Abstract
Objective
Treatment of type 1 diabetes has been intensified aiming at normalizing blood glucose, which may increase the risk of severe hypoglycemia (SH). We aimed to compare the incidence of SH events in the four Nordic countries Denmark, Iceland, Norway and Sweden, and to assess the influence of hemoglobin A1c (HbA1c) and treatment modalities on the frequency of SH; particularly, to explore if a HbA1c target ≤6.7% (50 mmol/mol) is feasible.
Research design and methods
Data on children below 15 years with a diabetes duration more than 1 year, registered in the national childhood diabetes databases in the four Nordic countries from 2008 to 2012, were compiled. Data completeness was more than 95%.
Results
Totally 8806 (48% females) patients with 29 715 person years were included, mean age and diabetes duration were 11 years and 5.1 years, respectively. The overall rate of SH was 6.0 per 100 patient-years, and did not change during the study period. The Swedish population constantly had the lowest SH incidence while it decreased significantly in the Danish population. HbA1c decreased significantly over time (p<0.01), while the number of pump users increased (p<0.01). Stratifying for HbA1c levels showed the lowest risk of SH in patients with HbA1c ≤6.7% (≤50 mmol/mol), but in the statistical models adjusting for possible confounders the difference between the HbA1c groups disappeared. Pump users had the lowest SH risk, also after adjusting for possible confounders.
Conclusions
Risk of SH differs between the Nordic countries with the lowest risk in Sweden. Pump therapy was associated with decreased risk of SH. The low HbA1c group had the same or a lower risk of SH compared with the highest HbA1c groups. A target HbA1c ≤6.7% (≤50 mmol/mol) seems achievable without increasing the risk of SH.
Keywords: Severe hypoglycemia, Nordic countries, HbA1c, insulin pump, target HbA1c.
Significance of this study.
What is already known about this subject?
The incidence of severe hypoglycemia (SH) in children with type 1 diabetes is decreasing, and during the last two decades there has been a shift from a strong negative association between hemoglobin A1c (HbA1c) and SH to no association.
What are the new findings?
The incidence of SH differs between the Nordic countries despite equal access to healthcare.
Pump therapy is associated with decreased risk of SH.
HbA1c below 50 mmol/mol is not a risk factor for SH.
How might these results change the focus of research or clinical practice?
Focus should be on causes of differences in SH between countries.
The HbA1c target may be reduced to 6.7% (50 mmol/mol) and below.
Introduction
To reduce long-term complications in type 1 diabetes (T1D) blood glucose (BG) should be close to normal.1 Accordingly, the new guidelines from the International Society of Pediatric and Adolescent Diabetes in 2014 and the American Diabetes Association in 2014 recommend a haemoglobin A1c (HbA1c) below 7.5% (58 mmol/mol) in children below 18 years.2 3 The National Institute for Health and Care Excellence recommend a HbA1c level of 6.5% (48 mmol/mol) or lower (https://www.nice.org.uk/guidance/qs125/chapter/Quality-statement-2-Education-and-information), and some centres in Scandinavia have changed their HbA1c target to 6.7% (50 mmol/mol). It is unknown if this target level can be obtained without increasing the risk of severe hypoglycemia (SH) events. Reducing the HbA1c target without increasing the SH risk has previously been of opposing demands, particularly in young children.1 4 5 Many children and their parents fear SH, and tend to increase mean BG, thereby deteriorating metabolic control.6 7 Over the last one or two decades, insulin treatment regimens have been intensified, and treatment modalities have changed from regular insulin to analog insulin or continuous subcutaneous insulin infusion.8–10 In the same period, an increased number of BG measurements and insulin bolus injections have been recommended.3 These changes may all contribute to reduce the frequency of SH. Thus, a report from the German and Austrian ‘Diabetes Patienten Verlaufsdokumentation’ (DPV) database has shown a decrease in the relative risk of SH, particularly in the groups with the lowest HbA1c,11 a study from the Danish Registry of Childhood and Adolescent Diabetes (DanDiabKids) showed a significant decrease in SH in the period 2008–2012 with unchanged HbA1c,9 and a multicenter and multinational study from the Hvidoere Study Group showed significant differences in the frequency of SH between diabetes centers in different countries.12 Finally, a recent multicenter study, including the DPV database and large centers from Australia and the USA, reported the SH incidence independent of HbA1c,13 but investigations in other large longitudinal cohorts were called for. No studies have compared incidence data of SH between complete national childhood databases in countries with equal access to healthcare.
We aimed to (1) compare the incidence of SH events in children with T1D in the four Nordic countries in the period 2008–2012 and (2) examine the influence of HbA1c and treatment modalities on the frequency of SH, and particularly, explore if a HbA1c target ≤6.7% (50 mmol/mol) is possible without increasing the risk of SH.
Research design and methods
Data on children below 15 years of age registered in the national childhood diabetes databases in the four Nordic countries—Denmark, Iceland, Norway and Sweden—from 1 January 2008 to 31 December 2012 were compiled. All 89 Nordic centers—18 in Denmark, 1 in Iceland, 27 in Norway, 43 in Sweden—treating T1D in children registered data in the national databases.14 Only children with a diabetes duration of more than 1 year were included. Data ascertainment concerning incident cases with the diagnosis T1D was 99% in the Danish registry (DanDiabKids), almost 100% in the Icelandic registry, 92%–93% in the Norwegian registry for childhood diabetes (Norwegian Childhood Diabetes Registry) and almost 100% in the Swedish registry for childhood diabetes (SweDiabKids).9 14 Data completeness of the different variables varied between 96% and 99%. HbA1c and SH data completeness was 98% and 99%, respectively.
Data on gender, age, diabetes duration, HbA1c, insulin dose per kilogram per day, treatment modality, weight, height and SH were registered once a year at the visit closest to the patient’s date of birth in Denmark and the first visit of the year in Norway. In Iceland and Sweden, all variables were registered at each visit every third month, but data for this study were the last dataset of the year, apart from SH and HbA1c. SH events were registered at each visit and summed up at the last visit of the year; HbA1c for this study was the individual mean of the four registrations collected during the year.
HbA1c was measured centrally in Denmark, Iceland and Norway, while it was decentralized in Sweden. Denmark and Norway used a high-pressure liquid chromatographic method (Tosoh Bioscience, South San Francisco, California, USA). In Iceland, HbA1c was measured with the DCA 2000 Vantage Analyzer (Siemens AG, Erlangen, Germany). In Sweden HbA1c was measured with the DCA 2000 Vantage Analyzer, or with local laboratory methods.15 All laboratories in the Nordic countries are validated through the International Federation of Clinical Chemistry and Laboratory Medicine reference program several times a year.15 16
SH was defined in accordance with the guidelines of the International Society of Pediatric and Adolescent Diabetes as an event associated with severe neuroglycopenia resulting in coma or seizure and requiring parenteral therapy (glucagon or intravenous glucose).17
Body mass index (BMI) was calculated as weight in kilograms divided by height in square meters, and BMI SD score (BMISDS) was calculated from the Swedish population-based longitudinal reference values from birth to 18 years of age for height and weight.18
Ethics
The registries in Denmark, Norway, and Sweden have national quality registry status. In Denmark, patient consent is not required before registration. In Norway, parents have to provide written, informed consent. Parents and patients in Sweden are informed about the registry before agreeing to be included. The Icelandic registry has been approved by the National Bioethics Committee and the Data Protection Authority. The project has been approved by the database steering committees in the four countries.
Statistical analysis
The dataset consisted of multiple records for each patient in the four Nordic countries. The number of contributing patient-years was calculated based on time from first to last registered visit. For descriptive analysis, mean and SD were calculated for continuous variables and percentages for categorical variables. Multilevel mixed-effects models were used to test if HbA1c (linear regression) and treatment (logistic regression) changed during the period. The categorical variable calendar year was used to assess changes in SH. Predictors of SH were identified using univariate and multivariate negative binomial regression models,19 with country as fixed effect and patient as random effect. Three multivariate models were tested: model 1, including the independent variables gender, age and diabetes duration; model 2, model 1 plus the variable year; model 3, model 2 plus the variables BMISDS and HbA1c/treatment modality. Results are presented as incidence risk ratio with 95% CIs. A sensitivity test was conducted excluding patients in partial remission defined as an insulin dose <0.4 IU/kg/day to test a possible impact of endogenous insulin production. A two-sided p value <0.05 was considered statistically significant. Analyses were performed using STATA V.13 and SPSS V.21.
Results
A total of 8806 (48% females) patients with T1D duration of more than 1 year were enrolled into the study, resulting in 29 715 person years with 1775 SH events in 1087 patients. Thus 89.6% of the patients had no SH, 9.1% had one to two SH and 1.3% had three or more SH. Mean age was 11 years and mean diabetes duration was 5.1 years (table 1). Excluding children with a daily insulin dose of less than 0.4 IU/kg did not change the results; thus results presented are for the whole cohort.
Table 1.
Baseline data based on the treatment data presented
| Total group | Sweden | Denmark | Norway | Iceland | p Value | |
| n (patient-years) | 29 715 | 16 901 | 5700 | 6871 | 243 | |
| n (patients) | 8806 | 4138 | 2150 | 2438 | 80 | |
| Gender | <0.01 | |||||
| Male | 4534 (52%) | 2182 (53%) | 1051 (49%) | 1264 (52%) | 37 (47%) | |
| Female | 4270 (48%) | 1956 (47%) | 1099 (51%) | 1174 (48%) | 41 (53%) | |
| Age groups | <0.01 | |||||
| 0–4 years | 273 (3%) | 156 (4%) | 39 (2%) | 75 (3%) | 3 (4%) | |
| 5–9 years | 1948 (22%) | 1111 (27%) | 401 (19%) | 422 (17%) | 14 (17%) | |
| 10–14 years | 6585 (75%) | 2871 (69%) | 1710 (80%) | 1941 (80%) | 63 (79%) | |
| Treatment | <0.01 | |||||
| Pump | 4869 (57%) | 1984 (50%) | 1244 (61%) | 1601 (68%) | 40 (51%) | |
| Pen | 3607 (43%) | 2003 (50%) | 803 (39%) | 762 (32%) | 39 (49%) | |
| Duration* | 5.1 (3.1) | 5.1 (3.0) | 5.2 (3.2) | 5.2 (3.1) | 5.1 (3.0) | 0.46 |
| HbA1c (mmol/mol)* | 65 (13) | 61 (11) | 67 (13) | 71 (14) | 70 (10) | <0.01 |
| HbA1c (%)* | 8.1 (1.2) | 7.7 (1.0) | 8.2 (1.2) | 8.6 (1.3) | 8.5 (0.9) | <0.01 |
| Insulin dose (IU/kg/day)* | 0.89 (0.3) | 0.86 (0.3) | 0.95 (0.3) | 0.89 (0.3) | 0.90 (0.3) | <0.01 |
*Mean±1SD.
HbA1c, hemoglobin A1c.
Incidence of SH over time
The highest incidence of SH was in 2009 with seven SH events per 100 patient-years, and the lowest incidence was in 2012 with five SH events per 100 patient-years. In total, the SH incidence did not change significantly in the Nordic countries over the 5-year study period, and the total incidence of SH in the whole study period was 6.0 per 100 patient-years. Comparing the individual years, significant variations could be shown. The Swedish population had the lowest SH incidence, while the SH incidence was stable between 7 and 10 SH events per 100 patient-years in the Norwegian population. The SH incidence decreased in the Danish population (p<0.01). The same decrease was seen as a trend in the Icelandic population (figure 1).
Figure 1.
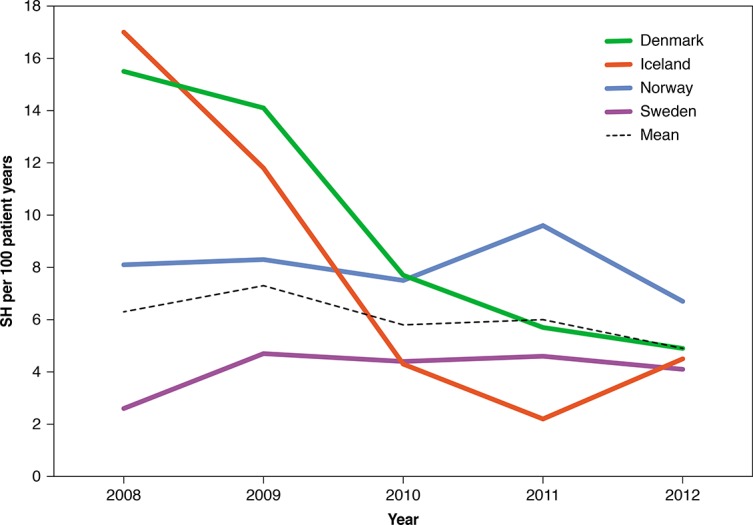
Unadjusted severe hypoglycemia (SH) in the four countries between 2008 and 2012.
Hemoglobin A1c
Mean HbA1c decreased significantly during the period (p<0.001), primarily caused by a steady decrease in HbA1c in the Swedish population, while it was unchanged in the other countries. HbA1c was significantly lower in the Swedish population (total mean 7.9% (62.3 mmol/mol)) throughout the period, compared with total mean HbA1c in Denmark 8.1% (65.4 mmol/mol), Norway 8.4% (68.6 mmol/mol) and Iceland 8.5% (69.4 mmol/mol).
Hemoglobin A1c and SH
The incidence of SH was lowest in the group with the lowest HbA1c (HbA1c ≤6.7% (≤50 mmol/mol), (p=0.04) (table 2). When adjusting for gender, age and diabetes duration in the multivariate analysis, there was no significant difference in the SH incidence risk ratios between the HbA1c groups. However, there was a trend towards lower SH incidence risk ratio in the group with the lowest HbA1c (p=0.07) (table 3).
Table 2.
Risk ratio of SH with 95% CI and p values for different risk factors
| Risk factor | Univariate analysis—SH risk ratio 95% CI |
p Value |
| Gender | 1.0 (0.9 to 1.2) | 0.91 |
| Age (years) | ||
| 0–4 | 1.1 (0.8 to 1.5) | 0.62 |
| 5–9 | 0.9 (0.8 to 1.1) | 0.24 |
| 10–14 | 1.0 | |
| Duration (years) | ||
| 1–2 | 0.8 (0.7 to 1.0) | 0.06 |
| 2–3 | 0.9 (0.7 to 1.1) | 0.16 |
| 3–4 | 1.0 (0.8 to 1.2) | 0.99 |
| 4–5 | 1.0 (0.8 to 1.2) | 0.83 |
| >5 | 1.0 | |
| Year | ||
| 2008 | 1.3 (1.0 to 1.6) | 0.02 |
| 2009 | 1.5 (1.3 to 1.8) | <0.01 |
| 2010 | 1.2 (0.98 to 1.4) | 0.07 |
| 2011 | 1.3 (1.0 to 1.5) | 0.02 |
| 2012 | 1 | |
| HbA1c (mmol/mol) | ||
| ≤50 | 0.7 (0.6 to 0.9) | 0.04 |
| >50−≤60 | 0.9 (0.7 to 1.0) | 0.08 |
| >60−≤70 | 1.0 | |
| >70 | 1.0 (0.9 to 1.2) | 0.91 |
| Treatment | ||
| Pen versus pump | 1.3 (1.1 to 1.5) | <0.01 |
| Insulin dose (IU/kg/day) | ||
| ≤0.6 | 0.8 (0.6 to 1.1) | 0.07 |
| >0.6−≤0.8 | 1.0 | |
| >0.8−≤1.0 | 1.3 (1.1 to 1.6) | <0.01 |
| >1.0−≤1.2 | 1.3 (1.1 to 1.6) | <0.01 |
| ≥1.2 | 1.2 (1.0 to 1.5) | 0.09 |
| BMISDS | ||
| ≤ −2 | 0.8 (0.4 to 1.8) | 0.59 |
| > −2 to ≤−1 | 0.8 (0.6 to 1.1) | 0.16 |
| >−1 to ≤0 | 0.8 (0.7 to 0.98) | 0.03 |
| 0−≤1 | 1.0 | |
| >1−<2 | 1.1 (0.9 to 1.3) | 0.25 |
| ≥2 | 1.0 (0.8 to 1.3) | 0.85 |
Second column showing results of univariate analyses.
BMISDS, body mass index SD score; HbA1c, hemoglobin A1c; SH, severe hypoglycemia.
Table 3.
Risk ratio of SH with 95% CI and p values in the different HbA1c groups and in pen versus pump users
| Risk factor | Model 1—SH risk ratio (95% CI), p Value |
Model 2—SH risk ratio (95% CI), p Value |
Model 3—SH risk ratio (95% CI), p Value |
| HbA1c (mmol/mol) | |||
| ≤50 | 0.8 (0.6 to 1.0), p=0.07 | 0.8 (0.6 to 1.0), p=0.09 | 0.8 (0.6 to 1.1), p=0.25 |
| >50−≤60 | 0.9 (0.7 to 1.0), p=0.13 | 0.9 (0.8 to 1.0), p=0.14 | 0.9 (0.8 to 1.1), p=0.31 |
| >60−≤70 | 1.0 | 1.0 | 1.0 |
| >70 | 1.0 (0.9 to 1.2), p=0.95 | 1.0 (1.0 to 1.2), p=0.90 | 1.0 (0.9 to 1.2), p=0.93 |
| Treatment | |||
| Pen versus pump | 1.3 (1.2 to 1.5), p<0.01 | 1.3 (1.1 to 1.5), p<0.01 | 1.3 (1.0 to 1.4), p<0.01 |
HbA1c, hemoglobin A1c; SH, severe hypoglycemia.
Pump therapy
The number of patients on pump therapy increased significantly from 39% in 2008 to 59% in 2012 (p<0.001). Sweden had the lowest percentage of pump users and the lowest increase during the 5 year study period (from 36% to 51%) while Denmark had the largest increase in patients on pump therapy. In 2012, 71% of the patients were on pump therapy in Denmark and Norway (figure 2).
Figure 2.
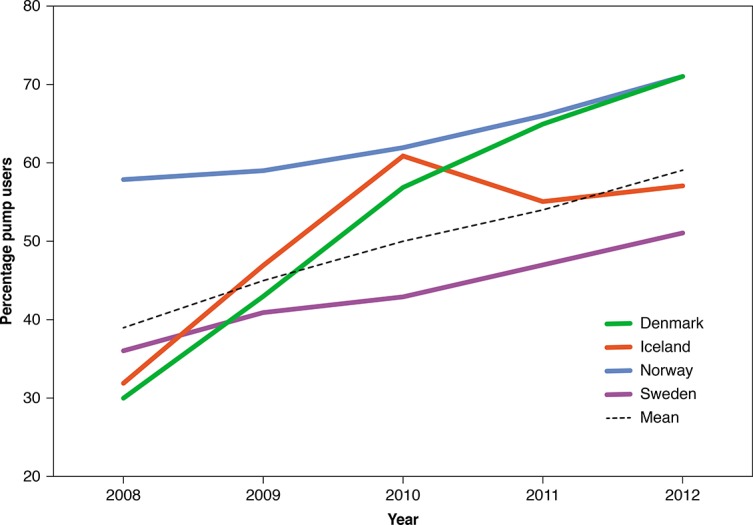
Percentage of patients on pump therapy in the four countries in the period between 2008 and 2012.
Pump therapy and SH
Patients on pen therapy had a higher risk of SH compared with patients on pump therapy with a risk ratio (95 CI) of 1.3 (1.1 to 1.5) (p<0.01) (table 2). This was partly caused by a significantly lower risk of SH in Danish children on pump therapy, and partly by a slightly lower SH risk in Swedish children on pump therapy. The lower risk of SH in pump users persisted in the multivariate models (table 3).
Other variables and SH
The incidence of SH was independent of gender and age groups 1–4, 5–9 and 10–14 years. The SH risk was independent of diabetes duration (table 2). Children with a need of an insulin below 0.6 IU/kg/day had the lowest SH risk (table 2).
Discussion
In the study period between 2008 and 2012, the total incidence of SH among children with T1D in the Nordic countries Denmark, Iceland, Norway and Sweden was stable despite a significant decrease of the mean HbA1c. A large variation in the SH incidence between the countries was shown with the lowest incidence in Sweden. The SH rate was independent of the HbA1c level, although, with a tendency to lower SH rate in children with the lowest HbA1c. Pump treatment appeared to reduce the SH risk ratio. Our results obtained in ‘real life’ T1D care indicate that a HbA1c target ≤6.7% (50 mmol/mol) may be possible without increasing the risk of SH.
The mean rate of six SH events per 100 patient-years was lower than the number reported in a multicenter study from the Hvidoere Group in children below 11 years of age.12 Two studies, one from an Australian regional childhood database, including children with T1D with an age below 18 and a multicenter US study including children between 6 and 18 years with diabetes duration of more than 2 years reported SH incidences comparable to our study.20 21 The Australian study demonstrated reduced risk of SH in patients 12–18 years on pump treatment,21 while the US study revealed fewer SH episodes in the group with ‘excellent glycemic control’,20 which was characterized by more frequent pump use, more BG per day and a lower insulin dose per day. Interestingly, a Finish single-center study from the 1990s, including only pen-treated patients,22 but also a study from the DPV database,11 found lower SH rates than in our study.
There were large unexplained variations in SH between the four countries. Particularly in Denmark, a large decline in the SH rate could be demonstrated from 16 per 100 patient-years in 2008 to five in 2012. During that period a large increase in patients on pump therapy was observed.9 However, the SH rate in Sweden was stable and low (between 2 and 5 per 100 patient-years) and in Norway the SH rate was stable at a higher level (between 7 and 10 per 100 patient-years), despite a lower percentage of patients using insulin pump in Sweden compared with Denmark and Norway. When analyzed in toto, there appeared to be an association between insulin pump use and lower rates of SH; however, this association was not consistently seen across individual countries nor did it explain the variance in rates of sever hypoglycemia between countries. Other variables explaining variations in SH between countries may be different glucose monitoring regimens, including sensor-augmented insulin pump with threshold suspend feature,23 24 different insulin regimens, including daily insulin dose, ratio between long-acting/short acting insulin, use of long-acting insulin analog,9 number of insulin bolus per day and different management guidelines.3 25 Initiatives such as quality improvement programs26 may also have contributed to reduce the risk of SH in Sweden.
During the period 2008–2012 we observed a significant overall reduction in HbA1c levels without any change in SH events, but there was a large intercountry variation in mean HbA1c. Older studies found a negative association between SH incidence and metabolic control.4 5 The relationship between SH and metabolic control has been weaker in studies from recent years,11 21 and a new multicenter, multinational study found the SH rate to be independent of HbA1c.13 A recent US multicenter study showed lower rates of SH in the patients with HbA1c below 7% (53 mmol/mol) compared with patients with a HbA1c level of more than 9% (75 mmol/mol).20 Thus, during the last two decades, there has been a shift from a strong negative association between HbA1c and SH4 5 to nearly no association,11 21 and finally, no association between HbA1c and SH as demonstrated in our study and the new multicenter study including four countries.13
In accordance with the Australian and the DPV study,11 20 we did not find any association between gender and SH. We did not find any association between age and SH in accordance with the Australian study,21 in contrast to the DPV study,11 but the age ranges in the two studies were 1–18 years21 and 1–20 years,11 respectively. Children with insulin requirement below 0.6 IU/kg/day had lower risk of SH, indicating a protective role of residual beta cell function or perhaps more physiological insulin dosing.27 28
Strength of the study
The study population consisted of data on the majority of children with T1D in the four Nordic countries in a 5-year period. Data completeness was high and data represented ‘real-life’ T1D care. All four countries had comparable healthcare systems and free access to healthcare including technical aids such as insulin pump.
Limitations of the study
Despite representatives from the four countries gather at an annual conference to harmonize data collection practice, and one or two national conferences are held every year, data collection may not be 100% uniform in the 89 centers. Moreover, some SH events may be forgotten although families are generally highly affected by SH events. In this study, patient-years are calculated as time between first and last registered visit. SH are reported retrospectively, also at the first visit. Thus, our approach slightly underestimates the patient-years for estimating SH incidence, and thus slightly overestimates the SH incidence. Data completeness concerning number of BG per day and bolus of insulin per day, bolus/basal insulin ratio and physical activity, all factors that may impact on the SH rate was too low to be useful. Due to sample size differences, the Swedish results have a much higher impact on the total data material than results from Iceland.
Conclusion
The total incidence of SH in the Nordic countries remained stable in spite of a significant decrease in HbA1c in the period 2008–2012. Although all the four countries offer equal access to healthcare, the incidence of SH and HbA1c varied. The low incidence of SH in pump users compared with pen users and the low incidence of SH in the Swedish population warrants further examinations. A target HbA1c ≤6.7% (≤50 mmol/mol) may be achievable without increasing the risk of SH.
Acknowledgments
We thank the Icelandic Thorvaldsens Foundation, The Danish Society for Diabetes in Childhood and Adolescence (DanDiabKids), the Norwegian Study group in Childhood Diabetes and the Steering Committee of Swedish Paediatric Diabetes Quality Registry, SwedDiabKids.
Footnotes
Contributors: NHB analyzed data, wrote and edited the manuscript and is the guarantor of the work; AKD analyzed data, contributed to the discussion, reviewed and edited the manuscript; KA, RB, AJ, US, TS, AVT, JS contributed to the discussion, reviewed and edited the manuscript. All authors contributed to data collection. US was the guarantor of the Swedish data.
Funding: This project was supported by the Health Research Fund of Central Denmark Region, and the Swedish Board of Health and Welfare and the Swedish Association of Local Authorities and Regions. The Norwegian Childhood Diabetes Registry is financed by the South-Eastern Norway Regional Health Authority. The Danish registry is funded by the Danish Regions’ Clinical Quality Development Programme. The funding sources had no role in data collection or analysis.
Competing interests: None declared.
Patient consent: Only in Norway parents have to provide written informed concent.
Ethics approval: The project has been approved by the database steering committees of the four countries.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: No additional data are available.
References
- 1. Nathan DM, Genuth S, Lachin J, et al. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993;329:977–86. 10.1056/NEJM199309303291401 [DOI] [PubMed] [Google Scholar]
- 2. Chiang JL, Kirkman MS, Laffel LM, et al. Type 1 diabetes through the life span: a position statement of the American Diabetes Association. Diabetes Care 2014;37:2034–54. 10.2337/dc14-1140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rewers MJ, Pillay K, de Beaufort C, et al. ISPAD Clinical Practice Consensus Guidelines 2014. Assessment and monitoring of glycemic control in children and adolescents with diabetes. Pediatr Diabetes 2014;15(Suppl 20):102–14. 10.1111/pedi.12190 [DOI] [PubMed] [Google Scholar]
- 4. Adverse events and their association with treatment regimens in the diabetes control and complications trial. Diabetes Care 1995;18:1415–27. [DOI] [PubMed] [Google Scholar]
- 5. Davis EA, Keating B, Byrne GC, et al. Hypoglycemia: incidence and clinical predictors in a large population-based sample of children and adolescents with IDDM. Diabetes Care 1997;20:22–5. 10.2337/diacare.20.1.22 [DOI] [PubMed] [Google Scholar]
- 6. Johnson SR, Cooper MN, Davis EA, et al. Hypoglycaemia, fear of hypoglycaemia and quality of life in children with Type 1 diabetes and their parents. Diabet Med 2013;30:1126–31. 10.1111/dme.12247 [DOI] [PubMed] [Google Scholar]
- 7. Lawton J, Waugh N, Barnard KD, et al. Challenges of optimizing glycaemic control in children with Type 1 diabetes: a qualitative study of parents' experiences and views. Diabet Med 2015;32:1063–70. 10.1111/dme.12660 [DOI] [PubMed] [Google Scholar]
- 8. Bulsara MK, Holman CD, Davis EA, et al. The impact of a decade of changing treatment on rates of severe hypoglycemia in a population-based cohort of children with type 1 diabetes. Diabetes Care 2004;27:2293–8. 10.2337/diacare.27.10.2293 [DOI] [PubMed] [Google Scholar]
- 9. Fredheim S, Johansen A, Thorsen SU, et al. Nationwide reduction in the frequency of severe hypoglycemia by half. Acta Diabetol 2015;52:591–9. 10.1007/s00592-014-0697-5 [DOI] [PubMed] [Google Scholar]
- 10. Johnson SR, Cooper MN, Jones TW, et al. Long-term outcome of insulin pump therapy in children with Type 1 diabetes assessed in a large population-based case-control study. Diabetologia 2013;56:2392–400. 10.1007/s00125-013-3007-9 [DOI] [PubMed] [Google Scholar]
- 11. Karges B, Rosenbauer J, Kapellen T, et al. Hemoglobin A1c levels and risk of severe hypoglycemia in children and young adults with type 1 diabetes from Germany and Austria: a trend analysis in a cohort of 37,539 patients between 1995 and 2012. PLoS Med 2014;11:e1001742 10.1371/journal.pmed.1001742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. de Beaufort CE, Lange K, Swift PG, et al. Metabolic outcomes in young children with type 1 diabetes differ between treatment centers: the Hvidoere Study in Young Children 2009. Pediatr Diabetes 2013;14:422–8. 10.1111/j.1399-5448.2012.00922.x [DOI] [PubMed] [Google Scholar]
- 13. Haynes A, Hermann JM, Miller KM, et al. Severe hypoglycemia rates are not associated with HbA1c: a cross-sectional analysis of 3 contemporary pediatric diabetes registry databases. Pediatr Diabetes 2016 10.1111/pedi.12477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hanberger L, Birkebaek N, Bjarnason R, et al. Childhood diabetes in the Nordic countries: a comparison of quality registries. J Diabetes Sci Technol 2014;8:738–44. 10.1177/1932296814531479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hanberger L, Åkesson K, Samuelsson U. Glycated haemoglobin variations in paediatric type 1 diabetes: the impact of season, gender and age. Acta Paediatr 2014;103:398–403. 10.1111/apa.12530 [DOI] [PubMed] [Google Scholar]
- 16. Hanas R, John G; International HBA1c Consensus Committee. 2010 consensus statement on the worldwide standardization of the hemoglobin A1C measurement. Diabetes Care 2010;33:1903–4. 10.2337/dc10-0953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ly TT, Maahs DM, Rewers A, et al. ISPAD Clinical Practice Consensus Guidelines 2014. Assessment and management of hypoglycemia in children and adolescents with diabetes. Pediatr Diabetes 2014;15(Suppl 20):180–92. 10.1111/pedi.12174 [DOI] [PubMed] [Google Scholar]
- 18. Wikland KA, Luo ZC, Niklasson A, et al. Swedish population-based longitudinal reference values from birth to 18 years of age for height, weight and head circumference. Acta Paediatr 2002;91:739–54. 10.1111/j.1651-2227.2002.tb03322.x [DOI] [PubMed] [Google Scholar]
- 19. Bulsara MK, Holman CD, Davis EA, et al. Evaluating risk factors associated with severe hypoglycaemia in epidemiology studies-what method should we use? Diabet Med 2004;21:914–9. 10.1111/j.1464-5491.2004.01250.x [DOI] [PubMed] [Google Scholar]
- 20. Campbell MS, Schatz DA, Chen V, et al. A contrast between children and adolescents with excellent and poor control: the T1D exchange clinic registry experience. Pediatr Diabetes 2014;15:110–7. 10.1111/pedi.12067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cooper MN, O'Connell SM, Davis EA, et al. A population-based study of risk factors for severe hypoglycaemia in a contemporary cohort of childhood-onset type 1 diabetes. Diabetologia 2013;56:2164–70. 10.1007/s00125-013-2982-1 [DOI] [PubMed] [Google Scholar]
- 22. Tupola S, Rajantie J, Mäenpää J. Severe hypoglycaemia in children and adolescents during multiple-dose insulin therapy. Diabet Med 1998;15:695–9. [DOI] [PubMed] [Google Scholar]
- 23. Ly TT, Nicholas JA, Retterath A, et al. Effect of sensor-augmented insulin pump therapy and automated insulin suspension vs standard insulin pump therapy on hypoglycemia in patients with type 1 diabetes: a randomized clinical trial. JAMA 2013;310:1240–7. 10.1001/jama.2013.277818 [DOI] [PubMed] [Google Scholar]
- 24. Bergenstal RM, Klonoff DC, Garg SK, et al. Threshold-based insulin-pump interruption for reduction of hypoglycemia. N Engl J Med 2013;369:224–32. 10.1056/NEJMoa1303576 [DOI] [PubMed] [Google Scholar]
- 25. Cameron FJ, Wherrett DK. Care of diabetes in children and adolescents: controversies, changes, and consensus. Lancet 2015;385:2096–106. 10.1016/S0140-6736(15)60971-0 [DOI] [PubMed] [Google Scholar]
- 26. Peterson A, Hanberger L, Akesson K, et al. Improved results in paediatric diabetes care using a quality registry in an improvement collaborative: a case study in Sweden. PLoS One 2014;9:e97875 10.1371/journal.pone.0097875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sørensen JS, Johannesen J, Pociot F, et al. Residual β-Cell function 3-6 years after onset of type 1 diabetes reduces risk of severe hypoglycemia in children and adolescents. Diabetes Care 2013;36:3454–9. 10.2337/dc13-0418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Steffes MW, Sibley S, Jackson M, et al. Beta-cell function and the development of diabetes-related complications in the diabetes control and complications trial. Diabetes Care 2003;26:832–6. 10.2337/diacare.26.3.832 [DOI] [PubMed] [Google Scholar]


