/10.7.721. [DOI] [PubMed] [Google Scholar]
- 17.Esteller M, Sparks A, Toyota M, Sanchez-Cespedes M, Capella G, Peinado MA, Gonzalez S, Tarafa G, Sidransky D, Meltzer SJ, et al. Analysis of Adenomatous polyposis coli promoter hypermethylation in human cancer. Cancer Res. 2000;60:4366–4371. [PubMed] [Google Scholar]
- 18.Tsuchiya T, Tamura G, Sato K, Endoh Y, Sakata K, Jin Z, Motoyama T, Usuba O, Kimura W, Nishizuka S, et al. Distinct methylation patterns of two APC gene promoters in normal and cancerous gastric epithelia. Oncogene. 2000;19:3642–3646. doi: 10.1038/sj.onc.1203704. [DOI] [PubMed] [Google Scholar]
- 19.Arnold CN, Goel A, Niedzwiecki D, Dowell JM, Wasserman L, Compton C, Mayer RJ, Bertagnolli MM, Boland CR. APC promoter hypermethylation contributes to the loss of APC expression in colorectal cancers with allelic loss on 5q. Cancer Biol Ther. 2004;3:960–964. doi: 10.4161/cbt.3.10.1113. [DOI] [PubMed] [Google Scholar]
- 20.Deng G, Song GA, Pong E, Sleisenger M, Kim YS. Promoter methylation inhibits APC gene expression by causing changes in chromatin conformation and interfering with the binding of transcription factor CCAAT-binding factor. Cancer Res. 2004;64:2692–2698. doi: 10.1158/0008-5472.CAN-03-3000. [DOI] [PubMed] [Google Scholar]