Abstract
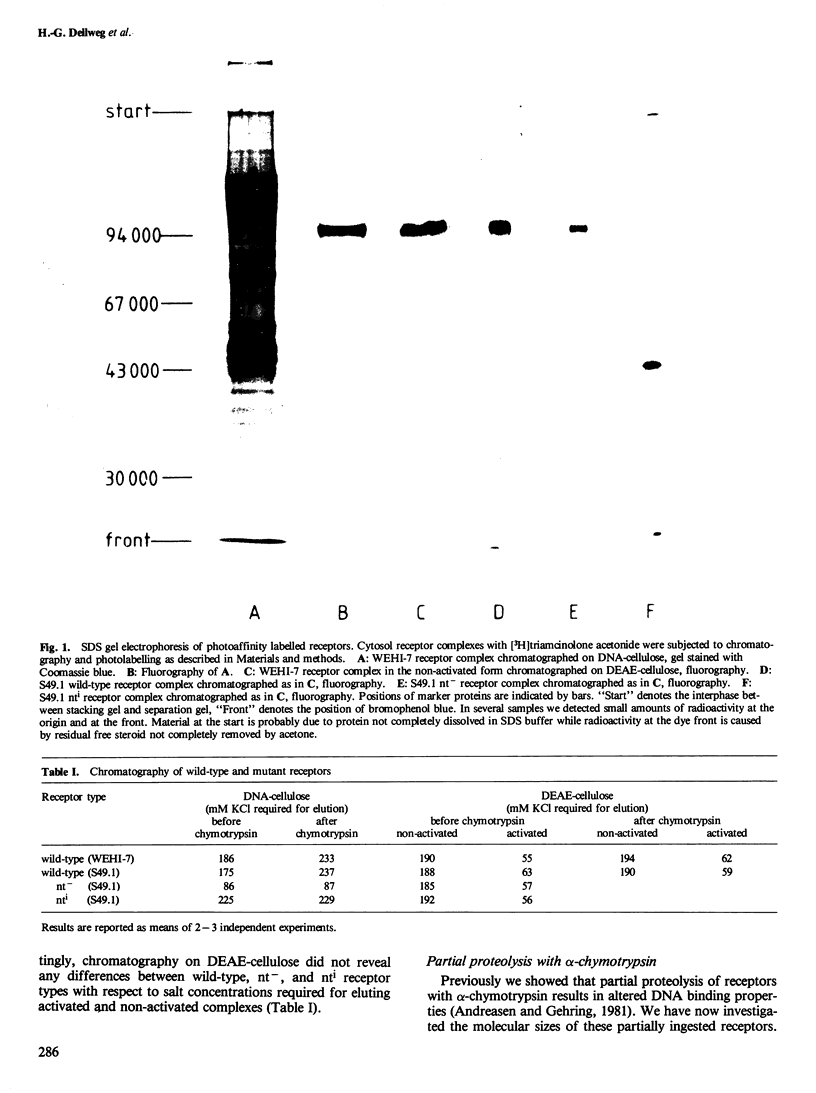
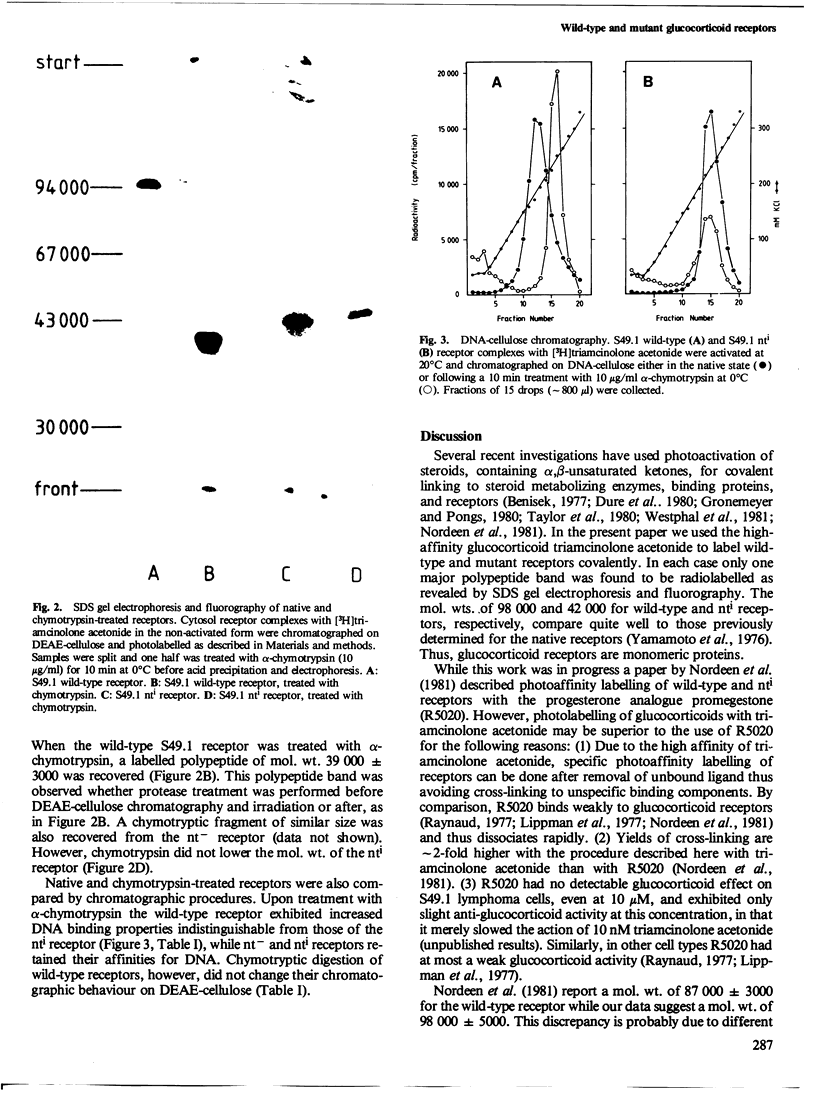
[3H]Triamcinolone acetonide was used to tag covalently specific glucocorticoid receptors by photoaffinity labelling at lambda greater than or equal to 320 nm. Receptors of wild-type mouse lymphoma cells and two glucocorticoid resistant mutants of "nuclear transfer deficient" (nt-) and "increased nuclear transfer" (nti) phenotypes, respectively, were used. Wild-type and nt- receptors yielded radiolabelled polypeptide bands of mol. wt. 98 000 as revealed by gel electrophoresis under denaturing conditions and fluorography. In contrast, the nti receptor had a mol. wt. of 42 000. Partial proteolysis of the wild-type receptor with alpha-chymotrypsin resulted in a fragment of mol. wt. 39 000 which still contained the steroid binding site but had increased affinity for DNA indistinguishable from that of the nti receptor. Chymotrypsin thus removed a domain from the wild-type receptor polypeptide which is involved in modulating DNA binding. The same domain is missing from the nti receptor.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andreasen P. A., Gehring U. Activation and partial proteolysis of variant glucocorticoid receptors, studied by two-phase partitioning. Eur J Biochem. 1981 Dec;120(3):443–449. doi: 10.1111/j.1432-1033.1981.tb05722.x. [DOI] [PubMed] [Google Scholar]
- Beato M., Feigelson P. Glucocorticoid-binding proteins of rat liver cytosol. I. Separation and identification of the binding proteins. J Biol Chem. 1972 Dec 25;247(24):7890–7896. [PubMed] [Google Scholar]
- Benisek W. F. Labeling of delta5-3-ketosteroid isomerase by photoexcited steroid ketones. Methods Enzymol. 1977;46:469–479. doi: 10.1016/s0076-6879(77)46056-7. [DOI] [PubMed] [Google Scholar]
- Dure L. S., 4th, Schrader W. T., O'Malley B. W. Covalent attachment of a progestational steroid to chick oviduct progesterone receptor by photoaffinity labelling. Nature. 1980 Feb 21;283(5749):784–786. doi: 10.1038/283784a0. [DOI] [PubMed] [Google Scholar]
- Gehring U. Genetic analysis of glucocorticoid action in neoplastic lymphoid cells. Adv Enzyme Regul. 1978;17:343–361. doi: 10.1016/0065-2571(79)90021-9. [DOI] [PubMed] [Google Scholar]
- Gehring U. Specific receptors control steroid sensitivity in lymphoma cell hybrids. Mol Cell Endocrinol. 1980 Dec;20(3):261–274. doi: 10.1016/0303-7207(80)90042-8. [DOI] [PubMed] [Google Scholar]
- Gehring U., Tomkins G. M. A new mechanism for steroid unresponsiveness: loss of nuclear binding activity of a steroid hormone receptor. Cell. 1974 Nov;3(3):301–306. doi: 10.1016/0092-8674(74)90145-7. [DOI] [PubMed] [Google Scholar]
- Gorski J., Gannon F. Current models of steroid hormone action: a critique. Annu Rev Physiol. 1976;38:425–450. doi: 10.1146/annurev.ph.38.030176.002233. [DOI] [PubMed] [Google Scholar]
- Gronemeyer H., Pongs O. Localization of ecdysterone on polytene chromosomes of Drosophila melanogaster. Proc Natl Acad Sci U S A. 1980 Apr;77(4):2108–2112. doi: 10.1073/pnas.77.4.2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris A. W., Bankhurst A. D., Mason S., Warner N. L. Differentiated functions expressed by cultured mouse lymphoma cells. II. Theta antigen, surface immunoglobulin and a receptor for antibody on cells of a thymoma cell line. J Immunol. 1973 Feb;110(2):431–438. [PubMed] [Google Scholar]
- Higgins S. J., Gehring U. Molecular mechanisms of steroid hormone action. Adv Cancer Res. 1978;28:313–397. doi: 10.1016/s0065-230x(08)60650-8. [DOI] [PubMed] [Google Scholar]
- Katzenellenbogen B. S. Dynamics of steroid hormone receptor action. Annu Rev Physiol. 1980;42:17–35. doi: 10.1146/annurev.ph.42.030180.000313. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leach K. L., Dahmer M. K., Hammond N. D., Sando J. J., Pratt W. B. Molybdate inhibition of glucocorticoid receptor inactivation and transformation. J Biol Chem. 1979 Dec 10;254(23):11884–11890. [PubMed] [Google Scholar]
- Nordeen S. K., Lan N. C., Showers M. O., Baxter J. D. Photoaffinity labeling of glucocorticoid receptors. J Biol Chem. 1981 Oct 25;256(20):10503–10508. [PubMed] [Google Scholar]
- Okret S., Carlstedt-Duke J., Wrange O., Carlström K., Gustafsson J. A. Characterization of an antiserum against the glucocorticoid receptor. Biochim Biophys Acta. 1981 Oct 12;677(2):205–219. doi: 10.1016/0304-4165(81)90087-8. [DOI] [PubMed] [Google Scholar]
- Sakaue Y., Thompson E. B. Characterization of two forms of glucocorticoid hormone-receptor complex separated by DEAE-cellulose column chromatography. Biochem Biophys Res Commun. 1977 Jul 25;77(2):533–541. doi: 10.1016/s0006-291x(77)80012-0. [DOI] [PubMed] [Google Scholar]
- Schmidt T. J., Harmon J. M., Thompson E. B. 'Activation-labile' glucocorticoid-receptor complexes of a steroid-resistant variant of CEM-C7 human lymphoid cells. Nature. 1980 Jul 31;286(5772):507–510. doi: 10.1038/286507a0. [DOI] [PubMed] [Google Scholar]
- Stevens J., Eisen H. J., Stevens Y. W., Haubenstock H., Rosenthal R. L., Artishevsky A. Immunochemical differences between glucocorticoid receptors from corticoid-sensitive and -resistant malignant lymphocytes. Cancer Res. 1981 Jan;41(1):134–137. [PubMed] [Google Scholar]
- Stevens J., Stevens Y. W. Influence of limited proteolysis on the physicochemical and DNA-binding properties of glucocorticoid receptors from corticoid-sensitive and -resistant mouse lymphoma P1798. Cancer Res. 1981 Jan;41(1):125–133. [PubMed] [Google Scholar]
- Taylor C. A., Jr, Smith H. E., Danzo B. J. Photoaffinity labeling of rat androgen binding protein. Proc Natl Acad Sci U S A. 1980 Jan;77(1):234–238. doi: 10.1073/pnas.77.1.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westphal H. M., Fleischmann G., Beato M. Photoaffinity labeling of steroid binding proteins with unmodified ligands. Eur J Biochem. 1981 Sep;119(1):101–106. doi: 10.1111/j.1432-1033.1981.tb05582.x. [DOI] [PubMed] [Google Scholar]
- Wrange O., Gustafsson J. A. Separation of the hormone- and DNA-binding sites of the hepatic glucocorticoid receptor by means of proteolysis. J Biol Chem. 1978 Feb 10;253(3):856–865. [PubMed] [Google Scholar]
- Yamamoto K. R., Alberts B. M. Steroid receptors: elements for modulation of eukaryotic transcription. Annu Rev Biochem. 1976;45:721–746. doi: 10.1146/annurev.bi.45.070176.003445. [DOI] [PubMed] [Google Scholar]
- Yamamoto K. R., Gehring U., Stampfer M. R., Sibley C. H. Genetic approaches to steroid hormone action. Recent Prog Horm Res. 1976;32:3–32. doi: 10.1016/b978-0-12-571132-6.50008-7. [DOI] [PubMed] [Google Scholar]