Abstract
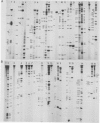
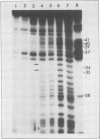
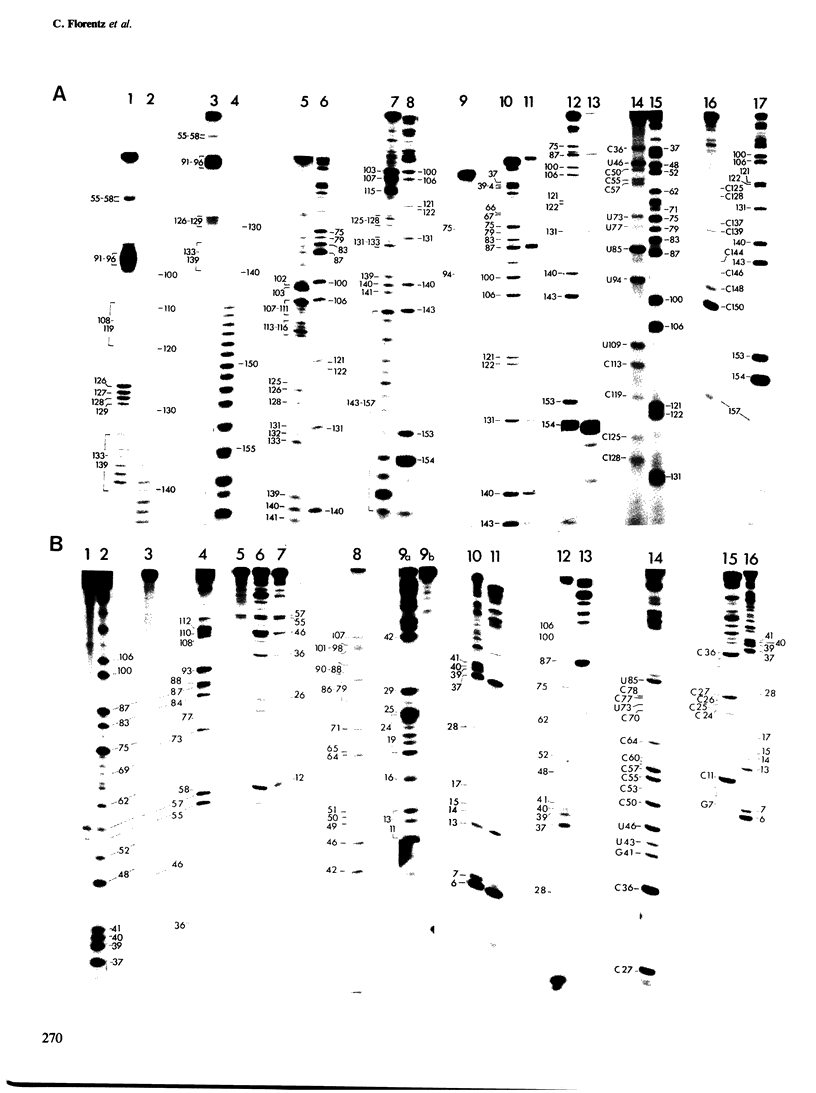
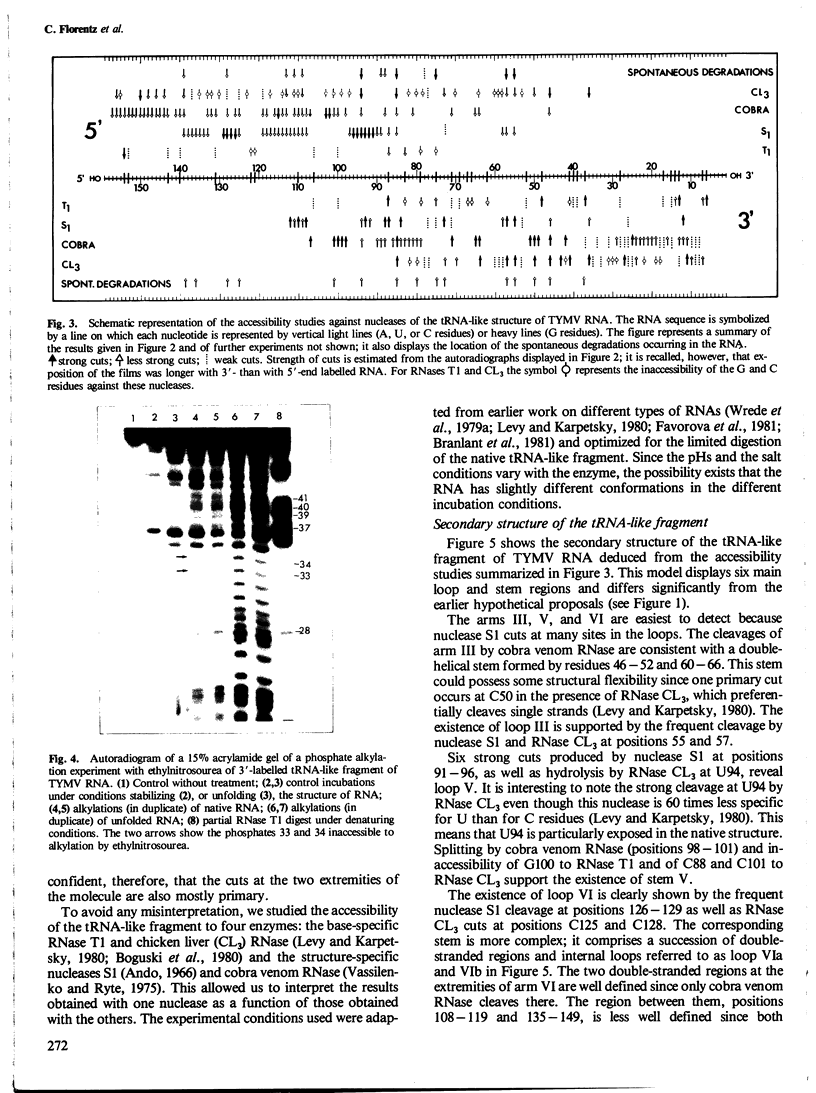
The secondary structure of the isolated tRNA-like sequence (n=159) present at the 3' OH terminus of turnip yellow mosaic virus RNA has been established from partial nuclease digestion with S1 nuclease and T1, CL3, and Naja oxiana RNases. The fragment folds into a 6-armed structure with two main domains. The first domain, of loose structure and nearest the 5' OH terminus, is composed of one large arm which extends into the coat protein cistron. The second, more compact domain, is composed of the five other arms and most probably contains the structure recognized by valyl-tRNA synthetase. In this domain three successive arms strikingly resemble the T[unk], anticodon, and D arms found in tRNA. Near the amino-acid accepting terminus, however, there is a new stem and loop region not found in standard tRNA. This secondary structure is compatible with a L-shaped three-dimensional organization in which the corner of the L and the anticodon-containing limb are similar to, and the amino-acid accepting region different from, that in tRNA. Ethylnitrosourea accessibility studies have shown similar tertiary structure features in the T[unk] loop of tRNAVal and in the homologous region of the viral RNA.
Keywords: ethylnitrosourea, nuclease digestion, secondary structure, tRNA-like, TYMV RNA
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahlquist P., Dasgupta R., Kaesberg P. Near identity of 3- RNA secondary structure in bromoviruses and cucumber mosaic virus. Cell. 1981 Jan;23(1):183–189. doi: 10.1016/0092-8674(81)90283-x. [DOI] [PubMed] [Google Scholar]
- Altwegg M., Kubli E. The nucleotide sequence of histidine tRNA gamma of Drosophila melanogaster. Nucleic Acids Res. 1980 Aug 11;8(15):3259–3262. doi: 10.1093/nar/8.15.3259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ando T. A nuclease specific for heat-denatured DNA in isolated from a product of Aspergillus oryzae. Biochim Biophys Acta. 1966 Jan 18;114(1):158–168. doi: 10.1016/0005-2787(66)90263-2. [DOI] [PubMed] [Google Scholar]
- Boguski M. S., Hieter P. A., Levy C. C. Identification of a cytidine-specific ribonuclease from chicken liver. J Biol Chem. 1980 Mar 10;255(5):2160–2163. [PubMed] [Google Scholar]
- Bonnet J., Ebel J. P., Shershneva L. P., Krutilina A. I., Venkstern T. V., Bayev A. A., Dirheirmer G. The corrected nucleotide sequence of valine tRNA from baker's yeast. Biochimie. 1974;56(9):1211–1213. doi: 10.1016/s0300-9084(74)80013-1. [DOI] [PubMed] [Google Scholar]
- Branlant C., Krol A., Ebel J. P., Gallinaro H., Lazar E., Jacob M. The conformation of chicken, rat and human U1A RNAs in solution. Nucleic Acids Res. 1981 Feb 25;9(4):841–858. doi: 10.1093/nar/9.4.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briand J. P., Jonard G., Guilley H., Richards K., Hirth L. Nucleotide sequence (n=159) of the amino-acid-accepting 3'-OH extremity of turnip-yellow-mosaic-virus RNA and the last portion of its coat-protein cistron. Eur J Biochem. 1977 Feb;72(3):453–463. doi: 10.1111/j.1432-1033.1977.tb11269.x. [DOI] [PubMed] [Google Scholar]
- Briand J. P., Keith G., Guilley H. Nucleotide sequence at the 5' extremity of turnip yellow mosaic virus genome RNA. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3168–3172. doi: 10.1073/pnas.75.7.3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butorin A. S., Remy P., Ebel J. P., Vassilenko S. K. Comparison of the hydrolysis patterns of several tRNAs by cobra venom ribonuclease in different steps of the aminoacylation reaction. Eur J Biochem. 1982 Jan;121(3):587–595. doi: 10.1111/j.1432-1033.1982.tb05827.x. [DOI] [PubMed] [Google Scholar]
- Crothers D. M., Seno T., Söll G. Is there a discriminator site in transfer RNA? Proc Natl Acad Sci U S A. 1972 Oct;69(10):3063–3067. doi: 10.1073/pnas.69.10.3063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donis-Keller H., Maxam A. M., Gilbert W. Mapping adenines, guanines, and pyrimidines in RNA. Nucleic Acids Res. 1977 Aug;4(8):2527–2538. doi: 10.1093/nar/4.8.2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebel J. P., Giegé R., Bonnet J., Kern D., Befort N., Bollack C., Fasiolo F., Gangloff J., Dirheimer G. Factors determining the specificity of the tRNA aminoacylation reaction. Non-absolute specificity of tRNA-aminoacyl-tRNA synthetase recognition and particular importance of the maximal velocity. Biochimie. 1973 May;55(5):547–557. doi: 10.1016/s0300-9084(73)80415-8. [DOI] [PubMed] [Google Scholar]
- Favorova O. O., Fasiolo F., Keith G., Vassilenko S. K., Ebel J. P. Partial digestion of tRNA--aminoacyl-tRNA synthetase complexes with cobra venom ribonuclease. Biochemistry. 1981 Feb 17;20(4):1006–1011. doi: 10.1021/bi00507a055. [DOI] [PubMed] [Google Scholar]
- Giegé R., Briand J. P., Mengual R., Ebel J. P., Hirth L. Valylation of the two RNA components of turnip-yellow mosaic virus and specificity of the tRNA aminoacylation reaction. Eur J Biochem. 1978 Mar;84(1):251–256. doi: 10.1111/j.1432-1033.1978.tb12163.x. [DOI] [PubMed] [Google Scholar]
- Guilley H., Jonard G., Kukla B., Richards K. E. Sequence of 1000 nucleotides at the 3' end of tobacco mosaic virus RNA. Nucleic Acids Res. 1979 Apr;6(4):1287–1308. doi: 10.1093/nar/6.4.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall T. C. Transfer RNA-like structures in viral genomes. Int Rev Cytol. 1979;60:1–26. doi: 10.1016/s0074-7696(08)61257-7. [DOI] [PubMed] [Google Scholar]
- Levy C. C., Karpetsky T. P. The purification and properties of chicken liver RNase: An enzyme which is useful in distinguishing between cytidylic and uridylic acid residues. J Biol Chem. 1980 Mar 10;255(5):2153–2159. [PubMed] [Google Scholar]
- Litvak S., Carr D. S., Chapeville F. TYMV RNA As a substrate of the tRNA nucleotidyltransferase. FEBS Lett. 1970 Dec 18;11(5):316–319. doi: 10.1016/0014-5793(70)80557-9. [DOI] [PubMed] [Google Scholar]
- Lockard R. E., Kumar A. Mapping tRNA structure in solution using double-strand-specific ribonuclease V1 from cobra venom. Nucleic Acids Res. 1981 Oct 10;9(19):5125–5140. doi: 10.1093/nar/9.19.5125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moras D., Comarmond M. B., Fischer J., Weiss R., Thierry J. C., Ebel J. P., Giegé R. Crystal structure of yeast tRNAAsp. Nature. 1980 Dec 25;288(5792):669–674. doi: 10.1038/288669a0. [DOI] [PubMed] [Google Scholar]
- Oberg B., Philipson L. Binding of histidine to tobacco mosaic virus RNA. Biochem Biophys Res Commun. 1972 Aug 21;48(4):927–932. doi: 10.1016/0006-291x(72)90697-3. [DOI] [PubMed] [Google Scholar]
- Prochiantz A., Haenni A. L. TYMV RNA as a substrate of tRNA maturation endonuclease. Nat New Biol. 1973 Feb 7;241(110):168–170. doi: 10.1038/newbio241168a0. [DOI] [PubMed] [Google Scholar]
- Renaud M., Dietrich A., Giegé R., Remy P., Ebel J. P. Interaction between yeast tRNAVal and yeast valyl-tRNA synthetase studied by monochromatic-ultraviolet-light-induced cross-linking. Eur J Biochem. 1979 Nov;101(2):475–483. doi: 10.1111/j.1432-1033.1979.tb19742.x. [DOI] [PubMed] [Google Scholar]
- Renaud M., Ehrlich R., Bonnet J., Remy P. Lack of correlation between affinity of the tRNA for the aminoacyl-tRNA synthetase and aminoacylation capacity as studied with modified tRNAPhe. Eur J Biochem. 1979 Oct;100(1):157–164. doi: 10.1111/j.1432-1033.1979.tb02044.x. [DOI] [PubMed] [Google Scholar]
- Rether B., Bonnet J., Ebel J. P. Studies on tRNA nucleotidyltransferase from baker's yeast. 1. Purification of the enzyme. Protection against thermal inactivation and inhibition by several substrates. Eur J Biochem. 1974 Dec 16;50(1):281–288. doi: 10.1111/j.1432-1033.1974.tb03896.x. [DOI] [PubMed] [Google Scholar]
- Rich A., Schimmel P. R. Structural organization of complexes of transfer RNAs with aminoacyl transfer RNA synthetases. Nucleic Acids Res. 1977;4(5):1649–1665. doi: 10.1093/nar/4.5.1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts R. J. Staphylococcal transfer ribonucleic acids. II. Sequence analysis of isoaccepting glycine transfer ribonucleic acids IA and IB from Staphylococcus epidermidis Texas 26. J Biol Chem. 1974 Aug 10;249(15):4787–4796. [PubMed] [Google Scholar]
- Silberklang M., Gillum A. M., RajBhandary U. L. The use of nuclease P1 in sequence analysis of end group labeled RNA. Nucleic Acids Res. 1977 Dec;4(12):4091–4108. doi: 10.1093/nar/4.12.4091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silberklang M., Prochiantz A., Haenni A. L., Rajbhandary U. L. Studies on the sequence of the 3'-terminal region of turnip-yellow-mosaic-virus RNA. Eur J Biochem. 1977 Feb;72(3):465–478. doi: 10.1111/j.1432-1033.1977.tb11270.x. [DOI] [PubMed] [Google Scholar]
- Stewart T. S., Roberts R. J., Strominger J. L. Novel species of tRNA. Nature. 1971 Mar 5;230(5288):36–38. doi: 10.1038/230036a0. [DOI] [PubMed] [Google Scholar]
- Thiyagarajan P., Ponnuswamy P. K. Solvent accessibility study on tRNAPhe. Biopolymers. 1979 Sep;18(9):2233–2247. doi: 10.1002/bip.1979.360180911. [DOI] [PubMed] [Google Scholar]
- Toots I., Metspalu A., Villems R., Saarma M. Location of single-stranded and double-stranded regions in rat liver ribosomal 5S RNA and 5.8S RNA. Nucleic Acids Res. 1981 Oct 24;9(20):5331–5343. doi: 10.1093/nar/9.20.5331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasilenko S. K., Babkina G. T. Vydelenie i svoistva ribonukleazy iz iada kobry. Biokhimiia. 1965 Jul-Aug;30(4):705–712. [PubMed] [Google Scholar]
- Vasilenko S. K., Ryte V. C. [Isolation of highly purified ribonuclease from cobra (Naja oxiana) venom]. Biokhimiia. 1975 May-Jun;40(3):578–583. [PubMed] [Google Scholar]
- Vassilenko S. K., Carbon P., Ebel J. P., Ehresmann C. Topography of 16 S RNA in 30 S subunits and 70 S ribosomes accessibility to cobra venom ribonuclease. J Mol Biol. 1981 Nov 15;152(4):699–721. doi: 10.1016/0022-2836(81)90123-6. [DOI] [PubMed] [Google Scholar]
- Vlassov V. V., Giegé R., Ebel J. P. Tertiary structure of tRNAs in solution monitored by phosphodiester modification with ethylnitrosourea. Eur J Biochem. 1981 Sep;119(1):51–59. doi: 10.1111/j.1432-1033.1981.tb05575.x. [DOI] [PubMed] [Google Scholar]
- Wrede P., Woo N. H., Rich A. Initiator tRNAs have a unique anticodon loop conformation. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3289–3293. doi: 10.1073/pnas.76.7.3289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrede P., Wurst R., Vournakis J., Rich A. Conformational changes of yeast tRNAPhe and E. coli tRNA2Glu as indicated by different nuclease digestion patterns. J Biol Chem. 1979 Oct 10;254(19):9608–9616. [PubMed] [Google Scholar]