Abstract
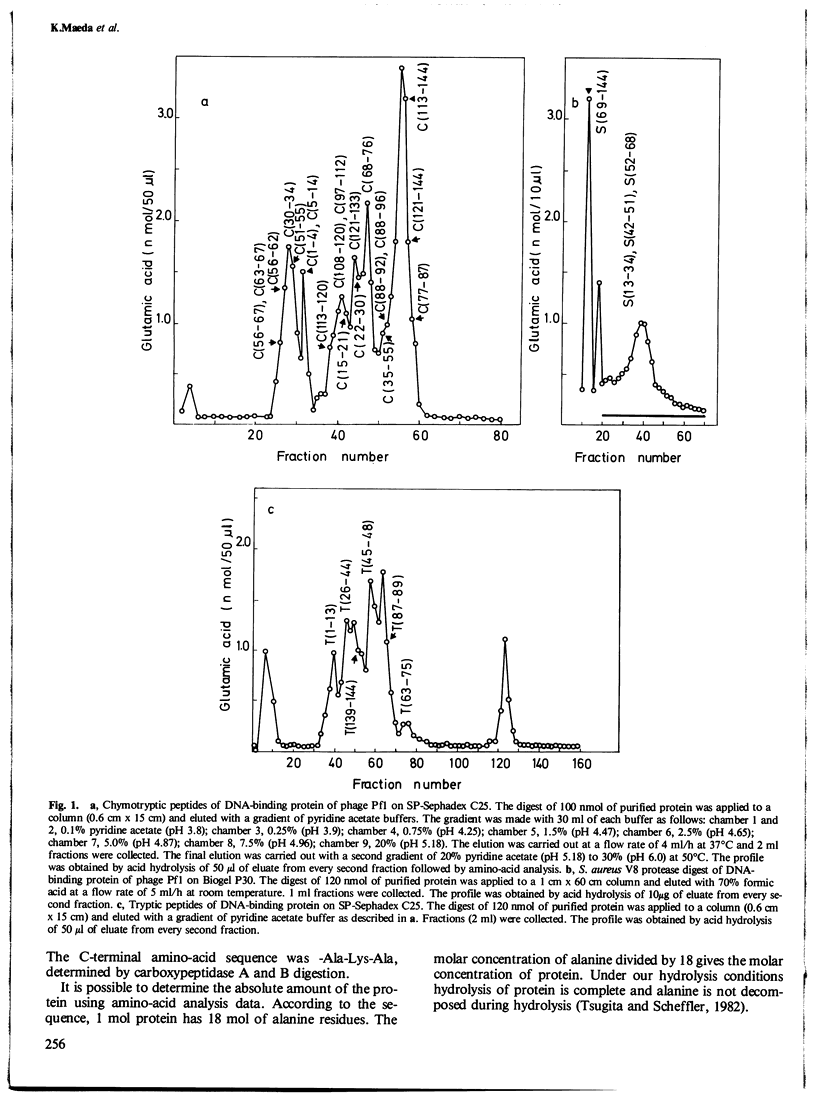
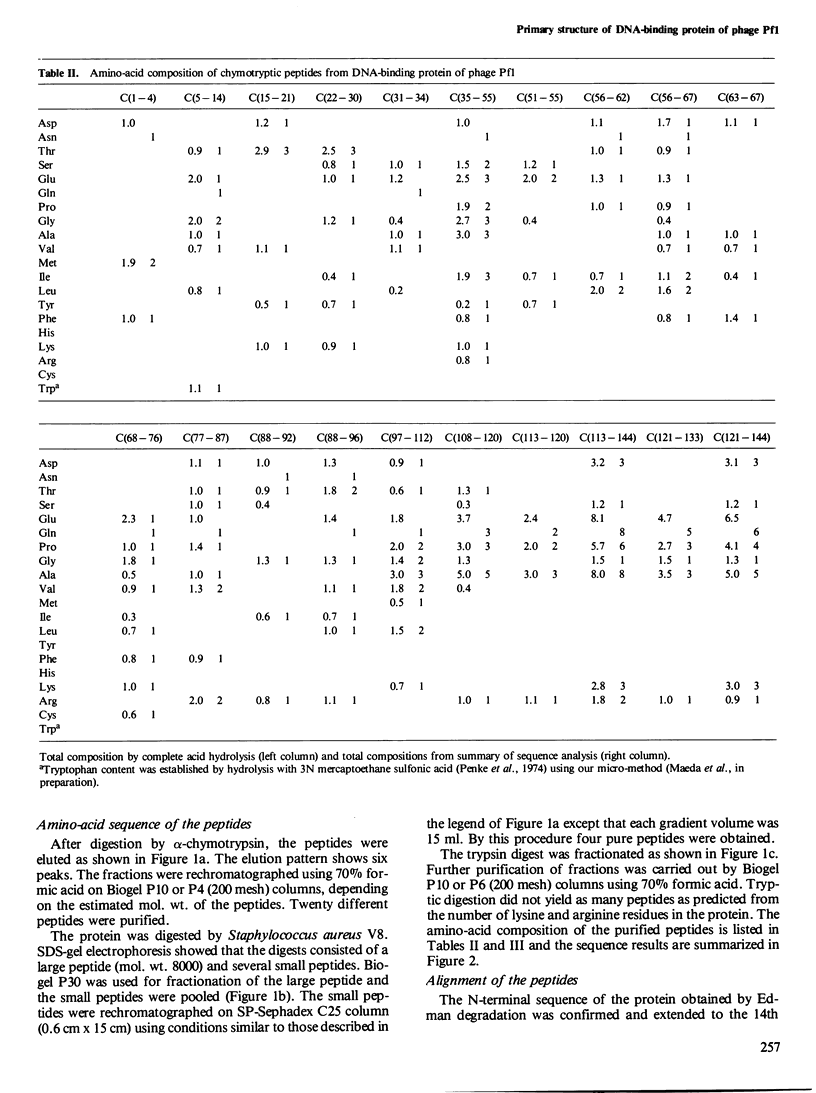
The amino-acid sequence of the single-stranded DNA-binding protein of bacteriophage Pf1 and the nucleotide sequence of the corresponding gene have been determined. The protein has 144 amino acids and a molecular weight of 15 400; the gene consists of 435 nucleotides. The amino-acid sequence was determined by Edman degradation, carboxypeptidase A, B, and P digestion of intact protein and of peptides derived by chymotrypsin, Staphylococcus aureus V8 protease, and trypsin digestion. The nucleotide sequence was determined by the dideoxy method after random cloning of fragments of Pf1 DNA into M13. No sequence homology could be established between the amino-acid sequence of the DNA-binding protein of Pseudomonas aeruginosa-specific bacteriophage Pf1 and bacteriophage fd of Escherichia coli.
Keywords: DNA sequence, Pf1 phage, protein sequence, single-stranded DNA-binding protein
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CANFIELD R. E. PEPTIDES DERIVED FROM TRYPTIC DIGESTION OF EGG WHITE LYSOZYME. J Biol Chem. 1963 Aug;238:2691–2697. [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Prediction of protein conformation. Biochemistry. 1974 Jan 15;13(2):222–245. doi: 10.1021/bi00699a002. [DOI] [PubMed] [Google Scholar]
- Coleman J. E., Oakley J. L. Physical chemical studies of the structure and function of DNA binding (helix-destabilizing) proteins. CRC Crit Rev Biochem. 1980 Jan;7(3):247–289. doi: 10.3109/10409238009105463. [DOI] [PubMed] [Google Scholar]
- Duckworth M. L., Gait M. J., Goelet P., Hong G. F., Singh M., Titmas R. C. Rapid synthesis of oligodeoxyribonucleotides VI. Efficient, mechanised synthesis of heptadecadeoxyribonucleotides by an improved solid phase phosphotriester route. Nucleic Acids Res. 1981 Apr 10;9(7):1691–1706. doi: 10.1093/nar/9.7.1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin T. W., Morton R. A. The spectrophotometric determination of tyrosine and tryptophan in proteins. Biochem J. 1946;40(5-6):628–632. doi: 10.1042/bj0400628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray C. W., Kneale G. G., Leonard K. R., Siegrist H., Marvin D. A. A nucleoprotein complex in bacteria infected with PF1 filamentous virus: identification and electron microscopic analysis. Virology. 1982 Jan 15;116(1):40–52. doi: 10.1016/0042-6822(82)90401-9. [DOI] [PubMed] [Google Scholar]
- HIRS C. H. The oxidation of ribonuclease with performic acid. J Biol Chem. 1956 Apr;219(2):611–621. [PubMed] [Google Scholar]
- Helene C., Maurizot J. C. Interactions of oligopeptides with nucleic acids. CRC Crit Rev Biochem. 1981;10(3):213–258. doi: 10.3109/10409238109113600. [DOI] [PubMed] [Google Scholar]
- Hill D. F., Petersen G. B. Nucleotide sequences in bacteriophage f1 DNA: nucleotide sequence of genes V, VII, and VIII. J Virol. 1980 Apr;34(1):40–50. doi: 10.1128/jvi.34.1.40-50.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isobe T., Yanagida M., Boosman A., Tsugita A. Characterization of the morphogenesis-dependent cleavage region of the major capsid protein (P23) of bacteriophage T4; sequence of an amber fragment of P23. J Mol Biol. 1978 Nov 5;125(3):339–356. doi: 10.1016/0022-2836(78)90407-2. [DOI] [PubMed] [Google Scholar]
- Kneale G. G., Marvin D. A. A nucleoprotein complex in bacteria infected with Pf1 filamentous virus: isolation and biochemical characterization. Virology. 1982 Jan 15;116(1):53–60. doi: 10.1016/0042-6822(82)90402-0. [DOI] [PubMed] [Google Scholar]
- Marvin D. A., Hohn B. Filamentous bacterial viruses. Bacteriol Rev. 1969 Jun;33(2):172–209. doi: 10.1128/br.33.2.172-209.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marvin D. A., Wachtel E. J. Structure and assembly of filamentous bacterial viruses. Philos Trans R Soc Lond B Biol Sci. 1976 Nov 30;276(943):81–98. doi: 10.1098/rstb.1976.0099. [DOI] [PubMed] [Google Scholar]
- McPherson A., Jurnak F., Wang A., Molineux I., Rich A. Structure of the DNA binding cleft of the gene 5 protein from bacteriophage fd. J Supramol Struct. 1979;10(4):457–465. doi: 10.1002/jss.400100408. [DOI] [PubMed] [Google Scholar]
- Meng M., Hogenkamp H. P. Purification, characterization, and amino acid sequence of thioredoxin from Corynebacterium nephridii. J Biol Chem. 1981 Sep 10;256(17):9174–9182. [PubMed] [Google Scholar]
- Messing J., Crea R., Seeburg P. H. A system for shotgun DNA sequencing. Nucleic Acids Res. 1981 Jan 24;9(2):309–321. doi: 10.1093/nar/9.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messing J., Gronenborn B., Müller-Hill B., Hans Hopschneider P. Filamentous coliphage M13 as a cloning vehicle: insertion of a HindII fragment of the lac regulatory region in M13 replicative form in vitro. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3642–3646. doi: 10.1073/pnas.74.9.3642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakashima Y., Dunker A. K., Marvin D. A., Konigsberg W. The amino acid sequence of a DNA binding protein, the gene 5 product of fd filamentous bacteriophage. FEBS Lett. 1974 Apr 1;40(2):290–292. doi: 10.1016/0014-5793(74)80246-2. [DOI] [PubMed] [Google Scholar]
- Penke B., Ferenczi R., Kovács K. A new acid hydrolysis method for determining tryptophan in peptides and proteins. Anal Biochem. 1974 Jul;60(1):45–50. doi: 10.1016/0003-2697(74)90129-8. [DOI] [PubMed] [Google Scholar]
- Pratt D., Laws P., Griffith J. Complex of bacteriophage M13 single-stranded DNA and gene 5 protein. J Mol Biol. 1974 Feb 5;82(4):425–439. doi: 10.1016/0022-2836(74)90239-3. [DOI] [PubMed] [Google Scholar]
- Ray D. S. Replication of bacteriophage M13. II. The role of replicative forms in single-strand synthesis. J Mol Biol. 1969 Aug 14;43(3):631–643. doi: 10.1016/0022-2836(69)90364-7. [DOI] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R., Barrell B. G., Smith A. J., Roe B. A. Cloning in single-stranded bacteriophage as an aid to rapid DNA sequencing. J Mol Biol. 1980 Oct 25;143(2):161–178. doi: 10.1016/0022-2836(80)90196-5. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schürmann P., Maeda K., Tsugita A. Isomers in thioredoxins of spinach chloroplasts. Eur J Biochem. 1981 May;116(1):37–45. doi: 10.1111/j.1432-1033.1981.tb05297.x. [DOI] [PubMed] [Google Scholar]
- Smilowitz H., Carson J., Robbins P. W. Association of newly synthesized major f1 coat protein with infected host cell inner membrane. J Supramol Struct. 1972;1(1):8–18. doi: 10.1002/jss.400010103. [DOI] [PubMed] [Google Scholar]
- Staden R. A new computer method for the storage and manipulation of DNA gel reading data. Nucleic Acids Res. 1980 Aug 25;8(16):3673–3694. doi: 10.1093/nar/8.16.3673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staden R. Sequence data handling by computer. Nucleic Acids Res. 1977 Nov;4(11):4037–4051. doi: 10.1093/nar/4.11.4037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarr G. E. Improved manual sequencing methods. Methods Enzymol. 1977;47:335–357. doi: 10.1016/0076-6879(77)47036-8. [DOI] [PubMed] [Google Scholar]



