Abstract
MicroRNAs (miRNAs/miRs) are 19–25 nucleotide-long, non-coding RNAs that regulate the expression of target genes at the post-transcriptional level. In the present study, the role of miR-340 in breast cancer (BC) was investigated. The overexpression of miR-340 significantly inhibited the proliferation, migration and invasion of human breast MDA-MB-231 cancer cells in vitro. The Rho-associated, coiled-coil containing protein kinase 1 (ROCK1) gene was identified as a target of miR-340; its expression was downregulated by overexpression of miR-340 by binding to its 3′-untranslated region. The short interfering RNA-mediated silencing of ROCK1 was also performed, which phenocopied the effects of miR-340 overexpression. An inhibitor of miR-340 was used to suppress miR-340 expression, which led to increased expression of ROCK1, thus improving the proliferation, migration and invasion of MDA-MB-231 cells. Data from the present study suggest that miR-340 inhibits MDA-MB-231 cell growth and its downregulation may lead to the progression and metastasis of BC. Thus, miR340 may act as a tumor-suppressor agent that could serve a key role in the diagnosis and therapy of BC.
Keywords: miRNA-340, breast cancer, ROCK1, growth, metastasis
Introduction
Breast cancer (BC) is the most common type of cancer in women worldwide and is the second-leading cause of mortality in women in the USA. In 2017, the projected number of new cases and number of mortalities of BC were 255,180 and 41,070, respectively in the USA (1). Although BC is detected at an earlier stage than it was in the past, the mortality rate of BC remains high; as many as 1 in 8 women may develop BC (2). Patients with triple-negative BC (TNBC) have the poorest outcome, owing to the high risk of metastatic progression and an absence of targeted treatments (3). The prognosis of BC can, therefore, be poor. The development of drug resistance and a poor understanding of the molecular mechanism by which BC progresses are two primary reasons behind the poor prognosis of BC (4–6). This frequent poor prognosis has led to a substantial interest in the quest for a novel predictive marker for BC.
MicroRNAs (miRNAs/miRs) are 19–25 nucleotide-long, non-coding RNAs that function as negative regulators of gene expression at the post-transcriptional level. miRNAs are transcribed by RNA polymerases II and III, and, following a series of cleavage steps, form mature miRNA. The regulatory function of miRNAs is mediated by the RNA-induced silencing complex (7). Dysregulation of miRNAs can lead to progression or inhibition of normal cell growth patterns, thus leading to oncogenesis (8). miR-340 suppresses cell migration and invasion by targeting myosin X in BC (9), whereas miR-340 suppresses prostate cancer cell growth by targeting high-mobility group nucleosome binding domain 5 (10). miR-340 suppresses glioblastoma multiforme cells by targeting the Rho-associated, coiled-coil containing protein kinase 1 (ROCK1) gene (11). A previous study revealed that decreased miR-340 expression in bone marrow was associated with liver metastasis in colorectal cancer (12). miR-340 inhibits the migration, invasion and metastasis of BC cells by targeting the Wnt pathway (13). The aforementioned studies suggest that miR-340 acts as a tumor suppressor.
ROCK1 belongs to the AGC family of serine/threonine kinases. The human ROCK1 gene is located on human chromosome 18, at position 18q11.1. ROCK1 normally regulates the actin cytoskeleton through the phosphorylation of substrates and modulation of actin-myosin contractility. It also acts as a key modulator in the formation of focal adhesions, cell motility and tumor cell invasion, thus contributing to the regulation of morphology, gene transcription, proliferation, differentiation, apoptosis and oncogenic transformation (14).
In the present study, ectopic expression of miR-340 was observed to inhibit cell proliferation, migration and invasion in vitro. ROCK1 was identified as a direct target of miR-340 and was shown to function as a tumor suppressor by downregulating ROCK1, suggesting that it has potential as a diagnostic and therapeutic marker in the treatment of BC.
Materials and methods
Cell lines, culture and transfection
The human BC MDA-MB-231 cell line was purchased from the Chinese Academy of Sciences (Shanghai, China). The cells were cultured in high-glucose Dulbecco's modified Eagle's medium (DMEM; Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) supplemented with 10% fetal bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc.), penicillin (100 U/ml) and streptomycin (100 µg/ml) (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany). The cells were incubated at 37°C in a humidified chamber with 5% CO2. MDA-MB-231 cells were cultured in 6-well plates. When the logarithmic cell growth reached 50–70% confluence, cells were transfected with miR-340 mimics, miR-340 inhibitor or short interfering RNA (siRNA) of ROCK1 (Shanghai Integrated Biotech Co., Ltd., Shanghai, China) at a concentration of 100 nM/l using Lipofectamine 2000 (Invitrogen; Thermo Fisher Scientific, Inc.), according to the manufacturer's protocol. All transfections were performed in triplicate. The sequences were as follows: miR-340 mimics sense, 5′-UUAUAAAGCAAUGAGACUGAUU-3′ and antisense, 5′-UCAGUCUCAUUGCUUUAUAAUU; NC mimics sense, 5′-UCACAACCUCCUAGAAAGAGUAGA-3′ and antisense, 5′-UACUCUUUCUAGGAGGUUGUGAUU; miR-340 inhibitor, 5′-AAUCAGUCUCAUUGCUUUAUAA-3′; inhibitor NC, 5′-AAUCAGUCUCAUUGCUUUAUAA-3′; si-ROCK1 sense, 5′-UGAUGCAAAGAUUGUACUCTT and antisense, 5′-GAGUACAAUCUUUGCAUCATT-3′.
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
The expression level of ROCK1 was analyzed by RT-qPCR. RNA was extracted from mimic-transfected cells using TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.). Complementary DNA was synthesized by RT using a PrimeScript RT-PCR kit, according to the manufacturer's protocol (Takara Bio, Inc., Otsu, Japan). qPCR was performed on a 7900HT Fast Real-Time PCR system (Applied Biosystems; Thermo Fisher Scientific, Inc.). The RT-qPCR was performed using KAPA SYBR® FAST Universal qPCR kit (cat. no. kk 4601; Kapa Biosystems, Inc., Wilmington, MA, USA). The qPCR steps were as follows: 5 min at 95°C, followed by 40 cycles of 95°C for 30 sec and 65°C for 45 sec. GAPDH was used as the reference. For quantitative analysis, relative gene expression levels were calculated using the 2−ΔΔCq method (10). Expression of messenger RNA (mRNA) was assessed by evaluating quantification cycle values. The primer sequences were: ROCK1 forward, 5′-AACATGCTGCTGGATAAATCTGG-3′ and reverse, 5′-TGTATCACATCGTACCATGCCT-3′; and GAPDH forward, 5′-ACAACTTTGGTATCGTGGAAGG-3′ and reverse, 5′-GCCATCACGCCACAGTTTC-3′. The experiment was performed in triplicate.
Cell proliferation assay
The effect of miR-340 and siRNA of ROCK1 on cell viability was measured using an MTT assay kit (Sigma-Aldrich; Merck KGaA) according to the manufacturer's protocol. In brief, cells (2×103 cells/well) transfected with miR-340 mimics, miR-340 inhibitors or siRNA of ROCK1 and their corresponding negative mimics were seeded into 96-well culture plates (BD Biosciences, Franklin Lakes, NJ, USA) and incubated overnight at 37°C in 5% CO2. Cell proliferation was assessed at 24, 48, 72, 96 and 120 h, following the addition of 5 mg/ml MTT solution. After 4 h of incubation, the medium was replaced with 150 µl dimethyl sulfoxide (Sigma-Aldrich; Merck KGaA) and vortexed for 10 min. The absorbance was measured using a microplate reader at 490 nm. Each experiment was performed in triplicate.
Colony formation assay
MDA-MB-231 cells (2×103 cells/well) were transfected with miR-340 mimics, miR-340 inhibitors, a siRNA targeting ROCK1 and their corresponding negative mimics. At 24 h post-transfection, the cells were digested with trypsin and resuspended into single-cell status (determined by microscopy). A total of 500 cells from each group were cultured in a 6-well plate for 14 days. The cells were then fixed with 4% paraformaldehyde and stained with freshly prepared 0.1% crystal violet stain for 10 min. Following rinsing with distilled water, the colonies that had formed in each well were counted under microscopy (inverted microscope; Olympus CKX41, Shibuya, Tokyo, Japan) using ×40 magnification. Each experiment was performed in triplicate.
Transwell invasion and migration assays
A Transwell invasion assay was performed to evaluate cell invasion ability. The filters (Corning Incorporated, Corning, NY, USA) were washed with serum-free DMEM and placed into the wells of a 24-well plate. The lower chamber contained DMEM with 10% FBS. For the upper chambers, 5×104 cells resuspended in 200 µl DMEM with 0.1% bovine serum albumin (Sigma-Aldrich; Merck KGaA) were plated in the top chamber of the Transwell chamber (Corning Incorporated) with a 2 mg/ml Matrigel-coated membrane containing 8-mm diameter pores. Plates were then incubated at 37°C in 5% CO2. After 18 h, cells remaining on the upper membrane surface were removed by scrubbing with a cotton swab, while cells on the lower surface of the membrane were fixed in 10% formalin at room temperature for 30 min and stained with 0.5% crystal violet. Images of six randomly selected fields of view were captured by inverted microscopy (LI-COR Biosciences, Lincoln, NE, USA) and the cells were counted at a magnification of ×200. For the migration assay, the transfected cells (2×104 cells per Transwell chamber) were placed in the top chamber without Matrigel. After 18 h, the migrated cells were lysed in glacial acetic acid and the solutions were transferred to a 96-well culture plate for the colorimetric reading of optical density at 560 nm. Each experiment was performed in triplicate.
Dual-luciferase reporter assay
HEK 293T human embryonic kidney cells were provided by the Department of Central Laboratory, Shanghai People's Tenth Hospital (Shanghai, China). The cells were stored in liquid nitrogen at −191°C. These cells were seeded in 24-well plates (BD Biosciences) and cultured until the cells reached 80–90% confluence. The 3′-untranslated region (UTR) of ROCK1 containing the putative miR-340 binding site was amplified from genomic DNA via PCR in a total volume of 50 µl according to the manufacturer's protocol. The reagents (Takara Bio, Inc., Otsu, Japan) used were 2X primer STAR GC buffer, dNTP Mixture, Primer1, Primer2, H20 and PimerSTAR HS DNA polymerase (Takara Bio, Inc.). The PCR steps were as follows: 94°C for 30 sec, 55°C for 30 sec and 72°C for 12 sec (30 cycles). The ROCK1 primers used were: Forward, 5′-AACATGCTGCTGGATAAATCTGG-3′ and reverse, 5′-TGTATCACATCGTACCATGCCT-3′. The corresponding mutant constructs were created by mutating the seed regions of the miR-340 binding sites (5′-UUUAUA-3′ to 5′-AAAUAU-3′). Fragments were subcloned into the Xhol site in the 3′-UTR of Renilla luciferase of the psiCHECK-2 reporter vector (Kapa Biosystems, Inc., Wilmington, MA, USA). HEK293T cells were transiently co-transfected with 0.2 µg psiCHECK-2/ROCK1 3′-UTR or psiCHECK-2/ROCK1 3′-UTR mutant receptor plasmids, together with 100 nmol/l miR-340 or negative control miRNA (miR-NC) using Lipofectamine 2000 (Invitrogen; Thermo Fisher Scientific, Inc.), according to the manufacturer's protocol. Luciferase activity was examined at 48 h after transfection using the Dual-Luciferase Reporter Assay system (Promega Corporation, Madison, WI, USA) and normalized to firefly luciferase activity. The ratio of Renilla:firefly luciferase was plotted. Three independent experiments were performed in triplicate.
Western blot analysis
Cells were lysed using radioimmunoprecipitation assay buffer (Sigma-Aldrich; Merck KGaA) and the protein concentrations were quantified using a Pierce BCA Protein Assay kit (Pierce; Thermo Fisher Scientific, Inc.). Proteins (50 µg) were separated using 10% SDS-PAGE and transferred to a nitrocellulose membrane (Beyotime Institute of Biotechnology, Jiangsu, China). The membrane was blocked with 5% skimmed milk for 1 h. The membrane was immunoblotted overnight at 4°C with primary antibodies against ROCK1 (dilution, 1:500; cat. no. 4035; Cell Signaling Technology, Inc., Danvers, MA, USA) and β-tubulin (dilution, 1:1,000; cat. no. CW0098M; CWBio, Jiangsu, China) as a loading control. The membrane was treated with a horseradish peroxidase-conjugated secondary antibody (dilution, 1:2,000; cat. nos. 35571 and 35569; Thermo Fisher Scientific, Inc.) for 1 h at room temperature. Immunoreactive protein bands were detected with an Odyssey Scanning System (LI-COR Biosciences, Lincoln, NE, USA).
Clinical data analysis
The Cancer Genome Atlas (TCGA) provides a large amount of clinical data concerning different cancer types (15). Data on invasive BC were obtained from TCGA and analyzed using starBase v2.0 (16) and PROGgeneV2 (17) to assess miRNA expression level and overall survival rate, respectively. miR-340 expression was compared against ROCK1 expression in 780 patient samples and miR-340 overall survival was assessed at median miRNA expression.
Statistical analysis
GraphPad Prism version 6.0 (GraphPad Software, Inc., La Jolla, CA, USA). Pearson correlations and Kaplan-Meier analysis were used for all statistical analysis. Data were presented as the mean ± standard deviation from at least three separate experiments. A t-test (two-tailed) was used to compare between groups. P<0.05 was considered to indicate a statistically significant difference.
Results
Patients and BC cell MDA-MB-231 expression data support miR-340 and ROCK1 axis
Previous studies have analyzed the role of miR-340 in the development and progression of BC (5,9). A significant negative correlation was observed between miR-340 and ROCK1 expression using the TCGA BC clinical patient dataset (r=0.286; P<0.01; Fig. 1A). Analysis of the TCGA dataset also highlighted the prolonged overall patient survival that correlated with miR-340 expression in BC (P<0.05; Fig. 1B).
Figure 1.
TCGA BC data analysis. (A) Scatter plot showing the negative correlation between the expression levels of miR-340 and ROCK1 in 748 samples of patients with BC in the TCGA dataset (Pearson correlation, r=−0.286; P<0.01). (B) Kaplan-Meier curve of overall survival in the TCGA BC dataset. To determine the groups of high and low expression, the dataset was divided at the median miRNA expression. P<0.05, high expression vs. low expression. TCGA, The Cancer Genome Atlas; BC, breast cancer; ROCK1, Rho-associated, coiled-coil containing protein kinase 1; NC, negative control; miR/miRNA, microRNA; HR, hazard ratio; Seq, sequence; RPM, reads per million; RSEM, RNA-Seq by Expectation-Maximization; hsa, Homo sapiens.
miR-340 suppresses MDA-MB-231 cell proliferation
The viability of cells transfected with miR-340 mimics and miR-340 inhibitors was compared with that of cells transfected with the corresponding miR-NCs at 24, 48, 72, 96 and 120 h post-transfection. Cells transfected with miR-340 mimics grew more slowly than control cells, whereas cells transfected with miR-340 inhibitors grew more rapidly than control cells at multiple time points (24, 48, 72, 96 and 120 h; Fig. 2A and B). Cells transfected with miR-340 mimics exhibited fewer colonies than NC groups, as determined by colony formation assays. miR-340 mimics decreased cell proliferation, whereas miR-340 inhibitors promoted it (Fig. 2C and D). These results suggest that the transient overexpression of miR-340 suppressed the proliferation and colony forming ability of MDA-MB-231 cells.
Figure 2.
MTT and colony formation assays. (A) Growth curve comparing MDA-MB-231 cells treated with miR-340 mimics vs. mimics NC. (B) Growth curve comparing MDA-MB-231 cells treated with miR-340 inhibitors vs. inhibitor NC. Post-transfection time is shown on the x-axis. Cells transfected with miR-340 exhibited inhibited proliferation, whereas miR-340 suppression markedly increased cell proliferation, when compared with that of the control. (C) Colony formation assays showing the effect of miR-340 mimics vs. mimic NC on MDA-MB-231 cell growth. (D) Colony formation assays showing the effect of miR-340 inhibitors vs. inhibitor NC on MDA-MB-231 cell growth. miR-340 mimics significantly suppressed cell proliferation when compared with that of NC mimics. P<0.05 for miR-340 mimics vs. mimic NC; miR-340 inhibitors vs. inhibitor NC. NC, negative control; miR, microRNA; OD, optical density.
miR-340 suppresses MDA-MB-231 cell migration and invasion
Cells transfected with miR-340 mimics, miR-340 inhibitors and their corresponding NCs were tested for their migratory and invasive abilities. Images were obtained using an inverted microscope at ×200 magnification. A total of three areas were randomly slected and the cells were counted (Fig. 3). The results demonstrated that miR-340 inhibited the migration and invasion of MDA-MB-231 cells whereas miR-340 inhibitor promoted the migration and invasion of MDA-MB-231 cells. These results indicate that the overexpression of miR-340 suppressed the migratory and invasive abilities of MDA-MB-231 cells.
Figure 3.
Effect of miR-340 on the migration and invasion of MDA-MB-231 cells. miR-340 mimics significantly suppressed both migration and invasion in MDA-MB-231 cells when compared with the NC mimics. Images were obtained on an inverted microscope with ×200 magnification. Solubilization with crystal violet was performed and spectrophotometric readings were obtained at an optical density value of 573 nm. P<0.05 for miR-340 mimics vs. mimic NC; miR-340 inhibitors vs. inhibitor NC. miR, microRNA; NC, negative control.
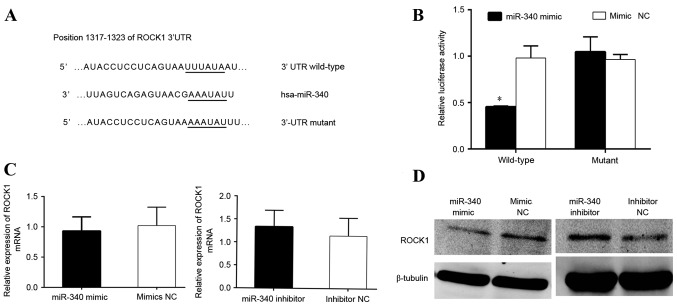
ROCK1 is a target gene of miR-340
Bioinformatics analysis revealed that miR-340 binds to putative target sequences at position 1,317–1,723 of the ROCK1 3′-UTR (Fig. 4A). To confirm that ROCK1 was a direct target of miR-340, luciferase reporter constructs containing wild-type and mutant 3′-UTR of the ROCK1 gene were engineered. The luciferase reporter assay revealed that miR-340 significantly decreased the luciferase activity of ROCK1 3′-UTR wild-type but not that of the 3′-UTR mutant in HEK293T cells (Fig. 4B). RT-qPCR and western blot analyses revealed that the overexpression of miR-340 significantly downregulated the expression of ROCK1 at the mRNA and protein levels in MDA-MB-231 cells (Fig. 4C and D).
Figure 4.
miR-340 negatively regulates ROCK1 by binding to the ROCK1 3′-UTR. (A) Binding sites of wild-type and mutant ROCK1 3′-UTR. (B) Luciferase reporter assay showing the inhibitory effect of miR-340 on ROCK1 3′-UTR luciferase activity (P<0.05; miR-340 mimics vs. mimic NC). (C) Reverse transcription-quantitative polymerase chain reaction showing ROCK1 mRNA level in MDA-MB-231 cells, comparing miR-340 mimics with miR-340 inhibitor. (D) Western blotting comparing ROCK1 protein level with NC. miR-340 mimics downregulated the expression of ROCK1, whereas miR-340 inhibitor upregulated ROCK1 expression. P<0.05 for miR-340 mimics vs. mimic NC; miR-340 inhibitors vs. inhibitor NC. ROCK1, Rho-associated, coiled-coil containing protein kinase 1; NC, negative control; miR, microRNA; UTR, untranslated region; mRNA, messenger RNA; hsa, Homo sapiens.
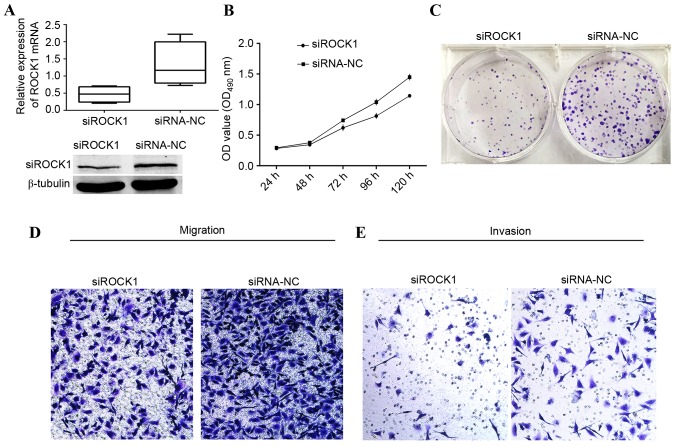
Expression of ROCK1 is downregulated by ROCK1-siRNA and the growth of MDA-MB-231 cells is inhibited by ROCK1-siRNA
The aforementioned results prompted an examination of whether the suppressive effect of miR-340 was mediated by repression of ROCK1 in MDA-MB-231 cells. The knockdown efficiency of ROCK1 was verified by RT-qPCR and western blot analyses. The expression level of ROCK1 was significantly decreased in MDA-MB-231 cells transfected with siRNA of ROCK1, when compared with that of the siRNA NC group, at the gene and protein levels (Fig. 5A). The effect of ROCK1 silencing on cell viability was determined by MTT assay over a period of 5 days. The growth curve revealed a clear decrease in cell number in the MDA-MB-231 cells that had been transfected with siRNA targeting ROCK1 from day 2, compared with that in the control group (Fig. 5B). The total number of colonies formed by MDA-MDA-231 cells transfected with 100 nM siRNA of ROCK1 was visibly decreased compared with that in the control group (Fig. 5C). Furthermore, ROCK1 silencing significantly suppressed the migration and invasion of MDA-MDA-231 cells (Fig. D and E), similar to the effect induced by miR-340. Taken together, these findings indicated that ROCK1 is a target of miR-340 that was involved in the suppression of the proliferation, migration and invasion of MDA-MB-231 cells.
Figure 5.
Knockdown of ROCK1 in MDA-MB-231 cells by siRNA. (A) Downregulation of ROCK1 by siRNA was confirmed by reverse transcription-quantitative polymerase chain reaction and western blotting in MDA-MB-231 cells. (B) Growth curve comparing siRNA of ROCK1 vs. siRNA of NC. (C) Colony formation assay comparing siRNA of ROCK1 vs. siRNA of NC. (D) Migration assay comparing siRNA of ROCK1 vs. siRNA of NC. (E) Invasion assay comparing siRNA of ROCK1 vs. siRNA of NC. All these show suppressive function of siRNA of ROCK1. P<0.05; siRNA-ROCK1 vs. siRNA NC. ROCK1, Rho-associated, coiled-coil containing protein kinase 1; NC, negative control; miR, microRNA; siRNA, short interfering RNA; OD, optical density.
Discussion
A number of studies have identified essential roles for miRNAs and genes in the cell growth and viability of BC (18–20). Normal tissue may have accumulated numerous detectable mutations, since breast tissue undergoes clonal expansion with more cell division compared with normal cells, which may lead to hypermutability (21). This clearly indicates the requirement for novel therapies to cure BC. With this hope, the current study aimed to investigate the role of miR-340 in the tumor growth and metastasis of BC.
Although there are numerous reports of aberrant miRNA expression in cancer (22,23), data on the involvement of miR-340 in cancer are limited and few potential targets have been identified. A previous study reported that the target genes of miRNAs have similar functions; miR-340 was observed to mimic the effects of transforming growth factor-β activation, inhibiting cell proliferation by modulating cell scattering and cell-cycle arrest in lung cancer (24). The expression of miR-340 in glioblastoma is responsible for a strong tumor-suppressive effect in long-term survivors by downregulating the NRAS proto-oncogene (25). miR-340 inhibits prostate cancer cell proliferation and metastasis by targeting the mouse double minute 2 homolog-tumor protein 53 signaling pathway (26). miR-340 inhibits esophageal cancer cell growth and invasion by targeting phosphoserine aminotransferase (27). miR-340 inhibits the migration, invasion and metastasis of BC cells by targeting the Wnt pathway (13). These studies, together with the results of the present study, confirm that miR-340 acts as a tumor-suppressor agent.
In the present study, ROCK1 was identified as a target of miR-340 and revealed that miR-340 overexpression is correlated with ROCK1 downregulation, leading to the inhibition of cell proliferation, migration and invasion. ROCK1 exists in a closed inactive conformation under quiescent conditions and is activated by direct binding of guanosine triphosphate-loaded Rho (28). ROCK activity and signaling are key elements in invasive and metastatic cancer cell behavior. ROCK kinase activity is greatly influenced by the expression of inhibitory proteins, including dihydropyrimidinase-like 2 and myosin-binding protein H (29). ROCK1 forms the stable actinomyosin filament bundles that initiate front-back and dendritic spine polarity (30). Furthermore, hypoxia-inducible factors mediate coordinated RhoA-ROCK1 expression and signaling in BC cells, which highlights the importance of targeting ROCK1 in the treatment of BC (31).
Although the exact mechanism underlying the miRNA-mediated regulation of ROCK1 is not clear, ROCK1 has been identified as a target of several miRNAs involved in carcinogenesis and tumor progression. In hepatocellular carcinoma, miRNA-145 suppresses cell proliferation and motility by inhibiting ROCK1 (32). The miRNA-mediated regulation of ROCK1 expression has also been observed in rectal cancer, in which miR-144 acts as a tumor suppressor by directly targeting ROCK1, and inhibiting the migration and proliferation of rectal cancer cells (33). Recently, miR-145 was reported to inhibit growth and migration by targeting ROCK1 in BC cells (34). These reports support the present findings, which indicate that the growth and invasion of MDA-MB-231 cells may be partly regulated by the miR-340-dependent modulation of ROCK1 expression.
In conclusion, the present study demonstrated that increased expression of miR-340 inhibited the proliferation, migration and invasion of MDA-MB-231 cells. The tumor-suppressor function of miR-340 was mediated by the downregulation of its downstream target gene ROCK1. These results suggest that miR-340 may act as a tumor-suppressor agent whose downregulation may contribute to the progression and metastasis of TNBC.
Acknowledgements
The present study was supported by grants from the National Natural Science Foundation of China (grant no. 82172240) and Shanghai Science Committee Foundation (grant no. 10411964700).
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67:7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 2.Oeffinger KC, Fontham ET, Etzioni R, Herzig A, Michaelson JS, Shih YC, Walter LC, Church TR, Flowers CR, LaMonte SJ, et al. Breast cancer screening for women at average risk: 2015 guideline update from the American Cancer Society. JAMA. 2015;314:1599–1614. doi: 10.1001/jama.2015.12783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Foulkes WD, Smith IE, Reis-Filho JS. Triple-negative breast cancer. N Engl J Med. 2010;363:1938–1948. doi: 10.1056/NEJMra1001389. [DOI] [PubMed] [Google Scholar]
- 4.Vu T, Claret FX. Trastuzumab: Updated mechanisms of action and resistance in breast cancer. Front Oncol. 2012;2:62. doi: 10.3389/fonc.2012.00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scaltriti M, Rojo F, Ocaña A, Anido J, Guzman M, Cortes J, Di Cosimo S, Matias-Guiu X, y Cajal S Ramon, et al. Expression of p95HER2, a truncated form of the HER2 receptor and response to anti-HER2 therapies in breast cancer. J Natl Cancer Inst. 2007;99:628–638. doi: 10.1093/jnci/djk134. [DOI] [PubMed] [Google Scholar]
- 6.Browne BC, O'Brien N, Duffy MJ, Crown J, O'Donovan N. HER-2 signaling and inhibition in breast cancer. Curr Cancer Drug Targets. 2009;9:419–438. doi: 10.2174/156800909788166484. [DOI] [PubMed] [Google Scholar]
- 7.Macfarlane LA, Murphy PR. MicroRNA: Biogenesis, function and role in cancer. Curr Genomics. 2010;11:537–561. doi: 10.2174/138920210793175895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pillai RS. MicroRNA function: Multiple mechanisms for a tiny RNA? RNA. 2005;11:1753–1761. doi: 10.1261/rna.2248605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen CP, Sun ZL, Lu X, Wu WX, Guo WL, Lu JJ, Han C, Huang JQ, Fang Y. miR-340 suppresses cell migration and invasion by targeting MYO10 in breast cancer. Oncol Rep. 2016;35:709–716. doi: 10.3892/or.2015.4411. [DOI] [PubMed] [Google Scholar]
- 10.Wei P, Qiao B, Li Q, Han X, Zhang H, Huo Q, Sun J. microRNA-340 suppresses tumorigenic potential of prostate cancer cells by targeting high-mobility group nucleosome-binding domain 5. DNA Cell Biol. 2016;35:33–43. doi: 10.1089/dna.2015.3021. [DOI] [PubMed] [Google Scholar]
- 11.Huang D, Qiu S, Ge R, He L, Li M, Li Y, Peng Y. miR-340 suppresses glioblastoma multiforme. Oncotarget. 2015;6:9257–9270. doi: 10.18632/oncotarget.3288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Takeyama H, Yamamoto H, Yamashita S, Wu X, Takahashi H, Nishimura J, Haraguchi N, Miyake Y, Suzuki R, Murata K, et al. Decreased miR-340 expression in bone marrow is associated with liver metastasis of colorectal cancer. Mol Cancer Ther. 2014;13:976–985. doi: 10.1158/1535-7163.MCT-13-0571. [DOI] [PubMed] [Google Scholar]
- 13.Mohammadi-Yeganeh S, Paryan M, Arefian E, Vasei M, Ghanbarian H, Mahdian R, Karimipoor M, Soleimani M. MicroRNA-340 inhibits the migration, invasion, and metastasis of breast cancer cells by targeting Wnt pathway. Tumour Biol. 2016;37:8993–9000. doi: 10.1007/s13277-015-4513-9. [DOI] [PubMed] [Google Scholar]
- 14.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 15.Cancer Genome Atlas Research Network. Weinstein JN, Collisson EA, Mills GB, Shaw KR, Ozenberger BA, Ellrott K, Shmulevich I, Sander C, Stuart JM. The cancer genome atlas pan-cancer analysis project. Nat Genet. 2013;45:1113–1120. doi: 10.1038/ng.2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li JH, Liu S, Zhou H, Qu LH, Yang JH. starBase v2.0: Decoding miRNA-ceRNA, miRNA-ncRNA and protein-RNA interaction networks from large-scale CLIP-Seq data. Nucleic Acids Res. 2014;42(Database issue):D92–D97. doi: 10.1093/nar/gkt1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goswami CP, Nakshatri H. PROGgeneV2: Enhancements on the existing database. BMC Cancer. 2014;14:970. doi: 10.1186/1471-2407-14-970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Iorio MV, Ferracin M, Liu CG, Veronese A, Spizzo R, Sabbioni S, Magri E, Pedriali M, Fabbri M, Campiglio M, et al. MicroRNA gene expression deregulation in human breast cancer. Cancer Res. 2005;65:7065–7070. doi: 10.1158/0008-5472.CAN-05-1783. [DOI] [PubMed] [Google Scholar]
- 19.Takahashi RU, Miyazaki H, Ochiya T. The roles of microRNAs in breast cancer. Cancers (Basel) 2015;7:598–616. doi: 10.3390/cancers7020598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells; Proc Natl Acad Sci USA; 2003; pp. 3983–3988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tomlinson IP. Mutations in normal breast tissue and breast tumours. Breast Cancer Res. 2001;3:299–303. doi: 10.1186/bcr311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shi L, Cheng Z, Zhang J, Li R, Zhao P, Fu Z, You Y. hsa-mir-181a and hsa-mir-181b function as tumor suppressors in human glioma cells. Brain Res. 2008;1236:185–193. doi: 10.1016/j.brainres.2008.07.085. [DOI] [PubMed] [Google Scholar]
- 23.Johnson CD, Esquela-Kerscher A, Stefani G, Byrom M, Kelnar K, Ovcharenko D, Wilson M, Wang X, Shelton J, Shingara J, et al. The let-7 microRNA represses cell proliferation pathways in human cells. Cancer Res. 2007;67:7713–7722. doi: 10.1158/0008-5472.CAN-07-1083. [DOI] [PubMed] [Google Scholar]
- 24.Gennarino VA, D'Angelo G, Dharmalingam G, Fernandez S, Russolillo G, Sanges R, Mutarelli M, Belcastro V, Ballabio A, Verde P, et al. Identification of microRNA-regulated gene networks by expression analysis of target genes. Genome Res. 2012;22:1163–1172. doi: 10.1101/gr.130435.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fiore D, Donnarumma E, Roscigno G, Iaboni M, Russo V, Affinito A, Adamo A, De Martino F, Quintavalle C, Romano G, et al. miR-340 predicts glioblastoma survival and modulates key cancer hallmarks through down-regulation of NRAS. Oncotarget. 2016;7:19531–19547. doi: 10.18632/oncotarget.6968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang K, Tang Y, He L, Dai Y. MicroRNA-340 inhibits prostate cancer cell proliferation and metastasis by targeting the MDM2-p53 pathway. Oncol Rep. 2016;35:887–895. doi: 10.3892/or.2015.4458. [DOI] [PubMed] [Google Scholar]
- 27.Morgan-Fisher M, Wewer UM, Yoneda A. Regulation of ROCK activity in cancer. J Histochem Cytochem. 2013;61:185–198. doi: 10.1369/0022155412470834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yan S, Jiang H, Fang S, Yin F, Wang Z, Jia Y, Sun X, Wu S, Jiang T, Mao A. MicroRNA-340 inhibits esophageal cancer cell growth and invasion by targeting phosphoserine aminotransferase 1. Cell Physiol Biochem. 2015;37:375–386. doi: 10.1159/000430361. [DOI] [PubMed] [Google Scholar]
- 29.Morgan-Fisher M, Wewer UM, Yoneda A. Regulation of ROCK activity in cancer. J Histochem Cytochem. 2013;61:185–198. doi: 10.1369/0022155412470834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Newell-Litwa KA, Badoual M, Asmussen H, Patel H, Whitmore L, Horwitz AR. ROCK1 and 2 differentially regulate actomyosin organization to drive cell and synaptic polarity. J Cell Biol. 2015;210:225–242. doi: 10.1083/jcb.201504046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gilkes DM, Xiang L, Lee SJ, Chaturvedi P, Hubbi ME, Wirtz D, Semenza GL. Hypoxia-inducible factors mediate coordinated RhoA-ROCK1 expression and signaling in breast cancer cells; Proc Natl Acad Sci USA; 2014; pp. E384–E393. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 32.Ding W, Tan H, Zhao C, Li X, Li Z, Jiang C, Zhang Y, Wang L. MiR-145 suppresses cell proliferation and motility by inhibiting ROCK1 in hepatocellular carcinoma. Tumour Biol. 2016;37:6255–6260. doi: 10.1007/s13277-015-4462-3. [DOI] [PubMed] [Google Scholar]
- 33.Cai SD, Chen JS, Xi ZW, Zhang LJ, Niu ML, Gao ZY. MicroRNA-144 inhibits migration and proliferation in rectal cancer by downregulating ROCK-1. Mol Med Rep. 2015;12:7396–7402. doi: 10.3892/mmr.2015.4391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zheng M, Sun X, Li Y, Zuo W. MicroRNA-145 inhibits growth and migration of breast cancer cells through targeting oncoprotein ROCK1. Tumour Biol. 2016;37:8189–8196. doi: 10.1007/s13277-015-4722-2. [DOI] [PubMed] [Google Scholar]