Abstract
Voltage-gated calcium channels (VGCCs) comprise five subtypes: The L-type; R-type; N-type; P/Q-type; and T-type, which are encoded by α1 subunit genes. Calcium ion channels also have confirmed roles in cellular functions, including mitogenesis, proliferation, differentiation, apoptosis and metastasis. An association between VGCCs, a reduction in proliferation and an increase in apoptosis in prostate cancer cells has also been reported. Therefore, in the present study, the online clinical database Oncomine was used to identify the alterations in the mRNA expression level of VGCCs in 19 cancer subtypes. Overall, VGCC family genes exhibited under-expression in numerous types of cancer, including brain, breast, kidney and lung cancers. Notably, the majority of VGCC family members (CACNA1C, CACNA1D, CACNA1A, CACNA1B, CACNA1E, CACNA1H and CACNA1I) exhibited low expression in brain tumors, with mRNA expression levels in the top 1–9% of downregulated gene rankings. A total of 5 VGCC family members (CACNA1A, CACNA1B, CACNA1E, CACNA1G and CACNA1I) were under-expressed in breast cancer, with a gene ranking in the top 1–10% of the low-expressed genes compared with normal tissue. In kidney and lung cancers, CACNA1S, CACNA1C, CACNA1D, CACNA1A and CACNA1H exhibited low expression, with gene rankings in the top 1–8% of downregulated genes. In conclusion, the present findings may contribute to the development of new cancer treatment approaches by identifying target genes involved in specific types of cancer.
Keywords: voltage-gated calcium channels, cancer, under-expression, potential biomarker, meta-analysis, Oncomine
Introduction
Conventional studies on ion channels have primarily focused on the crucial roles these channels perform in excitatory cell types, including neurons, cardiomyocytes and secretory cells (1). The roles of ion channels in various cell functions, including mitogenesis, cell proliferation, differentiation, apoptosis and metastasis are now well recognized (2–4). The expression of ion channel transcripts has been highlighted as a potential biomarker of certain types of cancer, including prostate cancer and breast cancer (5–8). Therefore, the investigation of the functional roles of ion channels in cancer development may identify novel approaches for tumor prognosis (9,10).
Calcium channels may be generally categorized into two major classes: Voltage-gated calcium channels (VGCCs) and ligand-gated calcium channels (LGCCs). VGCCs may be classified into five subtypes: L-type (10,11); N-Type (12); P-type (13–15); T-type (16–18); and R-type (19,20). These ion channels have been implicated in the progression of numerous cancers. Members of the LGCC class, namely the inositol trisphosphate receptor (21) and ryanodine (22), are also well documented to regulate certain processes occurring during cancer metastasis.
The role of calcium has been well documented in numerous cellular processes, including cell proliferation and inhibition and activation of various intracellular enzymes (23–25). The effect of calcium on these processes varies by location, extent and calcium homeostasis stage (26,27). The transcript levels of calcium channels may cause different domino effects on specific cell functions, as well as on cell proliferation, motility or even cell apoptosis (28).
The recessive inheritance pattern of tumor suppressor genes presents the greatest challenge in their identification. Another challenge is the diversity of their action mechanisms, which range from variations in structure and copy number to epigenetic changes and single base modifications. In addition, the molecular mechanism of action of a tumor suppressor gene may change depending on the tumor type. These variations affect the abnormal activation of oncogenes and/or the inactivation of tumor suppressor genes, resulting in tumor formation and development.
Expression levels of genes are currently recognized as potential biological markers (29–32). The recent publication of a public database (Oncomine; www.oncomine.org) containing mRNA expression profiles for various cancers has now led to the supply of a prodigious number of datasets, which may improve the identification of critical biomarkers in cancers and assist the development of improved molecular signatures, once in full operation (33,34). However, tumor suppressor genes tend to exhibit low or reduced expression in tumor tissue compared with normal tissue. A previous study specifically evaluated a tumor suppressor gene in breast cancer datasets from the Oncomine database and observed significant downregulation and low expression of the tumor suppressor gene ADAM metallopeptidase with thrombospondin type 1 motif 1 in breast carcinomas, when compared with normal tissue (35). Another study revealed that the expression of sirtuin-3 (SIRT3), a mitochondria-localized tumor suppressor, was decreased in breast tumors relative to normal type-matched tissue. SIRT3 induced destabilization of hypoxia-inducible factor-1α, whereas knockdown of SIRT3 increased cancer cell growth and angiogenesis (36). The SIRT3 gene also exhibited lower expression in various tumor types, including clear cell hepatocellular, head and neck squamous cell, glioblastoma multiforme, testicular and prostate cancers (37).
VGCCs are likely to be possible targets for future clinical cancer treatments. The blockage of T-type calcium channels in HCT116 cells was shown to inhibit cell development and promote apoptosis (38). The T-type calcium channel also confers anti-proliferative properties in malignant tumor cells (39). Cells that highly express the majority of types of calcium channels are significantly reduced in their proliferation capabilities (40). The calcium voltage-gated channel subunit α1 G (CACNA1G; Cav3.1) gene, a T-type α subunit of a calcium channel, has been studied in colorectal cancer for its involvement in methylation and silencing. CACNA1G is also considered a potential tumor suppressor gene, which may reduce the effects of hypermethylation of CpG islands, thereby attenuating cancer development (41). Another study reported an association between the CACNA1G gene, a reduction in proliferation and an increase in apoptosis in prostate cancer cells. However, the molecular mechanisms of the role of calcium channels as tumor suppressors remain unclear and require additional study (42).
The authors of the present study previously performed a meta-analysis on public microarray datasets and demonstrated the existence of voltage-gated calcium gene signatures in patients with cancer (43). However, compared with normal tissue, certain VGCC family genes exhibited low expression in tumor tissue. In addition, at present, the way in which VGCC family genes function as tumor suppressors in cancer development remains largely unknown. To the best of our knowledge, the present study is the first exploration of the reduced expression of VGCC genes in cancer development, and the present findings indicated that VGCC genes may be prospective targets for cancer treatment. Based on bioinformatics screening, the under-expression of VGCCs at the mRNA level has raised the question of their possible association with the development and progression of cancer. This potential association between VGCCs and cancer was investigated using Oncomine, a clinical online microarray database (44). The aim of the present study was to examine the expression of VGCC genes in various types and subtypes of cancer, and to identify critical indicators supporting prospective research on the role of VGCC channels in cancer onset and progression.
Materials and methods
Meta-analysis
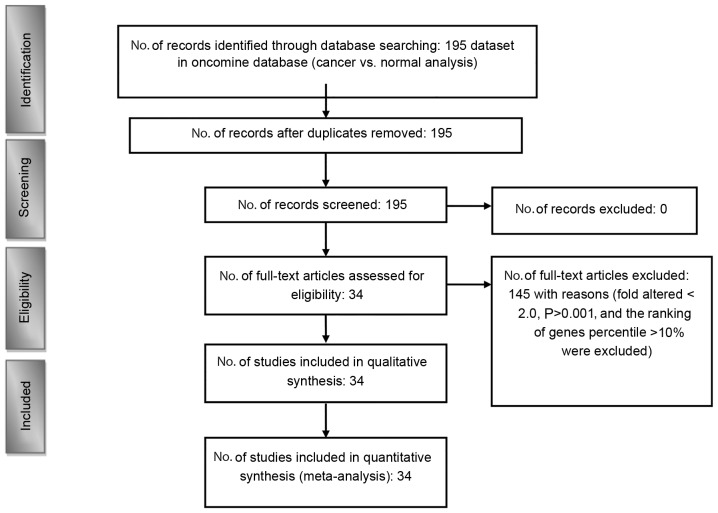
According to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis guidelines, a meta-analysis of public microarray data was performed to analyze the under-expression of VGCC mRNA in clinical cancer tissue (Fig. 1) (45,46). Oncomine (www.oncomine.org) was used to conduct a systematic analysis of all public cancer microarray data (44). The Oncomine Platform Overview Q1 2014 website comprises >700 independent datasets with ~90,000 microarray experiments. Analyses of these data determined the under-expression of VGCC genes in diverse types and subtypes of cancer.
Figure 1.
Flow chart describing the process of data identification and accumulation for the quantitative analysis.
Threshold specifications were set to evaluate the potential tumor suppressor genes in the datasets that could control VGCC transcript expression in cancer tissue. Samples that showed under-expression with a fold change <-2.0, P<0.001 and a gene rank in the top 10% were included in the analysis. A fold-change-based standard for under-expression was used to establish the linear model association between mRNA levels and VGCC gene expression in cancer tissues compared with normal expression levels in matched tissue. The P-values were used to determine the gene rank percentile, indicating the degree of expression. Ultimately, 34 studies were retained, comprising 4,443 samples (47,48). To investigate VGCC expression in cancer cell lines, gene expression data on VGCC genes across a panel of 967 cancer cell lines was downloaded and analyzed from the Cancer Cell Line Encyclopedia (CCLE) (49).
Results and Discussion
Voltage-gated calcium channel family in cancer development
VGCCs consist of the α1 subunit, which controls the construction of calcium selective pores. Five subtypes of VGCC are recognized, consisting of the L-type, R-type, N-type, P/Q-type and T-type, which are encoded by the α1 subunit genes (50,51). CACNA1S, CACNA1C, CACNA1D and CACNA1F encode the L-type, CACNA1E encodes the R-type, CACNA1B encodes the N-type, CACNA1A encodes the P/Q type, and CACNA1G, CACNA1H and CACNA1I encode the T-type (52). Tumor suppressor genes have been well documented as cell division regulators that control protein expression. A mutation of these anti-oncogenes may affect cell proliferation, cell cycle and apoptosis, which is then likely to trigger the onset of a specific type of cancer (53). In cancer tissues, abnormal cell growth is mainly caused by the inactivation or lack of tumor suppressor genes (54). Tumor suppressor genes are also a type of cell growth inhibitor and suppress tumor formation genes. Tumor suppressor genes are also under-expressed or downregulated in tumor tissues compared with normal tissue (53).
Previous studies have specifically used the Oncomine database to screen and evaluate tumor suppressor genes. For example, protein tyrosine phosphatase, receptor type D was expressed at significantly lower levels in glioma relative to matched tissue in the brain (55). Another study using the Oncomine database revealed that the level of castor zinc finger 1 (CASZ1) expression was lower in numerous cancer cell types, including gastric intestinal-type adenocarcinoma, head and neck squamous cell carcinoma and clear cell renal cell carcinoma. CASZ1 was also revealed to function as a tumor-suppressor in neuroblastoma (56). A high expression of CASZ1 may interrupt the cancer development process of other types of tissue and play a role in their tumorigenesis (57).
The present bioinformatics analysis revealed that CACNA1S, CACNA1C, CACNA1D and CACNA1A were abundantly expressed in normal tissue but not in cancer tissue; thus, they could serve as tumor suppressor gene markers for specific subtypes of cancer. For these specific types and subtypes of cancer, patients may be supplemented with healthy food or a calcium agonist. This would enable the regulation of CACNA1S, CACNA1C, CACNA1D and CACNA1A signal transduction, thereby offering a method to treat cancer and even cardiovascular disease (58–61), obesity (62) and diabetes (63–65). However, no global method currently exists to screen for the under-expression of VGCCs in diverse types and subtypes of cancers. Therefore, the present study used Oncomine, an online clinical microarray database, to calculate the changes in mRNA expression levels of VGCCs in 20 types of cancer, and to determine a suitable threshold based on P-values (<0.001), fold change (<-2.0) and gene rank (<top 10%) to select satisfactory datasets. Fig. 1 showed VGCC gene under-expression in 20 cancers, including the corresponding fold changes, P-values and top gene ranks.
A bioinformatics approach was performed in the current study to determine the functions of VGCC genes in the development of cancer, and to determine whether VGCC genes are potential tumor suppressor genes similar to PTEN (66) or SIRT3. The VGCC genes showed downregulation in 18 of the 20 examined cancer tissues compared with normal tissue (Fig. 1). Overall, VGCC family genes showed under-expression in numerous types of cancers, including brain, breast, kidney and lung cancers. Notably, when compared with their expression in normal tissue, the majority of VGCC family members (CACNA1C, CACNA1D, CACNA1A, CACNA1B, CACNA1E, CACNA1H and CACNA1I) were found to be downregulated in brain tumors, with an mRNA expression level in the top 1–9% of the gene rankings. In total, 5 of the VGCC family members (CACNA1A, CACNA1B, CACNA1E, CACNA1G and CACNA1I) were under-expressed in breast cancer, with a gene ranking in the top 1–10% of the expressed genes relative to normal tissue. In kidney cancer and lung cancer, CACNA1S, CACNA1C, CACNA1D, CACNA1A and CACNA1H had low levels of expression, with a gene ranking in the top 1–8% of the expressed genes. In addition, certain VGCC family genes were under-expressed in other cancer subtypes, including colorectal, lymphoma, ovarian and bladder cancers.
L-type calcium channel family
The L-type calcium channel is encoded by four genes: Cav1.1 (CACNA1S); Cav1.2 (CACNA1C); Cav1.3 (CACNA1D); and Cav1.4 (CACNA1F). These genes are generally located in different areas, including skeletal muscle, smooth muscle, bone (osteoblasts) and ventricular myocytes. Previous studies explored the role of L-type calcium channels primarily in the physiology and pharmacology fields (67,68). Therefore, their functions are largely unknown in terms of their role in cancer progression. Notably, compared with our previous studies, CACNA1S was overexpressed relative to normal tissue samples in acute myeloid leukemia and brain and central nervous system (CNS) tumors (1). The present data revealed downregulated CACNA1S in renal oncocytoma, with a −3.920-fold change, as well as in head and neck squamous cell carcinoma, (3.670-fold change) and in squamous cell lung carcinoma (−2.038-fold change), compared with matched normal tissue (Table I). In addition, CACNA1S ranked in the top 5% of downregulated genes in head-neck and renal cancer (Fig. 2). Thus, CACNA1S showed increased mRNA expression in a number of cancer tissues but decreased expression in others. These findings indicated that cell context-specific alterations in CACNA1S expression may play a critical role in cancer biology.
Table I.
L-type calcium channel expression in cancers.
| Gene and cancer type | Patient number, n | P-value (cancer/normal) | t-test (cancer/normal) | Fold (cancer/normal) | Gene ranking, % | Database reference | (Refs.) |
|---|---|---|---|---|---|---|---|
| CACNA1S Renal | |||||||
| Renal oncocytoma | 92 | 1.77×10–16 | −26.050 | −3.920 | 303 (top 3) | Clin Cancer Res 2005/08/15 | (94) |
| Papillary renal cell carcinoma | 92 | 4.88×10–15 | −21.978 | −2.934 | 324 (top 3) | Clin Cancer Res 2005/08/15 | (94) |
| Head-neck | |||||||
| Head and neck squamous cell carcinoma | 54 | 6.96×10–9 | −7.318 | −3.670 | 748 (top 6) | Cancer Res 2004/01/01 | (95) |
| Tongue squamous cell carcinoma | 58 | 5.26×10–6 | −5.261 | −6.638 | 324 (top 4) | BMC Cancer 2009/01/12 | (96) |
| Lung | |||||||
| Squamous cell lung carcinoma | 93 | 8.64×10–7 | −6.038 | −2.038 | 601 (top 7) | Cancer Res 2005/04/15 | (97) |
| CACNA1C Brain | |||||||
| Glioblastoma | 180 | 9.26×10–21 | −14.125 | −4.483 | 484 (top 3) | Cancer Cell 2006/04/01 | (70) |
| Oligodendroglioma | 180 | 1.39×10–14 | −10.049 | −3.024 | 304 (top 2) | Cancer Cell 2006/04/01 | (70) |
| Anaplastic astrocytoma | 180 | 7.46×10–11 | −8.854 | −3.166 | 72 (top 1) | Cancer Cell 2006/04/01 | (70) |
| Diffuse astrocytoma | 180 | 4.59×10–4 | −4.878 | −2.505 | 619 (top 4) | Cancer Cell 2006/04/01 | (70) |
| Glioblastoma | 84 | 1.77×10–4 | −11.513 | −2.550 | 1,173 (top 6) | J Clin Oncol 2008/06/20 | (71) |
| Lymphoma | |||||||
| Centroblastic lymphoma | 336 | 3.77×10–18 | −13.615 | −2.381 | 17 (top 1) | Nat Genet 2005/04/01 | (98) |
| Ovarian | |||||||
| Ovarian serous adenocarcinoma | 53 | 1.72×10–14 | −13.253 | −27.228 | 218 (top 2) | Cancer Sci 2009/08/01 | (99) |
| Bladder | |||||||
| Infiltrating bladder urothelial carcinoma | 157 | 9.94×10–12 | −7.365 | −3.364 | 675 (top 6) | J Clin Oncol 2006/02/10 | (100) |
| Superficial bladder cancer | 157 | 2.00×10–10 | −8.288 | −6.787 | 750 (top 6) | J Clin Oncol 2006/02/10 | (100) |
| Prostate | |||||||
| Prostatic intraepithelial neoplasia epithelia | 101 | 2.80×10–5 | −4.635 | −3.139 | 134 (top 2) | Nat Genet 2007/01/01 | (101) |
| Prostate carcinoma epithelia | 101 | 7.53×10–4 | −3.381 | −2.192 | 524 (top 5) | Nat Genet 2007/01/01 | (101) |
| Renal | |||||||
| Papillary renal cell carcinoma | 67 | 4.95×10–6 | −5.711 | −2.297 | 308 (top 2) | BMC Cancer 2009/05/18 | (102) |
| Salivary gland | |||||||
| Salivary gland adenoid cystic carcinoma | 22 | 5.15×10–6 | −5.962 | −22.108 | 257 top 3) | Am J Pathol 2002/10/01 | (103) |
| Cervix | |||||||
| Cervical squamous cell carcinoma | 45 | 1.25×10–4 | −8.154 | −2.377 | 1,775 (top 10) | Gynecol Oncol 2008/03/01 | (104) |
| Colorectal | |||||||
| Rectosigmoid adenocarcinoma | 237 | 5.06×10–4 | −3.880 | −2.273 | 1861 (top 10) | TCGA 2011/09/08 | |
| CACNA1D Lung | |||||||
| Squamous cell lung carcinoma | 156 | 7.16×10–31 | −19.447 | −4.801 | 51 (top 1) | PLoS One 2010/04/22 | (105) |
| Lung adenocarcinoma | 246 | 2.15×10–24 | −13.057 | −2.443 | 45 (top 1) | Cancer Res 2012/01/01 | (106) |
| Brain | |||||||
| Glioblastoma | 180 | 3.23×10–16 | −10.515 | −2.850 | 1,122 (top 6) | Cancer Cell 2006/04/01 | (70) |
| Anaplastic astrocytoma | 180 | 3.49×10–6 | −5.415 | −2.094 | 1,722 (top 9) | Cancer Cell 2006/04/01 | (70) |
| Diffuse astrocytoma | 180 | 0.001 | −4.432 | −2.814 | 1,117 (top 6) | Cancer Cell 2006/04/01 | (70) |
| Myeloma | |||||||
| Monoclonal gammopathy of undetermined | |||||||
| significance | 78 | 3.65×10–6 | −4.889 | −2.563 | 435 (top 3) | Blood 2007/02/15 | (107) |
| Smoldering myeloma | 78 | 2.30×10–4 | −4.507 | −3.487 | 552 (top 3) | Blood 2007/02/15 | (107) |
| Sarcoma | |||||||
| Dedifferentiated liposarcoma | 54 | 2.88×10–4 | −4.694 | −4.980 | 102 (top 1) | Cancer Res 2005/07/01 | (108) |
| Malignant fibrous histiocytoma | 54 | 6.13×10–4 | −3.790 | −4.255 | 514 (top 5) | Cancer Res 2005/07/01 | (108) |
| Renal | |||||||
| Renal Wilms tumor | 67 | 3.71×10–4 | −6.265 | −2.958 | 144 (top 1) | BMC Cancer 2009/05/18 | (102) |
| CACNA1F Lymphoma | |||||||
| Anaplastic large cell lymphoma | 60 | 1.14×10–12 | −13.057 | −2.044 | 43 (top 1) | J Clin Invest 2007/03/01 | (73) |
Figure 2.
Under-expression of 10 voltage-gated calcium channel genes in 19 types of cancers. The comparison of cancer vs. normal tissue was shown at different expression levels based on P<0.001, a fold change >2.0 and a gene rank percentile <10%, to screen the microarray datasets. The color alterations represent the changes in level of gene rank percentage.
CACNA1C has been revealed to affect the pathophysiology of psychiatric disease (69). The present bioinformatics analysis confirmed that CACNA1C exhibited low expressions in the majority of types of cancer, including brain, lymphoma, ovarian, bladder, prostate, renal, salivary gland, cervix and colorectal cancers, compared with normal tissue (Table I). In addition, 9 of the 20 types of cancer showed downregulation in which CACNA1C was ranked in the top 5% of genes that had low expression (Fig. 2). Compared with normal tissue, CACNA1C was significantly downregulated in the majority of brain tumor types, including diffuse astrocytoma, glioblastoma, anaplastic astrocytoma and oligodendroglia, with P-values ranging between 1.77×10−4 and 9.26×10−21 and a gene ranking between 1 and 6%. Lastly, CACNA1C expression decreased in centroblastic lymphoma, with a −2.381-fold increase, a P-value of 3.77×10−18 and a gene ranking in the top 1% relative to matched normal tissue sections. Compared with our previous study (43), CACNA1C was overexpressed compared with normal tissue samples in gastric, pancreas, brain, colorectal, breast, uterus, skin and prostate cancers and leukemia. Notably, up- and downregulated CACNA1C was observed in brain, prostate and colorectal tumors compared with normal tissue. The conflicting expression profiles of CACNA1C in the same types of cancer may be due to the broad range of categories for each cancer subtype. For example, our previous study (43) indicated that CACNA1C had high expression in prostate carcinoma; however, in the present study, CACNA1C had low expression in prostatic intraepithelial neoplasia epithelia relative to normal type-matched tissue. In addition, CACNA1C had high expression in colon and renal adenoma; however, in the present study, CACNA1C had low expression in rectosigmoid adenocarcinoma relative to normal matched tissue. In addition, CACNA1C was up- and downregulated in brain glioblastoma. The small sample size in the original publications (70,71) may explain this discrepancy. Collectively, our data suggested that alterations in CACNA1C expression may have an adverse effect on tissue homeostasis, which may result in tumorigenesis.
CACNA1D regulates cell firing (21) and is associated with prostate cancer; however, the presence of CACNA1D in other cancer subtypes requires confirmation. The present study revealed that CACNA1D expression levels decreased in lung, brain, myeloma, sarcoma and renal cancer. In 5 of the 20 cancer tissue sections, CACNA1D was downregulated and presented in the top 10% of downregulated genes (Fig. 2). In addition, CACNA1D levels also markedly declined in squamous cell lung carcinoma and lung adenocarcinoma, with fold changes ranging between-2.443 and −4.801, P-values ranging between 2.15×10−24 and 7.16×10−31 and gene rankings all in the top 1% relative to normal type-matched tissue. In brain tumors, consisting of glioblastoma, anaplastic astrocytoma and diffuse astrocytoma, CACNA1D was substantially downregulated, with fold changes between 2.814 and −2.850, P-values between 0.001 and 3.23×10−16 and gene rankings in the top 6–9%. To the best of our knowledge, these findings regarding the low expression of CACNA1D in myeloma, sarcoma and renal tumors (Table I) are novel findings. In our previous study (43), CACNA1D specifically had low expression in myeloma, sarcoma and renal tumors and was highly expressed in prostate, breast, colorectal, bladder, gastric, uterine and esophageal tumors. In addition, CACNA1D was up- and downregulated in brain and lung cancer. CACNA1D was upregulated in lung carcinoid tumors, but under-expressed in squamous cell lung carcinoma and lung adenocarcinoma. Based on the current data, CACNA1D may be a useful marker for potential tumor suppressor genes in cancer development; however, its mechanism of action in cancer requires additional investigation in prospective studies.
CACNA1F was reported to be involved in human physiology and photoreceptors, although its role in cancer biology is generally unknown. In the present analysis, CACNA1F was under-expressed in anaplastic large cell lymphoma with a fold change of −2.044, P-value of 1.14×10−12, and gene ranking in the top 1% (Table I). In our previous study, CACNA1F was overexpressed in testicular teratoma relative to normal matched tissue samples (72). Therefore, CACNA1F had increased mRNA expression in testis cancer but decreased expression in lymphoma. These findings indicated that cell context-specific alterations in CACNA1F expression may play a critical role in cancer biology.
P/Q-type calcium channel family
The P/Q calcium channel is encoded by Cav2.1 (CACNA1A), located in Purkinje cells and cerebellar granule cells (73). CACNA1A is known to be involved in neurological disease (74); compared with normal tissue, CACNA1A is downregulated in brain, colorectal, gastric and breast cancers (Fig. 1). In 4 of the 20 cancer types studied, the expression of CACNA1A was low, with a gene ranking in the top 10% of downregulated genes (Fig. 1). Brain tumors, consisting of glioblastoma, anaplastic astrocytoma, astrocytoma, atypical teratoid/rhabdoid tumor, malignant glioma, primitive neuroectodermal tumor, classic medulloblastoma, desmoplastic medulloblastoma and oligodendroglioma, all showed significant downregulation (P<0.05) of CACNA1A in comparison to type-matched tissues. In silico analysis of brain datasets showed a −2.084-fold change in CACNA1A expression levels, with P-values ranging between 2.84×10−4 and 8.77×10−18 and a gene ranking in the top 1–5%. Colorectal cancers, including colorectal carcinoma and colon adenoma, all showed the most significant decreases in expression of CACNA1A relative to control samples, with-2.807- to −3.376-fold downregulation, P-values between 3.39×10−5 and 5.25×10−6 and gene rankings in the top 8–10% (Table II). Compared with our previous studies (43), CACNA1A specifically had low expression in colorectal, esophageal and gastric cancers, but also had high expression in leukemia, and sarcoma, ovarian, uterine, lung and cervical tumors. In addition, it was also identified that CACNA1A was up- and downregulated in brain tumors. Overall, our bioinformatics analysis verified that CACNA1A is likely to be useful as a possible cancer therapeutic target for colorectal, esophagus, gastric and breast cancers.
Table II.
P-type calcium channel expression in cancers.
| Gene and cancer type | Patient number, n | P-value (cancer/normal) | t-test (cancer/normal) | Fold (cancer/normal) | Gene ranking, % | Database reference | (Refs.) |
|---|---|---|---|---|---|---|---|
| CACNA1A Brain | |||||||
| Glioblastoma | 180 | 8.77×10–18 | −10.656 | −3.039 | 841 (top 5) | Cancer Cell 2006/04/01 | (70) |
| Anaplastic astrocytoma | 180 | 3.02×10–8 | −6.935 | −2.188 | 637 (top 4) | Cancer Cell 2006/04/01 | (70) |
| Brain glioblastoma | 557 | 2.09×10–13 | −24.201 | −6.900 | 330 (top 3) | TCGA 2013/06/03 | |
| Glioblastoma | 84 | 4.22×10–6 | −8.968 | −2.424 | 585 (top 3) | J Clin Oncol 2008/06/20 | (71) |
| Astrocytoma | 51 | 2.54×10–5 | −5.241 | −7.344 | 148 (top 3) | Cancer Res 2001/09/15 | (109) |
| Atypical teratoid/rhabdoid tumor | 85 | 9.05×10–5 | −7.761 | −523.335 | 24 (top 1) | Nature 2002/01/24 | (11) |
| Malignant glioma, NOS | 85 | 2.07×10–4 | −5.012 | −139.970 | 80 (top 2) | Nature 2002/01/24 | (11) |
| Primitive neuroectodermal tumor, NOS | 85 | 4.64×10–4 | −10.153 | −704.199 | 34 (top 1) | Nature 2002/01/24 | (11) |
| Classic medulloblastoma | 85 | 5.73×10–4 | −5.011 | −51.831 | 411 (top 8) | Nature 2002/01/24 | (11) |
| Desmoplastic medulloblastoma | 85 | 8.98×10–4 | −3.937 | −49.283 | 199 (top 4) | Nature 2002/01/24 | (11) |
| Oligodendroglioma | 42 | 1.09×10–4 | −6.523 | −2.168 | 289 (top 4) | Oncogene 2003/07/31 | (110) |
| Astrocytoma | 42 | 2.84×10–4 | −5.138 | −2.084 | 141 (top 2) | Oncogene 2003/07/31 | (110) |
| Colorectal | |||||||
| Colorectal carcinoma | 82 | 5.25×10–6 | −5.293 | −2.807 | 1,830 (top 10) | Clin Exp Metastasis 2010/02/01 | (111) |
| Colon adenoma | 64 | 3.39×10–5 | −4.402 | −3.376 | 1,451 (top 8) | Mol Cancer Res 2007/12/01 | (112) |
| Esophagus | |||||||
| Esophagus | 48 | 9.61×10–5 | −5.368 | −2.873 | 146 (top 1) | Gastroenterology 2006/09/01 | (20) |
| Gastric | |||||||
| Gastric intestinal type adenocarcinoma | 69 | 2.25×10–4 | −3.737 | −2.370 | 1,040 (top 6) | Eur J Cancer 2009/02/01 | (113) |
| Gastric mixed adenocarcinoma | 69 | 3.40×10–4 | −5.809 | −6.673 | 426 (top 3) | Eur J Cancer 2009/02/01 | (113) |
| Breast | |||||||
| Invasive ductal breast carcinoma | 30 | 9.34×10–4 | −3.536 | −2.205 | 189 (top 1) | BMC Cancer 2007/03/27 | (114) |
NOS, not otherwise specified.
N-type calcium channel family
The N-type calcium channel is encoded by an α1 subunit termed CACNA1B (Cav2.2), which is localized in the brain and peripheral nervous system. In neuropathic pain, CACNA1B serves to preserve neuronal firing and neurotransmitter release (74). However, CACNA1B has not yet been associated with cancer. The bioinformatics data derived in the present study regarding brain and breast cancer showed that CACNA1B ranked in the top 1 and 5% of genes with low expression, respectively. Brain tumors, including glioblastoma, oligodendroglia, anaplastic astrocytoma, diffuse astrocytoma and glioblastoma all showed significant downregulation (P<0.05) of CACNA1B relative to control samples. In silico analyses of brain datasets showed under-expression of CACNA1A, ranging between −4.336 and −8.235-fold decreases in transcript expression, P-values between 1.05×10−5 and 3.47×10−19 and gene rankings in the top 1–5%. For CACNA1B expression in male breast carcinoma, the data also showed decreases, with a −2.067-fold change and a P-value of 3.41×10−5 relative to control tissue (Table III). Compared with our previous results (43), CACNA1B specifically had low expression in brain cancer, but also had high expression in prostate tumors. In addition, CACNA1B was also upregulated in intraductal cribriform breast adenocarcinoma, but downregulated in male breast carcinoma. Finally, the present data indicated that CACNA1B was moderately expressed in brain cancer and male breast cancer. Understanding the underlying mechanisms behind CACNA1B action in cancer progression may assist in determining prospective therapeutic targets for the treatment of brain cancer and male breast cancer.
Table III.
N-type calcium channel expression in cancers.
| Gene and cancer type | Patient number, n | P-value (cancer/normal) | t-test (cancer/normal) | Fold (cancer/normal) | Gene ranking, % | Database reference | (Refs.) |
|---|---|---|---|---|---|---|---|
| CACNA1B | |||||||
| Brain | |||||||
| Glioblastoma | 180 | 3.47×10–19 | −12.113 | −8.235 | 656 (in top 4) | Cancer Cell 2006/04/01 | (70) |
| Oligodendroglioma | 180 | 2.37×10–11 | −7.840 | −4.336 | 947 (top 5) | Cancer Cell 2006/04/01 | (70) |
| Anaplastic astrocytoma | 180 | 1.37×10–9 | −7.611 | −5.134 | 223 (top 2) | Cancer Cell 2006/04/01 | (70) |
| Diffuse astrocytoma | 180 | 1.40×10–6 | −6.720 | −4.929 | 33 (top 1) | Cancer Cell 2006/04/01 | (70) |
| Glioblastoma | 84 | 1.05×10–5 | −19.805 | −5.922 | 691 (top 4) | J Clin Oncol 2008/06/20 | (71) |
| Breast | |||||||
| Male breast carcinoma | 593 | 3.41×10–5 | −4.423 | −2.067 | 827 (top 5) | TCGA2011/09/02 | |
T-type calcium channel family
The T-type calcium channel consists of three subtypes [Cav3.1 (CACNA1G), Cav3.2 (CACNA1H) and Cav3.3 (CACNA1I)], which are located in neurons, pacemaker cells and osteocytes. The functions of the T-type calcium channel remain generally unknown. CACNA1G exhibited low expression in numerous cancers, including ovarian serous adenocarcinoma and mucinous breast carcinoma, with a gene ranking in the top 3–5% of genes with low expression, fold changes between −6.893 and −2.209, and P-values between 2.42×10−5 and 4.27×10−10 (Table IV). Compared with our previous results (43), CACNA1G specifically had low expression in ovarian, renal, brain and bladder cancer, but also exhibited high expression in sarcoma, lung, uterine and prostate tumors. CACNA1G was also revealed to be upregulated in invasive lobular breast carcinoma but downregulated CACNA1G was observed in mucinous breast carcinoma and mantle cell lymphoma. In addition, CACNA1G was upregulated in rectosigmoid adenocarcinoma but downregulated in colorectal carcinoma.
Table IV.
T-type calcium channel expression in cancers.
| Gene and cancer type | Patient number, n | P-value (cancer/normal) | t-test (cancer/normal) | Fold (cancer/normal) | Gene ranking, % | Database reference | (Refs.) |
|---|---|---|---|---|---|---|---|
| CACNA1G Ovarian | |||||||
| Ovarian serous adenocarcinoma | 53 | 4.27×10–10 | −8.514 | −6.893 | 685 (top 5) | Cancer Sci 2009/08/01 | (99) |
| Colorectal | |||||||
| Colorectal carcinoma | 82 | 6.45×10–7 | −6.447 | −3.266 | 1,537 (top 8) | Clin Exp Metastasis 2010/02/01 | (111) |
| Brain | |||||||
| Anaplastic astrocytoma | 180 | 4.26×10–6 | −5.111 | −1.976 | 1,771 (top 10) | Cancer Cell 2006/04/01 | (70) |
| Breast | |||||||
| Mucinous breast carcinoma | 593 | 2.42×10–5 | −7.311 | −2.209 | 438 (top 3) | TCGA 2011/09/02 | |
| Mantle cell lymphoma | 336 | 7.74×10–4 | −3.839 | −2.085 | 207 (top 3) | Nat Genet 2005/04/01 | (98) |
| Renal | |||||||
| Renal oncocytoma | 92 | 1.76×10–15 | −3.710 | −2.201 | 362 (top 3) | Clin Cancer Res 2005/08/15 | (94) |
| Chromophobe renal cell carcinoma | 1.24×10–7 | −9.936 | −2.096 | 490 (top 4) | Clin Cancer Res 2005/08/15 | (94) | |
| Bladder | |||||||
| Infiltrating bladder Urothelial carcinoma | 157 | 2.53×10–15 | −9.913 | −6.640 | 380 (top 4) | J Clin Oncol 2006/02/10 | (100) |
| Superficial bladder cancer | 157 | 1.49×10–7 | −5.754 | −4.595 | 1,158 (top 10) | J Clin Oncol 2006/02/10 | (100) |
| CACNA1H | |||||||
| Brain | |||||||
| Glioblastoma | 101 | 7.01×10–13 | −13.798 | −3.370 | 129 (top 1) | Cancer Cell 2006/05/01 | (115) |
| Ovarian | |||||||
| Ovarian serous adenocarcinoma | 53 | 1.44×10–8 | −7.924 | −9.621 | 1,021 (top 7) | Cancer Sci 2009/08/01 | (99) |
| Bladder | |||||||
| Infiltrating bladder urothelial carcinoma | 256 | 4.81×10–9 | −6.150 | −2.679 | 356 (top 2) | J Clin Oncol 2010/06/01 | (116) |
| Superficial bladder cancer | 256 | 2.62×10–8 | −5.851 | −2.292 | 1,295 (top 7) | J Clin Oncol 2010/06/01 | (116) |
| Lung | |||||||
| Lung adenocarcinoma | 203 | 1.43×10–4 | −4.251 | −2.152 | 609 (top 8) | Proc Natl Acad Sci USA 2001/11/20 | (117) |
| Squamous cell lung carcinoma | 203 | 3.80×10–4 | −3.779 | −4.318 | 423 (top 5) | Proc Natl Acad Sci USA 2001/11/20 | (117) |
| Breast | |||||||
| Invasive breast carcinoma stroma | 59 | 7.55×10–16 | −12.196 | −2.184 | 1,871 (top 10) | Nat Med 2008/05/01 | (18) |
| Invasive breast carcinoma | 158 | 1.75×10–4 | −11.625 | −4.405 | 758 (top 5) | Breast Cancer Res Treat 2011/03/04 | (118) |
| Brain | |||||||
| Brain glioblastoma | 557 | 4.91×10–9 | −19.105 | −8.779 | 946 (top 8) | TCGA 2013/06/03 | |
| CACNA1I | |||||||
| Brain | |||||||
| Glioblastoma | 557 | 3.13×10–5 | −8.788 | −7.373 | 185 (top 2) | TCGA 2013/06/03 | |
| Anaplastic oligodendroglioma | 54 | 0.001 | −5.915 | −2.371 | 620 (top 5) | Cancer Res2005/10/01 | (119) |
CACNA1H was downregulated in gastrointestinal stromal tumor, sarcoma and renal cancer (Fig. 1). CACNA1H presented in the top 1–3% of downregulated genes in glioblastoma and renal oncocytoma, respectively. Compared with normal tissue, the fold changes ranged between −3.370 and −2.201, and P-values ranged between 7.01×10−13 and 1.76×10−15. CACNA1H also exhibited low expression in bladder cancers, including infiltrating bladder urothelial carcinoma (fold change, −6.64; P<2.53×10−15) and superficial bladder cancer (fold change, −4.59; P=1.49×10−7). Notably, compared with our previous research, CACNA1H was specifically overexpressed relative to normal tissue samples in renal cancer, sarcoma and gastrointestinal stromal tumors (75). However, CACNA1H also had specific low expression in brain, ovarian, bladder, lung and breast cancer (Table IV).
CACNA1I exhibited low expression in invasive breast carcinoma and breast carcinoma stroma, with a gene ranking in the top 5 and 10%, P-values of 1.75×10−4 and 7.55×10−16, and fold changes between −4.405 and −2.184, respectively (Table IV). Notably, in our previous study, CACNA1I was overexpressed in invasive breast cancer, myxoid/round cell liposarcoma and esophageal adenocarcinoma relative to normal tissue samples (75). Compared with normal tissue, CACNA1I exhibited low expression in brain tumors, including brain glioblastoma, glioblastoma and anaplastic oligodendroglioma. Therefore, CACNA1I was proposed as a potential therapeutic target for these specific cancer subtypes.
R-type calcium channel family
Cav2.3 (CACNA1E) is the only subtype of R-type calcium channels and is localized in cerebellar granule and brain cells. Compared with normal tissue, CACNA1E showed fold changes between −3.061 and −2.027, P-values between 2.62×10−5 and 5–3.18×10−6, and a gene ranking in the top 1–3% of downregulated genes in gastric and brain cancer, respectively (Table V). CACNA1E was overexpressed in esophageal and uterine cancer relative to normal tissue samples (72,75); however, CACNA1E also had low expression in prostate, leukemia, and lung and breast cancers. Therefore, CACNA1E was proposed as a potential therapeutic target for these specific cancer subtypes.
Table V.
R-type calcium channel expression in cancers.
| Gene and cancer type | Patient number, n | P-value (cancer/normal) | t-test (cancer/normal) | Fold (cancer/normal) | Gene ranking, % | Database reference | (Refs.) |
|---|---|---|---|---|---|---|---|
| CACNA1E | |||||||
| Brain | |||||||
| Glioblastoma | 180 | 5.71×10–14 | −9.108 | −7.000 | 1,580 (top 9) | Cancer Cell 2006/04/01 | (70) |
| Anaplastic astrocytoma | 180 | 2.25×10–6 | −5.372 | −4.289 | 1,610 (top 9) | Cancer Cell 2006/04/01 | (70) |
| Glioblastoma | 84 | 3.18×10–6 | −9.375 | −2.027 | 566 (top 3) | J Clin Oncol 2008/06/20 | (71) |
| Gastric | |||||||
| Gastric mixed adenocarcinoma | 69 | 0.001 | −5.207 | −2.241 | 661 (top 4) | Eur J Cancer 2009/02/01 | (113) |
| Gastric cancer | 27 | 2.62×10–5 | −5.134 | −3.061 | 26 (top 1) | Med Oncol 2010/12/04 | (120) |
| Prostate | |||||||
| Prostate carcinoma epithelia | 101 | 7.00×10–6 | −4.835 | −2.496 | 91 (top 1) | Nat Genet 2007/01/01 | (101) |
| Prostatic intraepithelial neoplasia epithelia | 101 | 3.10×10–4 | −3.873 | −2.331 | 368 (top 4) | Nat Genet 2007/01/01 | (101) |
| Leukemia | |||||||
| Chronic lymphocytic leukemia | 111 | 4.10×10–5 | −5.849 | −2.097 | 402 (top 5) | J Clin Oncol 2004/10/01 | (121) |
| Lung | |||||||
| Small cell lung carcinoma | 203 | 6.11×10–5 | −4.747 | −4.842 | 554 (top 7) | Proc Natl Acad Sci USA 2001/11/20 | (117) |
VGCCs in clinical application
The present data established that the expression of calcium channel family genes in normal brain tissue is substantial in comparison to their expression in brain tumor tissue. Previous studies showed that calcium channel antagonists involved in the signaling pathway of meningioma may affect growth factor-mediated meningioma proliferation (75,76). Another study used human astrocytoma U-373 MG and human neuroblastoma SK-N-MC cell lines as models to investigate the effects of calcium channel antagonists, including verapamil, nifedipine and diltiazem, on cancer cell lines (77). Cell growth inhibition occurred with interference from agonist-induced intracellular Ca2+ mobilization (78). T-type (Cav3.1) calcium channel α1 subunit expression was changed in human glioma (76,79).
The effects of CACNA1A have been interpreted to represent a type of neurological disorder (80). Variations in the CACNA1A gene have been well documented to result in certain neurological diseases, including familial hemiplegic migraine type 1, sporadic hemiplegic type 1 and spinocerebellar ataxia type 6 (81–84). However, CACNA1A was revealed to be frequently expressed in adenocarcinomas and involved in cell proliferation and differentiation (85,86). CACNA1A under-expression in atypical teratoid/rhabdoid tumors (AT/RT), malignant glioma and primitive neuroectodermal tumors had P-values of 9.05×10−5, 2.07×10−4 and 4.64×10−4, and gene rankings in the top 1, 2 and 1%, respectively. These expression levels were listed in the top 1 percentile. AT/RT is a rare malignancy frequently identified in childhood. AT/RT may develop in the CNS, including the spinal cord (87).
CACNA1G was also identified as a novel tumor suppressor in lung cancer (86). CACNA1G has a significantly different expression in squamous cell carcinoma and adenocarcinomas (86). Furthermore, it plays a role in cell proliferation and differentiation (88). CACNA1G was inactivated in colorectal cancers, colorectal adenomas, gastric cancers and acute myelogenous leukemia (42). CACNA1C and CACNA1D are also involved in cancers and other types of disease. CACNA1D was under-expressed in VCaP-siERG cells (89). It was also proposed to be a biomarker in patients with prostate cancer (90). In addition, CACNA1C was under-expressed in hypoplastic left heart syndrome (91,92).
Cancer cell lines are widely accepted as in vitro models used to study tumorigenic molecular processes. They have been used to perform useful molecular and functional analyses to understand the role of signaling pathways in tumor initiation and progression and also to identify potential therapeutic targets (93). For this purpose, international cancer projects, including the CCLE, have currently created a wide collection of human cancer cell lines from different tumor types. To identify the expression of CACNA1S in various cell lines, genomic data from 967 cancer cell lines in the CCLE collection was analyzed. These data showed consistency with Fig. 2. CACNA1S was downregulated in kidney cell lines but exhibited high expression in brain and leukemia cell lines (Fig. 3). CACNA1C was downregulated in prostate and pancreatic cell lines but exhibited high expression in breast, skin and lymphoma cell lines. CACNA1D was downregulated in brain, CNS and renal cell lines but had high expression in breast, gastric and lung cell lines. CACNA1A was downregulated in brain cell lines but exhibited high expression in leukemia, ovarian and uterine cell lines. CACNA1B was downregulated in brain and breast cell lines, but exhibited high expression in lung cell lines. CACNA1G was downregulated in bladder and renal cell lines, but exhibited high expression in breast cell lines. CACNA1H was downregulated in bladder and brain cell lines, but had high expression in gastric and renal cell lines. CACNA1I was downregulated in brain cell lines, but exhibited high expression in breast cell lines. The CCLE data were used to confirm the differences in VGCC gene expression in cancer tissue (Fig. 3). These data may provide unprecedented potential for discovering novel biomarkers of cancer in the VGCC gene family.
Figure 3.
Plots showing voltage-gated calcium channel family gene mRNA expression z-scores vs. copy number value from 967 cell lines that were obtained through the Cancer Cell Line Encyclopedia. Each dot represents one cell line.
Tumor suppressor genes show low or under-expression in tumor tissue compared with normal tissue. Tumor suppressor gene changes result in the onset and progression of cancer. Determining the expression of these genes may therefore clarify the underlying mechanisms of oncogenesis. However, the tumor suppressor genes that are essential in numerous types of cancer remain poorly understood (45). In the present analysis, Oncomine was used to perform a meta-analysis of a clinical human microarray database. VGCCs were confirmed to play roles as tumor suppressor genes in specific cancers, including cancer tissue and cancer cell lines. Overall, VGCC family genes were under-expressed in numerous types of cancers, including brain, breast, kidney and lung cancers. Notably, the majority of VGCC family members (CACNA1C, CACNA1D, CACNA1A, CACNA1B, CACNA1E, CACNA1H and CACNA1I) were downregulated in brain tumors, with an mRNA expression level in the top 1–9% of the gene ranking. A total of 5 of the VGCC family members (CACNA1A, CACNA1B, CACNA1E, CACNA1G and CACNA1I) showed under-expression in breast cancer, with gene rankings in the top 1–10% of the expressed genes relative to normal tissues. In kidney cancer and lung cancer, CACNA1S, CACNA1C, CACNA1D, CACNA1A and CACNA1H exhibited low expression, with gene rankings in the top 1–8% of expressed genes. Collectively, the involvement of calcium channels in cancer development and progression remains largely obscure, although numerous studies have investigated this topic. The present findings may help clarify the role of VGCCs as tumor suppressors in cancer development and may contribute to the development of new cancer treatment approaches for specific types and subtypes of cancer.
Acknowledgements
Computational analysis and data mining was performed using the system provided by the Bioinformatics Core at the National Cheng Kung University, supported by the National Science Council, Taiwan. The authors thank the National Science Council of the Executive Yuan (grant no. 101-2320-B-034-001) and the Ministry of Science and Technology (grant nos. MOST103-2325-B006-012 and 104-2917-I-006-002). The authors also give special thanks to American Journal Experts and SCRIBENDI for the English editing services.
References
- 1.Sarkar P, Kumar S. Calcium sensing receptor modulation for cancer therapy. Asian Pac J Cancer Prev. 2012;13:3561–3568. doi: 10.7314/APJCP.2012.13.8.3561. [DOI] [PubMed] [Google Scholar]
- 2.Lang F, Föller M, Lang KS, Lang PA, Ritter M, Gulbins E, Vereninov A, Huber SM. Ion channels in cell proliferation and apoptotic cell death. J Membr Biol. 2005;205:147–157. doi: 10.1007/s00232-005-0780-5. [DOI] [PubMed] [Google Scholar]
- 3.Cuddapah VA, Sontheimer H. Ion channels and tranporters [corrected] in cancer. 2. Ion channels and the control of cancer cell migration. Am J Physiol Cell Physiol. 2011;301:C541–C549. doi: 10.1152/ajpcell.00102.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Long J, Zhang ZB, Liu Z, Xu YH, Ge CL. Loss of heterozygosity at the calcium regulation gene locus on chromosome 10q in human pancreatic cancer. Asian Pac J Cancer Prev. 2015;16:2489–2493. doi: 10.7314/APJCP.2015.16.6.2489. [DOI] [PubMed] [Google Scholar]
- 5.Azimi I, Roberts-Thomson SJ, Monteith GR. Calcium influx pathways in breast cancer: Opportunities for pharmacological intervention. Br J Pharmacol. 2014;171:945–960. doi: 10.1111/bph.12486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Loughlin KR. Calcium channel blockers and prostate cancer. Urol Oncol. 2014;32:537–538. doi: 10.1016/j.urolonc.2013.08.001. [DOI] [PubMed] [Google Scholar]
- 7.Chen Q, Zhang Q, Zhong F, Guo S, Jin Z, Shi W, Chen C, He J. Association between calcium channel blockers and breast cancer: A meta-analysis of observational studies. Pharmacoepidemiol Drug Saf. 2014;23:711–718. doi: 10.1002/pds.3645. [DOI] [PubMed] [Google Scholar]
- 8.George GP, Ramesh V, Mittal RD. Impact of total and ionized serum calcium on prostate cancer risk in North Indian men. Asian Pac J Cancer Prev. 2011;12:1257–1260. [PubMed] [Google Scholar]
- 9.Mahmoudi T, Karimi K, Arkani M, Farahani H, Nobakht H, Dabiri R, Asadi A, Zali MR. Parathyroid hormone gene rs6256 and calcium sensing receptor gene rs1801725 variants are not associated with susceptibility to colorectal cancer in Iran. Asian Pac J Cancer Prev. 2014;15:6035–6039. doi: 10.7314/APJCP.2014.15.15.6035. [DOI] [PubMed] [Google Scholar]
- 10.Stegmaier K, Ross KN, Colavito SA, O'Malley S, Stockwell BR, Golub TR. Gene expression-based high-throughput screening (GE-HTS) and application to leukemia differentiation. Nat Genet. 2004;36:257–263. doi: 10.1038/ng1305. [DOI] [PubMed] [Google Scholar]
- 11.Pomeroy SL, Tamayo P, Gaasenbeek M, Sturla LM, Angelo M, McLaughlin ME, Kim JY, Goumnerova LC, Black PM, Lau C, et al. Prediction of central nervous system embryonal tumour outcome based on gene expression. Nature. 2002;415:436–442. doi: 10.1038/415436a. [DOI] [PubMed] [Google Scholar]
- 12.Grasso CS, Wu YM, Robinson DR, Cao X, Dhanasekaran SM, Khan AP, Quist MJ, Jing X, Lonigro RJ, Brenner JC, et al. The mutational landscape of lethal castration-resistant prostate cancer. Nature. 2012;487:239–243. doi: 10.1038/nature11125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Storz MN, van de Rijn M, Kim YH, Mraz-Gernhard S, Hoppe RT, Kohler S. Gene expression profiles of cutaneous B cell lymphoma. J Invest Dermatol. 2003;120:865–870. doi: 10.1046/j.1523-1747.2003.12142.x. [DOI] [PubMed] [Google Scholar]
- 14.Bonome T, Levine DA, Shih J, Randonovich M, Pise-Masison CA, Bogomolniy F, Ozbun L, Brady J, Barrett JC, Boyd J, Birrer MJ. A gene signature predicting for survival in suboptimally debulked patients with ovarian cancer. Cancer Res. 2008;68:5478–5486. doi: 10.1158/0008-5472.CAN-07-6595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barretina J, Taylor BS, Banerji S, Ramos AH, Lagos-Quintana M, Decarolis PL, Shah K, Socci ND, Weir BA, Ho A, et al. Subtype-specific genomic alterations define new targets for soft-tissue sarcoma therapy. Nat Genet. 2010;42:715–721. doi: 10.1038/ng.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Skotheim RI, Lind GE, Monni O, Nesland JM, Abeler VM, Fosså SD, Duale N, Brunborg G, Kallioniemi O, Andrews PW, Lothe RA. Differentiation of human embryonal carcinomas in vitro and in vivo reveals expression profiles relevant to normal development. Cancer Res. 2005;65:5588–5598. doi: 10.1158/0008-5472.CAN-05-0153. [DOI] [PubMed] [Google Scholar]
- 17.Cutcliffe C, Kersey D, Huang CC, Zeng Y, Walterhouse D, Perlman EJ, Renal Tumor Committee of the Children's Oncology Group Clear cell sarcoma of the kidney: Up-regulation of neural markers with activation of the sonic hedgehog and Akt pathways. Clin Cancer Res. 2005;11:7986–7994. doi: 10.1158/1078-0432.CCR-05-1354. [DOI] [PubMed] [Google Scholar]
- 18.Finak G, Bertos N, Pepin F, Sadekova S, Souleimanova M, Zhao H, Chen H, Omeroglu G, Meterissian S, Omeroglu A, et al. Stromal gene expression predicts clinical outcome in breast cancer. Nat Med. 2008;14:518–527. doi: 10.1038/nm1764. [DOI] [PubMed] [Google Scholar]
- 19.Crabtree JS, Jelinsky SA, Harris HA, Choe SE, Cotreau MM, Kimberland ML, Wilson E, Saraf KA, Liu W, McCampbell AS, et al. Comparison of human and rat uterine leiomyomata: Identification of a dysregulated mammalian target of rapamycin pathway. Cancer Res. 2009;69:6171–6178. doi: 10.1158/0008-5472.CAN-08-4471. [DOI] [PubMed] [Google Scholar]
- 20.Hao Y, Triadafilopoulos G, Sahbaie P, Young HS, Omary MB, Lowe AW. Gene expression profiling reveals stromal genes expressed in common between Barrett's esophagus and adenocarcinoma. Gastroenterology. 2006;131:925–933. doi: 10.1053/j.gastro.2006.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Szatkowski C, Parys JB, Ouadid-Ahidouch H, Matifat F. Research Inositol 1,4,5-trisphosphate-induced Ca2+ signalling is involved in estradiol-induced breast cancer epithelial cell growth. Mol Cancer. 2010;9:156. doi: 10.1186/1476-4598-9-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mariot P, Prevarskaya N, Roudbaraki MM, Le Bourhis X, Van Coppenolle F, Vanoverberghe K, Skryma R. Evidence of functional ryanodine receptor involved in apoptosis of prostate cancer (LNCaP) cells. Prostate. 2000;43:205–214. doi: 10.1002/(SICI)1097-0045(20000515)43:3<205::AID-PROS6>3.3.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 23.Galione A, Evans AM, Ma J, Parrington J, Arredouani A, Cheng X, Zhu MX. The acid test: The discovery of two-pore channels (TPCs) as NAADP-gated endolysosomal Ca(2+) release channels. Pflugers Arch. 2009;458:869–876. doi: 10.1007/s00424-009-0682-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lipkin M, Newmark H. Effect of added dietary calcium on colonic epithelial-cell proliferation in subjects at high risk for familial colonic cancer. N Engl J Med. 1985;313:1381–1384. doi: 10.1056/NEJM198511283132203. [DOI] [PubMed] [Google Scholar]
- 25.Jin T, Zhang Z, Yang XF, Luo JS. S100A4 expression is closely linked to genesis and progression of glioma by regulating proliferation, apoptosis, migration and invasion. Asian Pac J Cancer Prev. 2015;16:2883–2887. doi: 10.7314/APJCP.2015.16.7.2883. [DOI] [PubMed] [Google Scholar]
- 26.Berridge MJ, Bootman MD, Roderick HL. Calcium signalling: Dynamics, homeostasis and remodelling. Nat Rev Mol Cell Biol. 2003;4:517–529. doi: 10.1038/nrm1155. [DOI] [PubMed] [Google Scholar]
- 27.Takeshige N, Yin G, Ohnaka K, Kono S, Ueki T, Tanaka M, Maehara Y, Okamura T, Ikejiri K, Maekawa T, et al. Associations between vitamin D receptor (VDR) gene polymorphisms and colorectal cancer risk and effect modifications of dietary calcium and vitamin D in a Japanese population. Asian Pac J Cancer Prev. 2015;16:2019–2026. doi: 10.7314/APJCP.2015.16.5.2019. [DOI] [PubMed] [Google Scholar]
- 28.Panner A, Wurster RD. T-type calcium channels and tumor proliferation. Cell Calcium. 2006;40:253–259. doi: 10.1016/j.ceca.2006.04.029. [DOI] [PubMed] [Google Scholar]
- 29.Zhong H, De Marzo AM, Laughner E, Lim M, Hilton DA, Zagzag D, Buechler P, Isaacs WB, Semenza GL, Simons JW. Overexpression of hypoxia-inducible factor 1alpha in common human cancers and their metastases. Cancer Res. 1999;59:5830–5835. [PubMed] [Google Scholar]
- 30.Linja MJ, Savinainen KJ, Saramäki OR, Tammela TL, Vessella RL, Visakorpi T. Amplification and overexpression of androgen receptor gene in hormone-refractory prostate cancer. Cancer Res. 2001;61:3550–3555. [PubMed] [Google Scholar]
- 31.Gillett C, Fantl V, Smith R, Fisher C, Bartek J, Dickson C, Barnes D, Peters G. Amplification and overexpression of cyclin D1 in breast cancer detected by immunohistochemical staining. Cancer Res. 1994;54:1812–1817. [PubMed] [Google Scholar]
- 32.Cole S, Bhardwaj G, Gerlach J, Mackie JE, Grant CE, Almquist KC, Stewart AJ, Kurz EU, Duncan AM, Deeley RG. Overexpression of a transporter gene in a multidrug-resistant human lung cancer cell line. Science. 1992;258:1650–1654. doi: 10.1126/science.1360704. [DOI] [PubMed] [Google Scholar]
- 33.Liu TH, Guo K, Liu RQ, Zhang S, Huang ZH, Liu YK. The high expressed serum soluble neural cell adhesion molecule, a high risk factor indicating hepatic encephalopathy in hepatocelular carcinoma patients. Asian Pac J Cancer Prev. 2015;16:3131–3135. doi: 10.7314/APJCP.2015.16.8.3131. [DOI] [PubMed] [Google Scholar]
- 34.Mao YY, Jing FY, Jin MJ, Li YJ, Ding Y, Guo J, Wang FJ, Jiang LF, Chen K. rs12904 polymorphism in the 3′UTR of EFNA1 is associated with colorectal cancer susceptibility in a Chinese population. Asian Pac J Cancer Prev. 2013;14:5037–5041. doi: 10.7314/APJCP.2013.14.9.5037. [DOI] [PubMed] [Google Scholar]
- 35.Martino-Echarri E, Fernández-Rodríguez R, Rodríguez-Baena FJ, Barrientos-Durán A, Torres-Collado AX, Plaza-Calonge Mdel C, Amador-Cubero S, Cortés J, Reynolds LE, Hodivala-Dilke KM, Rodríguez-Manzaneque JC. Contribution of ADAMTS1 as a tumor suppressor gene in human breast carcinoma. Linking its tumor inhibitory properties to its proteolytic activity on nidogen-1 and nidogen-2. Int J Cancer Cancer Cancer. 2013;133:2315–2324. doi: 10.1002/ijc.28271. [DOI] [PubMed] [Google Scholar]
- 36.Li YP, Tian FG, Shi PC, Guo LY, Wu HM, Chen RQ, Xue JM. 4-Hydroxynonenal promotes growth and angiogenesis of breast cancer cells through HIF-1α stabilization. Asian Pac J Cancer Prev. 2014;15:10151–10156. doi: 10.7314/APJCP.2014.15.23.10151. [DOI] [PubMed] [Google Scholar]
- 37.Kim HS, Patel K, Muldoon-Jacobs K, Bisht KS, Aykin-Burns N, Pennington JD, van der Meer R, Nguyen P, Savage J, Owens KM, et al. SIRT3 is a mitochondria-localized tumor suppressor required for maintenance of mitochondrial integrity and metabolism during stress. Cancer Cell. 2010;17:41–52. doi: 10.1016/j.ccr.2009.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dziegielewska B, Gray LS, Dziegielewski J. T-type calcium channels blockers as new tools in cancer therapies. Pflugers Arch. 2014;466:801–810. doi: 10.1007/s00424-014-1444-z. [DOI] [PubMed] [Google Scholar]
- 39.Lee JY, Park SJ, Park SJ, Lee MJ, Rhim H, Seo SH, Kim KS. Growth inhibition of human cancer cells in vitro by T-type calcium channel blockers. Bioorg Med Chem Lett. 2006;16:5014–5017. doi: 10.1016/j.bmcl.2006.07.046. [DOI] [PubMed] [Google Scholar]
- 40.Bertolesi GE, Shi CJ, Elbaum L, Jollimore C, Rozenberg G, Barnes S, Kelly ME. The Ca(2+) channel antagonists mibefradil and pimozide inhibit cell growth via different cytotoxic mechanisms. Mol Pharmacol. 2002;62:210–219. doi: 10.1124/mol.62.2.210. [DOI] [PubMed] [Google Scholar]
- 41.Toyota M, Ho C, Ohe-Toyota M, Baylin SB, Issa JP. Inactivation of CACNA1G, a T-type calcium channel gene, by aberrant methylation of its 5′ CpG island in human tumors. Cancer Res. 1999;59:4535–4541. [PubMed] [Google Scholar]
- 42.Díaz-Lezama N, Hernández-Elvira M, Sandoval A, Monroy A, Felix R, Monjaraz E. Ghrelin inhibits proliferation and increases T-type Ca2+ channel expression in PC-3 human prostate carcinoma cells. Biochem Biophys Res Commun. 2010;403:24–29. doi: 10.1016/j.bbrc.2010.10.100. [DOI] [PubMed] [Google Scholar]
- 43.Wang CY, Lai MD, Phan NN, Sun ZD, Lin YC. Meta-analysis of public microarray datasets reveals voltage-gated calcium gene signatures in clinical cancer patients. PLoS One. 2015;10:e0125766. doi: 10.1371/journal.pone.0125766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rhodes DR, Chinnaiyan AM. Integrative analysis of the cancer transcriptome. Nat Genet. 2005;37(Suppl):S31–S37. doi: 10.1038/ng1570. [DOI] [PubMed] [Google Scholar]
- 45.Zhou C, Ren Y, Wang K, Liu J, He JJ, Liu PJ. Intra-operative rectal washout with saline solution can effectively prevent anastomotic recurrence: A meta-analysis. Asian Pac J Cancer Prev. 2013;14:7155–7159. doi: 10.7314/APJCP.2013.14.12.7155. [DOI] [PubMed] [Google Scholar]
- 46.Alqumber MA, Dar SA, Haque S, Wahid M, Singh R, Akhter N. No association of the TGF-β1 29T/C polymorphism with breast cancer risk in caucasian and Asian populations: Evidence from a meta-analysis involving 55, 841 subjects. Asian Pac J Cancer Prev. 2014;15:8725–8734. doi: 10.7314/APJCP.2014.15.20.8725. [DOI] [PubMed] [Google Scholar]
- 47.Ni M, Chen Y, Lim E, Wimberly H, Bailey ST, Imai Y, Rimm DL, Liu XS, Brown M. Targeting androgen receptor in estrogen receptor-negative breast cancer. Cancer Cell. 2011;20:119–131. doi: 10.1016/j.ccr.2011.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.D'Ambrogio A, Nagaoka K, Richter JD. Translational control of cell growth and malignancy by the CPEBs. Nat Rev Cancer. 2013;13:283–290. doi: 10.1038/nrc3485. [DOI] [PubMed] [Google Scholar]
- 49.Barretina J, Caponigro G, Stransky N, Venkatesan K, Margolin AA, Kim S, Wilson CJ, Lehár J, Kryukov GV, Sonkin D, et al. The cancer cell line encyclopedia enables predictive modelling of anticancer drug sensitivity. Nature. 2012;483:603–607. doi: 10.1038/nature11003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ertel EA, Campbell KP, Harpold MM, Hofmann F, Mori Y, Perez-Reyes E, Schwartz A, Snutch TP, Tanabe T, Birnbaumer L, et al. Nomenclature of voltage-gated calcium channels. Neuron. 2000;25:533–535. doi: 10.1016/S0896-6273(00)81057-0. [DOI] [PubMed] [Google Scholar]
- 51.Cain SM, Snutch TP. Voltage-gated calcium channels and disease. Biofactors. 2011;37:197–205. doi: 10.1002/biof.158. [DOI] [PubMed] [Google Scholar]
- 52.Bidaud I, Mezghrani A, Swayne LA, Monteil A, Lory P. Voltage-gated calcium channels in genetic diseases. Biochim Biophys Acta. 2006;1763:1169–1174. doi: 10.1016/j.bbamcr.2006.08.049. [DOI] [PubMed] [Google Scholar]
- 53.Kavianpour M, Ahmadzadeh A, Shahrabi S, Saki N. Significance of oncogenes and tumor suppressor genes in AML prognosis. Tumour Biol. 2016;37:10041–10052. doi: 10.1007/s13277-016-5067-1. [DOI] [PubMed] [Google Scholar]
- 54.Cooper GM, Hausman RE. The cell. Sinauer Associates Sunderland; 2000. [Google Scholar]
- 55.Sanchez-Carbayo M, Socci ND, Lozano J, Saint F, Cordon-Cardo C. Defining molecular profiles of poor outcome in patients with invasive bladder cancer using oligonucleotide microarrays. J Clin Oncol. 2006;24:778–789. doi: 10.1200/JCO.2005.03.2375. [DOI] [PubMed] [Google Scholar]
- 56.Marta SC, Nicholas S, Juanjo L, Fabien S, Carlos CC. Defining molecular profiles of poor outcome in patients with invasive bladder cancer using oligonucleotide microarrays. Tumor Biol. 2006;27:47–47. doi: 10.1200/JCO.2005.03.2375. [DOI] [PubMed] [Google Scholar]
- 57.Schaldemose EL, Horjales-Araujo E, Demontis D, Børglum AD, Svensson P, Finnerup NB. No association of polymorphisms in the serotonin transporter gene with thermal pain sensation in healthy individuals. Mol Pain. 2014;10:76. doi: 10.1186/1744-8069-10-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stunnenberg BC, Deinum J, Links TP, Wilde AA, Franssen H, Drost G. Cardiac arrhythmias in hypokalemic periodic paralysis: Hypokalemia as only cause? Muscle Nerve. 2014;50:327–332. doi: 10.1002/mus.24225. [DOI] [PubMed] [Google Scholar]
- 59.Beitelshees AL, Navare H, Wang D, Gong Y, Wessel J, Moss JI, Langaee TY, Cooper-DeHoff RM, Sadee W, Pepine CJ, et al. CACNA1C gene polymorphisms, cardiovascular disease outcomes, and treatment response. Circ Cardiovasc Genet. 2009;2:362–370. doi: 10.1161/CIRCGENETICS.109.857839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Baig SM, Koschak A, Lieb A, Gebhart M, Dafinger C, Nürnberg G, Ali A, Ahmad I, Sinnegger-Brauns MJ, Brandt N, et al. Loss of Cav1. 3 (CACNA1D) function in a human channelopathy with bradycardia and congenital deafness. Nat Neurosci. 2011;14:77–84. doi: 10.1038/nn.2694. [DOI] [PubMed] [Google Scholar]
- 61.Johnson AD, Newton-Cheh C, Chasman DI, Ehret GB, Johnson T, Rose L, Rice K, Verwoert GC, Launer LJ, Gudnason V, et al. Association of hypertension drug target genes with blood pressure and hypertension in 86 588 individuals. Hypertension. 2011;57:903–910. doi: 10.1161/HYPERTENSIONAHA.110.158667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Madden E. Differential gene remodeling in overweight and obese patients with heart failure. 2013 [Google Scholar]
- 63.Kang H, Yang X, Chen R, Zhang B, Corona E, Schadt EE, Butte AJ. Integration of disease-specific single nucleotide polymorphisms, expression quantitative trait loci and coexpression networks reveal novel candidate genes for type 2 diabetes. Diabetologia. 2012;55:2205–2213. doi: 10.1007/s00125-012-2568-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Reinbothe TM, Alkayyali S, Ahlqvist E, Tuomi T, Isomaa B, Lyssenko V, Renström E. The human L-type calcium channel Cav1. 3 regulates insulin release and polymorphisms in CACNA1D associate with type 2 diabetes. Diabetologia. 2013;56:340–349. doi: 10.1007/s00125-012-2758-z. [DOI] [PubMed] [Google Scholar]
- 65.Reinbothe T, Lyssenko V, Lang S, Renström E. Polymorphisms in CACNA1D affect insulin release and channel expression and associate with type 2 diabetes; 46th Annual Meeting of the European-Association-for-the-Study-of-Diabetes (EASD); 2010.Springer; p. 296. [Google Scholar]
- 66.Qiu ZX, Zhao S, Li L, Li WM. Loss of expression of PTEN is associated with worse prognosis in patients with cancer. Asian Pac J Cancer Prev. 2015;16:4691–4698. doi: 10.7314/APJCP.2015.16.11.4691. [DOI] [PubMed] [Google Scholar]
- 67.Kamp TJ, Hell JW. Regulation of cardiac L-type calcium channels by protein kinase A and protein kinase C. Cir Res. 2000;87:1095–1102. doi: 10.1161/01.RES.87.12.1095. [DOI] [PubMed] [Google Scholar]
- 68.Moosmang S, Schulla V, Welling A, Feil R, Feil S, Wegener JW, Hofmann F, Klugbauer N. Dominant role of smooth muscle L-type calcium channel Cav1. 2 for blood pressure regulation. EMBO J. 2003;22:6027–6034. doi: 10.1093/emboj/cdg583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bhat S, Dao DT, Terrillion CE, Arad M, Smith RJ, Soldatov NM, Gould TD. CACNA1C (Ca v 1.2) in the pathophysiology of psychiatric disease. Prog Neurobiol. 2012;99:1–14. doi: 10.1016/j.pneurobio.2012.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sun L, Hui AM, Su Q, Vortmeyer A, Kotliarov Y, Pastorino S, Passaniti A, Menon J, Walling J, Bailey R, et al. Neuronal and glioma-derived stem cell factor induces angiogenesis within the brain. Cancer Cell. 2006;9:287–300. doi: 10.1016/j.ccr.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 71.Murat A, Migliavacca E, Gorlia T, Lambiv WL, Shay T, Hamou MF, de Tribolet N, Regli L, Wick W, Kouwenhoven MC, et al. Stem cell-related ‘self-renewal’ signature and high epidermal growth factor receptor expression associated with resistance to concomitant chemoradiotherapy in glioblastoma. J Clin Oncol. 2008;26:3015–3024. doi: 10.1200/JCO.2007.15.7164. [DOI] [PubMed] [Google Scholar]
- 72.Uchitel OD, Protti DA, Sanchez V, Cherksey BD, Sugimori M, Llinás R. P-type voltage-dependent calcium channel mediates presynaptic calcium influx and transmitter release in mammalian synapses; Proc Natl Acad Sci USA; 1992; pp. 3330–3333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Catterall WA. Structure and function of neuronal Ca2+ channels and their role in neurotransmitter release. Cell Calcium. 1998;24:307–323. doi: 10.1016/S0143-4160(98)90055-0. [DOI] [PubMed] [Google Scholar]
- 74.Piccaluga PP, Agostinelli C, Califano A, Rossi M, Basso K, Zupo S, Went P, Klein U, Zinzani PL, Baccarani M, et al. Gene expression analysis of peripheral T cell lymphoma, unspecified, reveals distinct profiles and new potential therapeutic targets. J Clin Invest. 2007;117:823–834. doi: 10.1172/JCI26833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gazulla J, Tintore M. P/Q-type voltage-dependent calcium channels in neurological disease. Neurologia. 2007;22:511–516. (In Spanish) [PubMed] [Google Scholar]
- 76.Jensen RL, Petr M, Wurster RD. Calcium channel antagonist effect on in vitro meningioma signal transduction pathways after growth factor stimulation. Neurosurgery. 2000;46:692–703. doi: 10.1097/00006123-200003000-00032. [DOI] [PubMed] [Google Scholar]
- 77.Lee YS, Sayeed MM, Wurster RD. Inhibition of cell growth and intracellular Ca2+ mobilization in human brain tumor cells by Ca2+ channel antagonists. Mol Chem Neuropathol. 1994;22:81–95. doi: 10.1007/BF03160097. [DOI] [PubMed] [Google Scholar]
- 78.Jensen RL, Origitano TC, Lee YS, Weber M, Wurster RD. In vitro growth inhibition of growth factor-stimulated meningioma cells by calcium channel antagonists. Neurosurgery. 1995;36:365–374. doi: 10.1227/00006123-199502000-00017. [DOI] [PubMed] [Google Scholar]
- 79.Latour I, Louw DF, Beedle AM, Hamid J, Sutherland GR, Zamponi GW. Expression of T-type calcium channel splice variants in human glioma. Glia. 2004;48:112–119. doi: 10.1002/glia.20063. [DOI] [PubMed] [Google Scholar]
- 80.Schorge S, Rajakulendran S. The P/Q channel in human disease: untangling the genetics and physiology. WIREs Membr Transp Signal. 2012;1:311–320. doi: 10.1002/wmts.22. [DOI] [Google Scholar]
- 81.Ophoff RA, Terwindt GM, Vergouwe MN, van Eijk R, Oefner PJ, Hoffman SM, Lamerdin JE, Mohrenweiser HW, Bulman DE, Ferrari M, et al. Familial hemiplegic migraine and episodic ataxia type-2 are caused by mutations in the Ca2+ channel gene CACNL1A4. Cell. 1996;87:543–552. doi: 10.1016/S0092-8674(00)81373-2. [DOI] [PubMed] [Google Scholar]
- 82.Zhuchenko O, Bailey J, Bonnen P, Ashizawa T, Stockton DW, Amos C, Dobyns WB, Subramony SH, Zoghbi HY, Lee CC, et al. Autosomal dominant cerebellar ataxia (SCA6) associated with small polyglutamine expansions in the alpha1A-voltage-dependent calcium channel. Nat Genet. 1997;15:62–69. doi: 10.1038/ng0197-62. [DOI] [PubMed] [Google Scholar]
- 83.Bürk K, Kaiser FJ, Tennstedt S, Schöls L, Kreuz FR, Wieland T, Strom TM, Büttner T, Hollstein R, Braunholz D, et al. A novel missense mutation in CACNA1A evaluated by in silico protein modeling is associated with non-episodic spinocerebellar ataxia with slow progression. Eur J Med Genet. 2014;57:207–211. doi: 10.1016/j.ejmg.2014.01.005. [DOI] [PubMed] [Google Scholar]
- 84.Sintas C, Carreño O, Corominas R, Serra SA, Vila M, Fernández-Castillo N, Toma C, Pons R, Llaneza M, Sobrido MJ, et al. Screening of cacna1a and ATP1A2 genes in hemiplegic migraine: Clinical, genetic and functional studies. J Headache Pain. 2013;14(Suppl 1):P26. doi: 10.1186/1129-2377-14-S1-P26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Verschuur-Maes AH, de Bruin PC, van Diest PJ. Epigenetic progression of columnar cell lesions of the breast to invasive breast cancer. Breast Cancer Res Treat. 2012;136:705–715. doi: 10.1007/s10549-012-2301-4. [DOI] [PubMed] [Google Scholar]
- 86.Castro M, Grau L, Puerta P, Gimenez L, Venditti J, Quadrelli S, Sánchez-Carbayo M. Multiplexed methylation profiles of tumor suppressor genes and clinical outcome in lung cancer. J Transl Med. 2010;8:86. doi: 10.1186/1479-5876-8-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Burger PC, Yu I, Tihan T, et al. Atypical teratoid/rhabdoid tumor of the central nervous system: A highly malignant tumor of infancy and childhood frequently mistaken for medulloblastoma: A pediatric oncology group study. Am J Surg Pathol. 1998;22:1083–1092. doi: 10.1097/00000478-199809000-00007. [DOI] [PubMed] [Google Scholar]
- 88.Roderick HL, Cook SJ. Ca2+ signalling checkpoints in cancer: Remodelling Ca2+ for cancer cell proliferation and survival. Nat Rev Cancer. 2008;8:361–375. doi: 10.1038/nrc2374. [DOI] [PubMed] [Google Scholar]
- 89.Tomlins SA, Laxman B, Varambally S, Cao X, Yu J, Helgeson BE, Cao Q, Prensner JR, Rubin MA, Shah RB, et al. Role of the TMPRSS2-ERG gene fusion in prostate cancer. Neoplasia. 2008;10:177–188. doi: 10.1593/neo.07822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chen R, Zeng X, Zhang R, Huang J, Kuang X, Yang J, Liu J, Tawfik O, Thrasher JB, Li B. Cav1.3 channel α1D protein is overexpressed and modulates androgen receptor transactivation in prostate cancers. Urol Oncol. 2014;32:524–536. doi: 10.1016/j.urolonc.2013.05.011. [DOI] [PubMed] [Google Scholar]
- 91.Liu Z, Christudass C, Zhang H, et al. A novel quantitative histomorphological tool to assess multiple biomarkers to predict prostate cancer aggression. Cancer Res. 2014;74(19 Suppl):S4013. doi: 10.1158/1538-7445.AM2014-4013. [DOI] [Google Scholar]
- 92.Ricci M, Xu Y, Hammond HL, Willoughby DA, Nathanson L, Rodriguez MM, Vatta M, Lipshultz SE, Lincoln J. Myocardial alternative RNA splicing and gene expression profiling in early stage hypoplastic left heart syndrome. PLoS One. 2012;7:e29784. doi: 10.1371/journal.pone.0029784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Dutton Regester K, Hayward NK. Reviewing the somatic genetics of melanoma: From current to future analytical approaches. Pigment Cell Melanoma Res. 2012;25:144–154. doi: 10.1111/j.1755-148X.2012.00975.x. [DOI] [PubMed] [Google Scholar]
- 94.Jones J, Otu H, Spentzos D, Kolia S, Inan M, Beecken WD, Fellbaum C, Gu X, Joseph M, Pantuck AJ, et al. Gene signatures of progression and metastasis in renal cell cancer. Clin Cancer Res. 2005;11:5730–5739. doi: 10.1158/1078-0432.CCR-04-2225. [DOI] [PubMed] [Google Scholar]
- 95.Ginos MA, Page GP, Michalowicz BS, Patel KJ, Volker SE, Pambuccian SE, Ondrey FG, Adams GL, Gaffney PM. Identification of a gene expression signature associated with recurrent disease in squamous cell carcinoma of the head and neck. Cancer Res. 2004;64:55–63. doi: 10.1158/0008-5472.CAN-03-2144. [DOI] [PubMed] [Google Scholar]
- 96.Estilo CL, O-charoenrat P, Talbot S, Socci ND, Carlson DL, Ghossein R, Williams T, Yonekawa Y, Ramanathan Y, Boyle JO, et al. Oral tongue cancer gene expression profiling: Identification of novel potential prognosticators by oligonucleotide microarray analysis. BMC Cancer. 2009;9:11. doi: 10.1186/1471-2407-9-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Talbot SG, Estilo C, Maghami E, Sarkaria IS, Pham DK, O-charoenrat P, Socci ND, Ngai I, Carlson D, Ghossein R, et al. Gene expression profiling allows distinction between primary and metastatic squamous cell carcinomas in the lung. Cancer Res. 2005;65:3063–3071. doi: 10.1158/0008-5472.CAN-04-1985. [DOI] [PubMed] [Google Scholar]
- 98.Basso K, Margolin AA, Stolovitzky G, Klein U, Dalla-Favera R, Califano A. Reverse engineering of regulatory networks in human B cells. Nat Genet. 2005;37:382–390. doi: 10.1038/ng1532. [DOI] [PubMed] [Google Scholar]
- 99.Yoshihara K, Tajima A, Komata D, et al. Gene expression profiling of advanced-stage serous ovarian cancers distinguishes novel subclasses and implicates ZEB2 in tumor progression and prognosis. Cancer Sci. 2009;100:1421–1428. doi: 10.1111/j.1349-7006.2009.01204.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sanchez-Carbayo M, Socci ND, Lozano J, Saint F, Cordon-Cardo C. Defining molecular profiles of poor outcome in patients with invasive bladder cancer using oligonucleotide microarrays. J Clin Oncol. 2006;24:778–789. doi: 10.1200/JCO.2005.03.2375. [DOI] [PubMed] [Google Scholar]
- 101.Tomlins SA, Mehra R, Rhodes DR, et al. Integrative molecular concept modeling of prostate cancer progression. Nat Genet. 2007;39:41–51. doi: 10.1038/ng1935. [DOI] [PubMed] [Google Scholar]
- 102.Yusenko MV, Kuiper RP, Boethe T, Ljungberg B, van Kessel AG, Kovacs G. High-resolution DNA copy number and gene expression analyses distinguish chromophobe renal cell carcinomas and renal oncocytomas. BMC Cancer. 2009;9:152. doi: 10.1186/1471-2407-9-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Frierson HF, Jr, El-Naggar AK, Welsh JB, Sapinoso LM, Su AI, Cheng J, Saku T, Moskaluk CA, Hampton GM. Large scale molecular analysis identifies genes with altered expression in salivary adenoid cystic carcinoma. Am J Pathol. 2002;161:1315–1323. doi: 10.1016/S0002-9440(10)64408-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Biewenga P, Buist MR, Moerland PD, Ver Loren van Themaat E, van Kampen AH, ten Kate FJ, Baas F. Gene expression in early stage cervical cancer. Gynecol Oncol. 2008;108:520–526. doi: 10.1016/j.ygyno.2007.11.024. [DOI] [PubMed] [Google Scholar]
- 105.Hou J, Aerts J, den Hamer B, van Ijcken W, den Bakker M, Riegman P, van der Leest C, van der Spek P, Foekens JA, Hoogsteden HC, et al. Gene expression-based classification of non-small cell lung carcinomas and survival prediction. PLoS One. 2010;5:e10312. doi: 10.1371/journal.pone.0010312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Okayama H, Kohno T, Ishii Y, Shimada Y, Shiraishi K, Iwakawa R, Furuta K, Tsuta K, Shibata T, Yamamoto S, et al. Identification of genes upregulated in ALK-positive and EGFR/KRAS/ALK-negative lung adenocarcinomas. Cancer Res. 2012;72:100–111. doi: 10.1158/0008-5472.CAN-11-1403. [DOI] [PubMed] [Google Scholar]
- 107.Wurmbach E, Chen YB, Khitrov G, Zhang W, Roayaie S, Schwartz M, Fiel I, Thung S, Mazzaferro V, Bruix J, et al. Genome-wide molecular profiles of HCV-induced dysplasia and hepatocellular carcinoma. Hepatology. 2007;45:938–947. doi: 10.1002/hep.21622. [DOI] [PubMed] [Google Scholar]
- 108.Detwiller KY, Fernando NT, Segal NH, Ryeom SW, D'Amore PA, Yoon SS. Analysis of hypoxia-related gene expression in sarcomas and effect of hypoxia on RNA interference of vascular endothelial cell growth factor A. Cancer Res. 2005;65:5881–5889. doi: 10.1158/0008-5472.CAN-04-4078. [DOI] [PubMed] [Google Scholar]
- 109.Rickman DS, Bobek MP, Misek DE, Kuick R, Blaivas M, Kurnit DM, Taylor J, Hanash SM. Distinctive molecular profiles of high-grade and low-grade gliomas based on oligonucleotide microarray analysis. Cancer Res. 2001;61:6885–6891. [PubMed] [Google Scholar]
- 110.Shai R, Shi T, Kremen TJ, Horvath S, Liau LM, Cloughesy TF, Mischel PS, Nelson SF. Gene expression profiling identifies molecular subtypes of gliomas. Oncogene. 2003;22:4918–4923. doi: 10.1038/sj.onc.1206753. [DOI] [PubMed] [Google Scholar]
- 111.Hong Y, Downey T, Eu KW, Koh PK, Cheah PY. A ‘metastasis-prone’ signature for early-stage mismatch-repair proficient sporadic colorectal cancer patients and its implications for possible therapeutics. Clin Exp Metastasis. 2010;27:83–90. doi: 10.1007/s10585-010-9305-4. [DOI] [PubMed] [Google Scholar]
- 112.Sabates-Bellver J, van der Flier LG, de Palo M, et al. Transcriptome profile of human colorectal adenomas. Mol Cancer Res. 2007;5:1263–1275. doi: 10.1158/1541-7786.MCR-07-0267. [DOI] [PubMed] [Google Scholar]
- 113.D'Errico M, de Rinaldis E, Blasi MF, Viti V, Falchetti M, Calcagnile A, Sera F, Saieva C, Ottini L, Palli D, et al. Genome-wide expression profile of sporadic gastric cancers with microsatellite instability. Eur J Cancer. 2009;45:461–469. doi: 10.1016/j.ejca.2008.10.032. [DOI] [PubMed] [Google Scholar]
- 114.Turashvili G, Bouchal J, Baumforth K, Wei W, Dziechciarkova M, Ehrmann J, Klein J, Fridman E, Skarda J, Srovnal J, et al. Novel markers for differentiation of lobular and ductal invasive breast carcinomas by laser microdissection and microarray analysis. BMC Cancer. 2007;7:55. doi: 10.1186/1471-2407-7-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lee J, Kotliarova S, Kotliarov Y, Li A, Su Q, Donin NM, Pastorino S, Purow BW, Christopher N, Zhang W, et al. Tumor stem cells derived from glioblastomas cultured in bFGF and EGF more closely mirror the phenotype and genotype of primary tumors than do serum-cultured cell lines. Cancer Cell. 2006;9:391–403. doi: 10.1016/j.ccr.2006.03.030. [DOI] [PubMed] [Google Scholar]
- 116.Lee JS, Leem SH, Lee SY, Kim SC, Park ES, Kim SB, Kim SK, Kim YJ, Kim WJ, Chu IS. Expression signature of E2F1 and its associated genes predict superficial to invasive progression of bladder tumors. J Clin Oncol. 2010;28:2660–2667. doi: 10.1200/JCO.2009.25.0977. [DOI] [PubMed] [Google Scholar]
- 117.Bhattacharjee A, Richards WG, Staunton J, Li C, Monti S, Vasa P, Ladd C, Beheshti J, Bueno R, Gillette M, et al. Classification of human lung carcinomas by mRNA expression profiling reveals distinct adenocarcinoma subclasses; Proc Natl Acad Sci USA; 2001; pp. 13790–13795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Gluck S, Ross JS, Royce M, McKenna EF, Jr, Perou CM, Avisar E, Wu L. TP53 genomics predict higher clinical and pathologic tumor response in operable early-stage breast cancer treated with docetaxel-capecitabine ± trastuzumab. Breast Cancer Res Treat. 2012;132:781–791. doi: 10.1007/s10549-011-1412-7. [DOI] [PubMed] [Google Scholar]
- 119.French PJ, Swagemakers SM, Nagel JH, Kouwenhoven MC, Brouwer E, van der Spek P, Luider TM, Kros JM, van den Bent MJ, Smitt PA Sillevis. Gene expression profiles associated with treatment response in oligodendrogliomas. Cancer Res. 2005;65:11335–11344. doi: 10.1158/0008-5472.CAN-05-1886. [DOI] [PubMed] [Google Scholar]
- 120.Wang Q, Wen YG, Li DP, Xia J, Zhou CZ, Yan DW, Tang HM, Peng ZH. Upregulated INHBA expression is associated with poor survival in gastric cancer. Med Oncol. 2012;29:77–83. doi: 10.1007/s12032-010-9766-y. [DOI] [PubMed] [Google Scholar]
- 121.Haslinger C, Schweifer N, Stilgenbauer S, Döhner H, Lichter P, Kraut N, Stratowa C, Abseher R. Microarray gene expression profiling of B-cell chronic lymphocytic leukemia subgroups defined by genomic aberrations and VH mutation status. J Clin Oncol. 2004;22:3937–3949. doi: 10.1200/JCO.2004.12.133. [DOI] [PubMed] [Google Scholar]