Abstract
Objective
There is a need for an improved biomarker for colorectal cancer (CRC) and advanced adenoma. We evaluated faecal microbial markers for clinical use in detecting CRC and advanced adenoma.
Design
We measured relative abundance of Fusobacterium nucleatum (Fn), Peptostreptococcus anaerobius (Pa) and Parvimonas micra (Pm) by quantitative PCR in 309 subjects, including 104 patients with CRC, 103 patients with advanced adenoma and 102 controls. We evaluated the diagnostic performance of these biomarkers with respect to faecal immunochemical test (FIT), and validated the results in an independent cohort of 181 subjects.
Results
The abundance was higher for all three individual markers in patients with CRC than controls (p<0.001), and for marker Fn in patients with advanced adenoma than controls (p=0.022). The marker Fn, when combined with FIT, showed superior sensitivity (92.3% vs 73.1%, p<0.001) and area under the receiver-operating characteristic curve (AUC) (0.95 vs 0.86, p<0.001) than stand-alone FIT in detecting CRC in the same patient cohort. This combined test also increased the sensitivity (38.6% vs 15.5%, p<0.001) and AUC (0.65 vs 0.57, p=0.007) for detecting advanced adenoma. The performance gain for both CRC and advanced adenoma was confirmed in the validation cohort (p=0.0014 and p=0.031, respectively).
Conclusions
This study identified marker Fn as a valuable marker to improve diagnostic performance of FIT, providing a complementary role to detect lesions missed by FIT alone. This simple approach may improve the clinical utility of the current FIT, and takes one step further towards a non-invasive, potentially more accurate and affordable diagnosis of advanced colorectal neoplasia.
Keywords: COLORECTAL CANCER, COLORECTAL ADENOMAS, COLORECTAL CANCER SCREENING, COLONIC MICROFLORA
Significance of this study.
What is already known on this subject?
Faecal immunochemical test (FIT) is recommended as a non-invasive screening test for colorectal cancer (CRC). Nevertheless, it is limited by its low sensitivity for advanced neoplasia.
There is a need for a simple, affordable, accurate and improved screening test for colorectal neoplasia.
Alternations in the gut microbial composition are associated with CRC and its precancerous neoplasia, with increased abundance of Fusobacterium and other bacteria. These microbial signatures may be used as biomarkers for the detection of colorectal neoplasia.
What are the new findings?
Fusobacterium is significantly increased in patients with CRC and advanced adenoma. Faecal quantification of Fusobacterium can serve as a biomarker to differentiate patients with CRC and advanced adenoma from controls.
Combining FIT with this marker significantly increases its detection rates for CRC with a sensitivity of 92.3% and a specificity of 93.0%, and for advanced adenoma with a sensitivity of 38.6% and a specificity of 89.0%. The combined test salvages more than 75% of the CRC samples missed by stand-alone FIT.
Further addition of two microbial markers does not improve the diagnostic performance. Fusobacterium quantification is key in supplementing FIT in diagnosing advanced colorectal neoplasia.
How might it impact on clinical practice in the foreseeable future?
Our study identifies faecal quantification of Fusobacterium to improve the diagnostic performance of FIT, and might have an impact on the diagnosis of colorectal neoplasia. This simple approach will enhance clinical applicability and utility of this finding. Our study takes one step further towards a non-invasive, potentially more accurate and affordable diagnosis of advanced colorectal neoplasia.
Introduction
Colorectal cancer (CRC) is the third most common cancer worldwide.1 Screening for CRC can reduce cancer mortality by identifying adenomas or early cancers that are highly treatable,2 3 and international guidelines have recommended several strategies for CRC screening.4 5 Nevertheless, a substantial proportion of the population has not undergone CRC screening, due to health seeking behaviours, public resources, healthcare accessibility and limitations of the screening tests. Conventional colonoscopy carries a small procedural risk, whereas flexible sigmoidoscopy is not effective in reducing proximal cancers.2 Stool-based occult blood tests have a moderate sensitivity to detect CRC as a population-based screening test, with a sensitivity of 69–86% for the faecal immunochemical test (FIT).6 Nevertheless, it has a low sensitivity for advanced adenoma.7 8 An accurate, non-invasive test with high sensitivities for both CRC and advanced adenoma is highly desirable.
As we know more about the metagenomic landscape of CRC, the use of microbial markers represents a tantalising possibility. This is supported by a number of studies, including ours, showing a distinctive gut microbiota among patients with CRC with several bacteria having a putative carcinogenic role.9–13 This includes Fusobacterium nucleatum which is able to promote colorectal carcinogenesis,14–16 whereas over-representation of other species from the Peptostreptococcus and Parvimonas genera have also been observed.9–12 Nevertheless, the potential utility of these microbial biomarkers in detecting colorectal neoplasia remains underexplored.
With this background, we evaluated the performance of three microbial markers with FIT as a diagnostic tool for CRC and advanced adenoma in a Chinese population. These markers are based on our previous metagenomic study on patients with CRC and controls,9 and target the genome of F. nucleatum (marker Fn), Peptostreptococcus anaerobius (marker Pa) and Parvimonas micra (marker Pm). We investigated the performance of these microbial markers in reference to FIT.
Materials and methods
Sample collection
Stool samples were retrieved from the research stool bank, collected from individuals undergoing colonoscopy at the Shaw Endoscopy Centre at the Prince of Wales Hospital, the Chinese University of Hong Kong (CUHK).17 The cohort included individuals presenting with digestive symptoms to the outpatient gastroenterology clinics, as well as asymptomatic individuals aged 50 years or above undergoing screening colonoscopy from the CUHK Jockey Club Bowel Cancer Education Centre. The exclusion criteria were: (1) use of antibiotics within the past 3 months; (2) on a vegetarian diet; (3) had a surgery or an invasive procedure within the past 3 months; (4) had an IBD; or (5) a past history of any cancer. These predefined exclusion criteria were important because they might independently alter the microbiota. After the written informed consent, we asked patients scheduled for colonoscopy to provide a stool sample before bowel preparation. After stool collection by the patients, samples were delivered to the hospital within 24 hours (mean 8.6 hours, SD 6.3 hours, range 0.4–23.3 hours) and stored at −80°C immediately until further analysis. Informed consent was obtained for all individuals.
Colonoscopy
Before the colonoscopy, the indications and risks were explained to each study participant. Polyethylene glycol (Klean-Prep; Helsinn Birex Pharmaceuticals Dublin, Ireland) was used as a standard bowel preparation regimen for each participant in split dosing. All colonoscopies were performed by experienced colonoscopists blinded to the FIT and microbial marker results. All procedures used air insufflation, and the colonoscopists aimed for a withdrawal time of 6 min or more.18 Lesions were removed or biopsied as deemed necessary by the colonoscopists. The specimens were sent for gross and microscopic examinations to a certified, accredited laboratory.
Definitions and clinical phenotypes
The clinical phenotype is defined by the endoscopic and pathological findings. The CRC stool samples were collected from patients with a colorectal adenocarcinoma confirmed by histology. Proximal tumours include those in the caecum, ascending colon, hepatic flexure, transverse colon or splenic flexure; whereas distal tumours include those in the descending colon, sigmoid colon or rectum. The CRC stage was assessed by the TNM system according to the American Joint Committee on Cancer Staging Manual, seventh edition. The advanced adenoma stool samples were collected from patients with adenomas 1 cm or greater in size, with a tubulovillous or villous component, or with high grade or severe dysplasia. Pathologists were blinded to the FIT or microbial marker results. Controls subjects were selected randomly from a healthy cohort of individuals undergoing screening colonoscopy with normal colorectal mucosae.
DNA preparation and storage
Stool samples were thawed on ice, and faecal DNA was extracted using the ZR Faecal DNA MiniPrep Kit (Zymo Research, USA) according to manufacturer's instructions. The extraction was performed with a spin column and the DNA was eluted in Tris-EDTA buffer, pH 8. Amplicons of the quantitative real-time PCR (qPCR) reactions were gel purified by using the QIAquick Gel Extraction Kit (Qiagen, USA). All DNA samples were stored at −20°C, and the DNA quantity was determined using the Thermo Scientific NanoDrop 2000c Spectrophotometer (Thermo Scientific, USA).
Quantitative real-time PCR
qPCR was used to determine the relative abundance of the markers. All reactions were assayed in 20 μL reaction volume containing 1× final concentration PowerUp SYBR Green Master Mix (Applied Biosystems, USA) in a 96-well optical PCR plate. Each reaction contained 40–80 ng of extracted faecal DNA and 200–250 nM of primers. Amplification and detection of DNA was performed with the Applied Biosystems 7500 Fast Real-Time PCR System (Applied Biosystems, USA) using the following reaction conditions: 50°C for 2 min, 95°C for 2 min, followed by 45 cycles of 95°C of 15 s and 60°C of 1 min. The primers for detecting Fn and total bacteria were used as previously described.19 Custom primers for detecting Pa and Pm were designed using AlleleID and Beacon Designer (PREMIER Biosoft, USA). The primers’ sequences were as follows: Fn, forward 5′-CAACCATTACTTTAACTCTACCATGTTCA-3′, reverse 5′-GTTGACTTTACAGAAGGAGATTATGTAAAAATC-3′, Pa, forward 5′-AGACGAATTCAAGTCAGTAAATACA-3′, reverse 5′-CTCCTATCCACCAGGATATCAA-3′, Pm, forward 5′-GTCACTACGGAAGAATTTGTC-3′, reverse 5′-GGCTTGAGCGATAATAACTTC-3′. The relative abundance of these markers was calculated in reference to the total bacterial DNA, determined by qPCR using the following primers: forward 5′-GCAGGCCTAACACATGCAAGTC-3′, reverse 5′-CTGCTGCCTCCCGTAGGAGT-3′. Each sample was assayed in triplicate in a single batch, and the mean of the three cycle threshold (Ct) values for each sample was used for subsequent analysis. The Ct value is defined by the number of cycles in qPCR required for the fluorescent signal to cross the threshold. The abundance of the microbial markers was calculated as a relative unit normalised to the total bacteria of that sample, using the 2−ΔCt method (where ΔCt=the average Ct value of each target − the average Ct value of total bacteria).
Faecal immunochemical test
The FIT was performed using the automated quantitative OC-Sensor test (Eiken Chemical, Japan). Frozen stool samples were allowed to thaw on ice. The test was performed by taking a sample using the probe with a serrated tip, which was poked into the whole stool and then pushed back into the system compatible OC-Auto sampling tubes. The volume of the device buffer was 2 mL. Tests were analysed using the automatic OC-Sensor μ-instrument (Eiken Chemical, Japan). Each test was analysed once and was considered positive at a cut-off value of 20 µg haemoglobin per gram of faeces (µg Hb/g), equivalent to a concentration of 100 ng of haemoglobin per millilitre (ng Hb/mL). The laboratory staff conducting the experiment was blinded to the colonoscopy and histology results, and had experience in performing at least 200 FITs. A standard for Faecal Immunochemical TesTs for Haemoglobin Evaluation Reporting (FITTER) checklist for reporting of our study using FIT has been presented in the online supplementary appendix.
gutjnl-2016-312766supp001.pdf (483.2KB, pdf)
Statistical analyses
The differences in microbial marker levels were determined by the Mann-Whitney U test. The performance of the markers was analysed by calculating the area under the receiver-operating characteristic curve (AUC), and compared using the Delong's test. The sensitivities and specificities were compared using the McNemar paired comparison test. The contingency tables were analysed using the Fisher's exact test. A nominal value of p<0.05 was taken as statistical significance. All the tests were performed by the R Project for Statistical Computing V.3.2.4.
Combining FIT and microbial markers
Combination of multiple biomarkers was performed by fitting the markers into a binary logistic regression model, which used a logit function from binomial distribution to link the composite score and outcome.21 The FIT result was a dichotomous variable, whereas the microbial marker results were continuous variables. We defined p as the probability that the sample was a case instead of a control, such that the logistic regression model could be written as 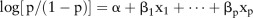 , where a represented the intercept, b represented the regression coefficient and x represented the marker in the model. The optimal cut-off value was determined by Youden's J index, which determined the maximum vertical distance between the receiver-operating characteristic curve and the diagonal line. It represented the maximum effectiveness of the marker, and was mathematically defined as
, where a represented the intercept, b represented the regression coefficient and x represented the marker in the model. The optimal cut-off value was determined by Youden's J index, which determined the maximum vertical distance between the receiver-operating characteristic curve and the diagonal line. It represented the maximum effectiveness of the marker, and was mathematically defined as 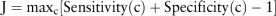 .
.
K-fold cross validation and model assessment
We performed internal and external validations to confirm whether combining FIT with the marker Fn could improve the diagnostic accuracy for CRC and advanced adenoma. For internal validation, we performed model fitting and K-fold cross validation on the discovery cohort (validation set). First, the logistic regression was used to fit the cohort. To get a robust result of the model fitness, we used the 10-fold cross validation and calculated the corresponding validated AUCs to compare the models. We repeated the entire procedure 1000 times to obtain the mean and CIs of the AUCs. We used the regression results fitted in the discovery cohort to compare the cross validation results of other models. For the external testing, we quantitated FIT and the marker Fn in an independent validation cohort (testing set). We applied the regression model fitted by the discovery cohort to obtain the corresponding AUCs, which were used to indicate the model performance in the validation cohort.
Results
Patient cohorts and quality control
The mean age of the 309 subjects in the discovery cohort was 61.8 years, with 109 (35.3%) female subjects (see online supplementary table S1). Two independent quantifications of total bacteria in the same stool samples correlated strongly, with a Spearman's correlation r=0.987 (p<0.001, online supplementary figure S1). Melt curve of each sample for all three biomarkers showed a single defined peak corresponding to the positive controls (see online supplementary figure S2). To further confirm the specificity of each designed marker, the qPCR products were visualised by agarose gel electrophoresis showing a single band of expected size with identity confirmed by Sanger sequencing.
Higher abundance of Fusobacterium in patients with CRC and advanced adenoma
The relative abundance of three microbial markers was determined in 309 individuals, including 104 patients with CRC, 103 patients with advanced adenoma and 102 healthy controls. The mean abundance of each marker was significantly higher in patients with CRC than controls (p<0.001, online supplementary figure S3). The relative abundance in patients with CRC compared with controls was 132-fold, 37-fold and 41-fold for the markers Fn, Pa and Pm, respectively.
As for patients with advanced adenoma, their relative abundance of marker Fn was significantly higher than the control group (3.8-fold, p=0.022, online supplementary figure S3A). Nevertheless, there was no significant difference in abundance between advanced adenoma and control groups for markers Pa (p=0.545) and Pm (p=0.232) (see online supplementary figure S3B, C).
These results suggested the potential of these markers for differentiating colorectal neoplasia and controls. Based on these results, we evaluated the three markers for classifying CRC and the marker Fn for classifying advanced adenoma from controls.
Performance of faecal microbial markers
We first evaluated the performance of the microbial markers in differentiating CRC from controls. At the best cut-off value of 1.5×10−6, the marker Fn provided a sensitivity of 72.1% and a specificity of 91.0%. The AUC value of the marker Fn was 0.83 (95% CI 0.78 to 0.89, figure 1A, online supplementary figure S4A). Its performance is significantly better than the marker Pa (p=0.004) and marker Pm (p=0.015), which had AUC values of 0.72 (95% CI 0.65 to 0.80) and 0.73 (95% CI 0.66 to 0.80), respectively (see online supplementary figure S5). Given the higher abundance of the marker Fn in patients with advanced adenoma, we evaluated its performance in differentiating advanced adenoma from controls. The AUC value for the marker Fn was 0.59 (95% CI 0.51 to 0.67), with a sensitivity of 32.7% and specificity of 87.0% (figure 1B, online supplementary figure S4B).
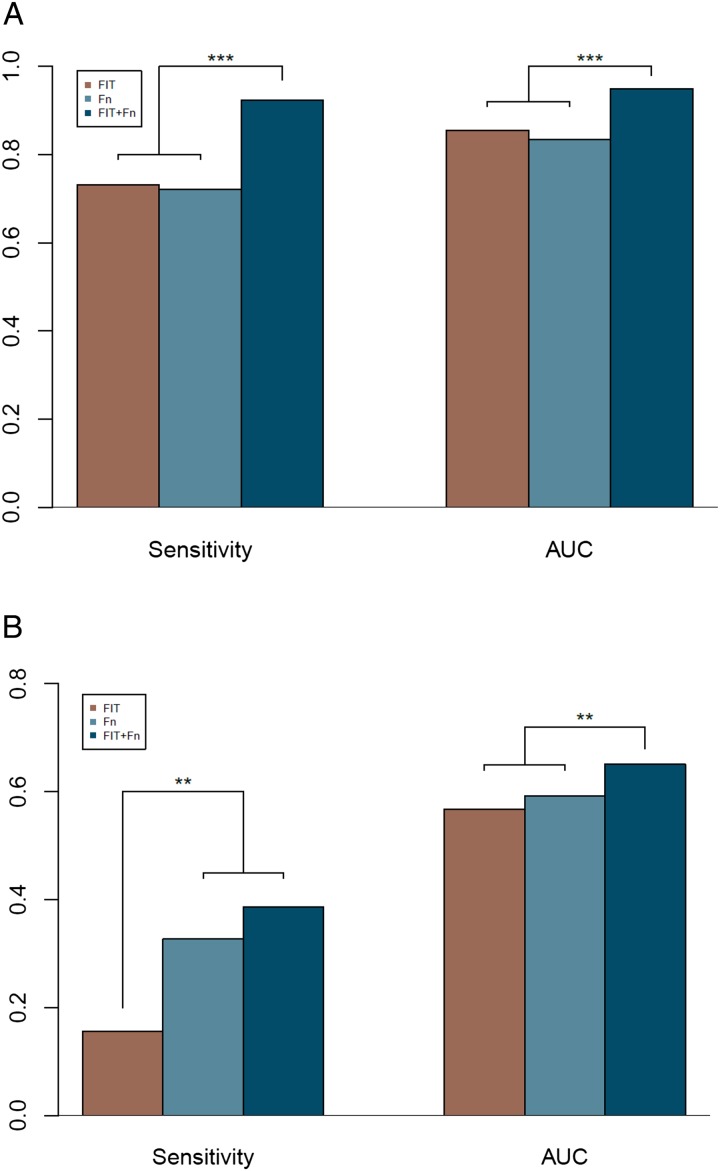
Figure 1.
The diagnostic performance of faecal immunochemical test (FIT), marker Fusobacterium nucleatum (Fn) and their combined test. Sensitivity and area under the receiver-operating characteristic curve (AUC) for diagnosing colorectal cancer (A) and advanced adenoma (B). ***p<0.001.
Combining FIT and microbial markers for diagnosis of CRC
As FIT is the current stool-based screening test of choice recommended by major international guidelines, we evaluated its accuracy in our cohort. At a threshold of 20 µg Hb/g, FIT detected 76 out of the 104 CRC samples. This was equivalent to a sensitivity of 73.1% at a specificity of 98.0% (figure 1A, online supplementary figure S4A).
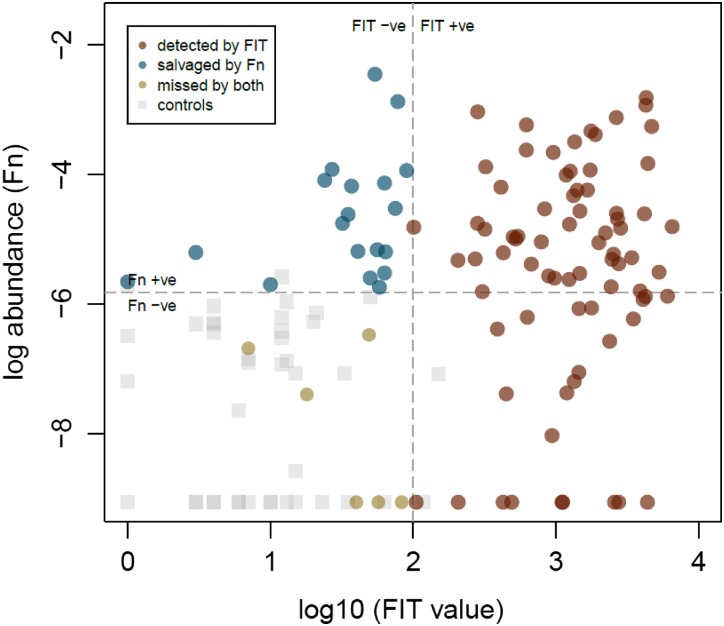
Next, we investigated whether the microbial markers may improve the performance of FIT. Notably, adding the quantitative marker Fn to FIT significantly improved its sensitivity in detecting CRC (92.3% vs 73.1%, p<0.001, table 1, figure 1A), at a threshold of 0.166 for the composite score. The performance of the combined test had an AUC of 0.95 (95% CI 0.92 to 0.98) which was significantly superior to that by FIT alone (AUC=0.86, 95% CI 0.81 to 0.90, p<0.001, table 2, figure 2A). The specificity was 98.0% for FIT and 93.0% for the combined test (p=0.074). The positive and negative predictive values of the combined test were 93.2% and 92.1%, respectively, with a false positive rate of 7.0%. The improved sensitivity translated into an additional detection of 20 CRC samples that had been missed by FIT alone (96/104 vs 76/104, figure 3). Comparing the cross validation result of combining FIT and marker Fn with regression result fitted by FIT, the combined test had much higher AUCs upon testing with 10 000 simulated cross validated AUCs (p<0.001).
Table 1.
The sensitivities and specificities of faecal immunochemical test (FIT), and the combined FIT and marker Fusobacterium nucleatum (Fn) test, for colorectal cancer and advanced adenoma
| FIT |
FIT+Fn
|
Comparison | |||||
|---|---|---|---|---|---|---|---|
| Findings | No. | Sensitivity (%) | Specificity (%) | No. | Sensitivity (%) | Specificity (%) | p Value |
| Colorectal cancer (n=104) | 76 | 73.1 (64.4–81.8) | 98.0 (95.1–100) | 96 | 92.3 (86.5–97.1) | 93.0 (88.0–97.0) | <0.001 |
| TNM stage | |||||||
| Stage 1 (n=22) | 15 | 68.2 (50.0–86.4) | Same as above | 19 | 86.4 (72.7–100.0) | Same as above | |
| Stage 2 (n=31) | 23 | 74.2 (58.1–90.3) | 29 | 93.6 (83.9–100.0) | |||
| Stage 3 (n=39) | 30 | 76.9 (64.1–89.7) | 37 | 94.9 (87.2–100.0) | |||
| Stage 4 (n=12) | 8 | 66.7 (41.7–91.7) | 11 | 91.7 (75.0–100.0) | |||
| Location | |||||||
| Proximal (n=28) | 23 | 82.1 (67.9–96.4) | Same as above | 26 | 92.9 (82.1–100.0) | Same as above | |
| Distal (n=76) | 53 | 69.7 (59.2–80.3) | 70 | 92.1 (85.5–97.4) | |||
| Advanced adenoma (n=103) | 16 | 15.5 (8.7–22.3) | 98.0 (95.1–100) | 39 | 38.6 (28.7–48.5) | 89.0 (83.0–95.0) | 0.007 |
Test performance was compared using the one-sided Delong's test to test for incremental gain in area under the receiver-operating characteristic curve (AUC).
Table 2.
Diagnostic performance of faecal immunochemical test (FIT), marker Fusobacterium nucleatum (Fn) and the combined test for colorectal cancer (CRC) and advanced adenoma
| Marker/AUC | Discovery cohort | Validation cohort | All samples |
|---|---|---|---|
| CRC model | |||
| FIT | 0.86 (0.81–0.90) | 0.85 (0.76–0.94) | 0.85 (0.81–0.89) |
| Fn | 0.83 (0.78–0.89) | 0.89 (0.80–0.98) | 0.85 (0.80–0.90) |
| FIT+Fn | 0.95 (0.92–0.98) | 0.96 (0.92–0.99) | 0.95 (0.92–0.98) |
| (FIT+Fn) vs FIT | p<0.001 | p=0.0014 | p<0.001 |
| Advanced adenoma model | |||
| FIT | 0.57 (0.53–0.61) | 0.56 (0.51–0.61) | 0.56 (0.53–0.59) |
| Fn | 0.59 (0.51–0.67) | 0.58 (0.49–0.67) | 0.59 (0.53–0.65) |
| FIT+Fn | 0.65 (0.58–0.73) | 0.63 (0.55–0.72) | 0.65 (0.59–0.70) |
| (FIT+Fn) vs FIT | p=0.007 | p=0.031 | p<0.001 |
The AUC values of the discovery cohort, the validation cohort and the combined cohort were shown, fitting the logistic regression model from the discovery cohort. The one-sided Delong's test was used to test for incremental gain in AUC for the combined test over FIT.
AUC, area under the receiver-operating characteristic curve.
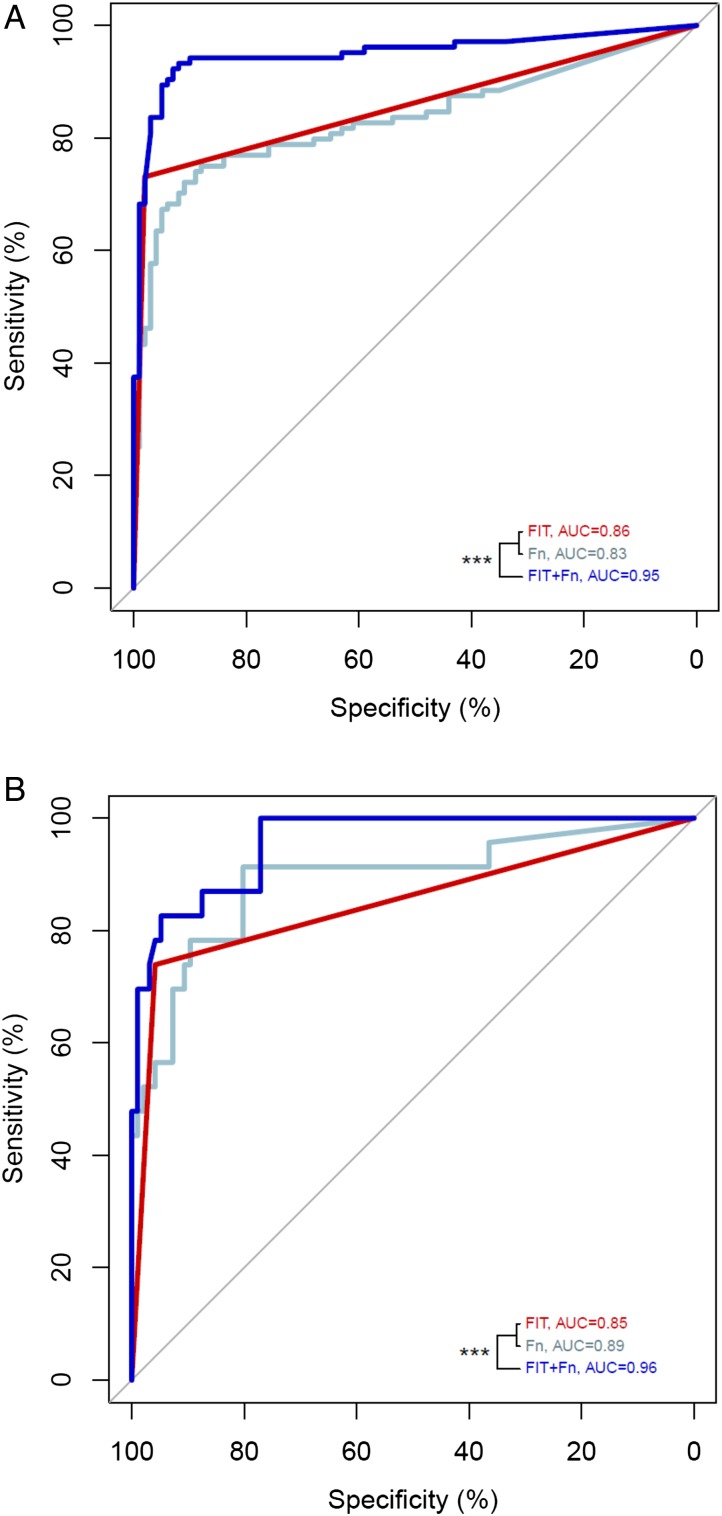
Figure 2.
The diagnostic performance of faecal immunochemical test (FIT), marker Fusobacterium nucleatum (Fn) and their combined test indicated by the receiver operating characteristic (ROC) curve analysis for colorectal cancer in the discovery (A) and validation (B) cohorts. ***p<0.001. AUC, area under the receiver-operating characteristic curve.
Figure 3.
The colorectal cancer samples detected by faecal immunochemical test (FIT) (red), missed by FIT and detected by marker Fusobacterium nucleatum (Fn) (blue), and missed by both tests (yellow). The dotted lines indicate the threshold of the individual test above which samples are regarded as positive.
Addition of the quantitative markers Pa and Pm to FIT individually improved its diagnostic performance (p<0.001 and p=0.026, respectively); nevertheless, their performances (AUC=0.92 and 0.89) were less than that of combined FIT and marker Fn. Further addition of the markers Pa and Pm did not improve performance of the combined FIT and marker Fn test (p=0.334, online supplementary figure S4A and table S2).
Relations with clinical stage and tumour location
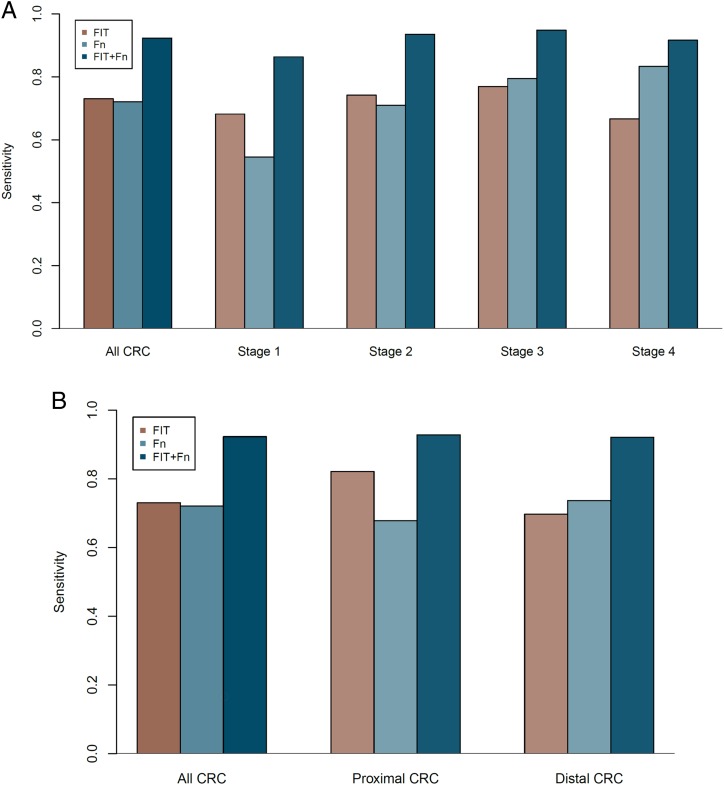
We sought to understand the performance of the markers in relation to the CRC stage and tumour location. The sensitivities for the combined FIT and marker Fn test were 86.4%, 93.6%, 94.9% and 91.7%, respectively, for stage 1–4 cancers. Despite nominally higher values for stage 2 and stage 3 cancers, analysis of the contingency tables showed no statistically significant difference between the CRC stages and detection rates of the combined test (p=0.707) (table 1, figure 4A).
Figure 4.
The sensitivities of faecal immunochemical test (FIT), marker Fusobacterium nucleatum (Fn) and the combined test stratified by clinical stage (A), and location of the colorectal cancer (CRC) (B).
Given the increasing proportion of proximal neoplasia, especially in the Asian population, it is important to assess the performance of markers for such lesions. The detection rates for proximal and distal tumours were similar for the marker Fn (68.2% vs 71.0%, p=0.793). Furthermore, the detection rates of the combined FIT and marker Fn test were similar between proximal and distal tumours (92.9% vs 92.1%, p=1.00, table 1, figure 4B).
Combining FIT and faecal Fusobacterium for diagnosis of advanced adenoma
As FIT is known to have a low sensitivity for advanced adenoma in the colorectum, we investigated whether adding microbial markers could improve its performance in detecting advanced adenoma. This is plausible given the higher relative abundance of the marker Fn in patients with advanced adenoma. Similar to the previously reported detection rates, FIT alone could detect only 16 advanced adenoma samples with a sensitivity of 15.5% and an AUC value of 0.57 (95% CI 0.53 to 0.61, online supplementary figure S6A). Addition of the marker Fn detected more than twice of the advanced adenoma samples (39/103, table 1, online supplementary figure S7), increasing the sensitivity to 38.6% at a specificity of 89.0%. This corresponded to a false positive rate of 11.0% at a threshold of 0.464 for the composite score. The AUC value of the combined test was increased to 0.65 (95% CI 0.58 to 0.73, p=0.007, online supplementary figure S6A and table S3). The combined model was cross validated, and it significantly improved the AUC while fitting all the data in the discovery cohort.
Validation in an independent cohort
To validate the association and performance of the marker Fn, its relative abundance was determined together with FIT in an independent cohort of 181 individuals. This testing set includes 23 patients with CRC, 62 patients with advanced adenoma and 96 controls. The associations of the marker Fn with CRC and advanced adenoma were replicated with higher abundance over the control group (p<0.001 and p=0.036, respectively, online supplementary figure S8). Consistent with the discovery cohort, the marker Fn has a comparable performance with FIT in diagnosing CRC with an AUC of 0.89 in the validation cohort (see online supplementary table S4). Addition of the marker Fn to FIT in the validation cohort significantly improved its AUC from 0.85 (95% CI 0.74 to 0.94) for FIT alone to 0.96 (95% CI 0.92 to 0.99) for the combined test (p=0.0014, table 2, figure 2B). The specificity was 94.8% corresponding to a false positive rate of 5.2%. As for the diagnosis of advanced adenoma, addition of the marker Fn to FIT increased the AUC from 0.55 to 0.63 in the validation cohort (p=0.031, online supplementary table S4 and figure S6B). Together with the superior cross validated AUCs for the combined test model (0.93 and 0.57 for CRC and advanced adenoma, respectively), these results suggested that this model of combining FIT with the marker Fn was robust across different cohorts for both CRC and advanced adenoma diagnoses. Combining all data with a total of 490 individuals, the AUCs of the combined test were 0.95 (95% CI 0.92 to 0.98) and 0.65 (95% CI 0.59 to 0.70) for CRC and advanced adenoma, respectively (table 2, online supplementary table S4).
Discussion
In this study, we have evaluated the performance of three microbial markers in differentiating patients with CRC and advanced adenoma from controls. Our results showed promise of using these markers for disease diagnosis, with a complementary role to FIT as the currently most accepted stool-based test. These markers were selected from three bacteria that were among the most significantly associated, and formed a co-occurrence network in the CRC microbiota.9 10 We observed consistent associations in all three markers, and identified Fn as a key marker that outperformed the other two. This is consistent with the increasing evidence for the bacterium's functional role in colorectal carcinogenesis,14–16 and extends the potential utility of this marker from patient prognosis22 to cancer diagnosis.
The most salient finding of this study is the significant detection gain upon addition of microbial markers to standard commercial FIT. Although faecal quantification of Fusobacterium alone had a comparable performance with that of FIT (AUC=0.83 vs 0.86, p>0.05), the most prominent gain was observed when the marker Fn was added to the FIT, resulting in a detection leap from 73.1% to 92.3% without significantly sacrificing its specificity. This translated into an additional detection of 20 cases which would have been missed by FIT alone, with a false positive rate of 7% (ie, specificity of 93.0%) comparable to most reported values for FIT.6 This combinational approach can lead to better diagnostic performance,23 and has been used in the multitarget stool DNA test which combines FIT with several molecular assays.24 Biologically, as some CRC or advanced adenoma may bleed minimally or intermittently, supplementing FIT with another molecular marker appears to be a logical approach to enhance the detection rate.
Another finding of this study is the increased sensitivity to detect advanced adenoma. Sensitivity is the most important characteristic for a screening test, as its primary role is to pick up samples for further diagnostic testing. This is a challenge to the standard FIT, as it is known to be insensitive in detecting advanced adenoma.7 8 This is exemplified by the suboptimal AUC of 0.57 at a threshold of 20 µg Hb/g. Adding the microbial marker Fn can increase the sensitivity of FIT for detecting advanced adenoma. This is supported by previous metagenomic studies, showing evidence of microbial dysbiosis with a higher Fusobacterium abundance in colorectal adenoma.10 25 Nevertheless, such microbial change in adenoma appears to be less prominent compared with CRC. This has resulted in a modest increase in Fusobacterium abundance and its diagnostic performance in the advanced adenoma group. Although we have used a statically robust method for both internal and external validations, further studies are required to test its performance across different populations.
We observed that the FIT sensitivities for CRC and advanced adenoma were at a lower end compared with some previous studies.6 7 We hypothesise several possible reasons. First, we used a threshold of 20 μg Hb/g (FIT100) instead of a lower value. Although this may have led to a lower sensitivity (73.1%) with a higher specificity (98%), we kept this value as recommended by the manufacturer. If we were to use lower cut-off values, the sensitivities for CRC and advanced adenoma would be 83.7% and 20.4% for a threshold of 10 μg Hb/g (FIT50), or 91.3% and 27.2% for a threshold of 5 μg Hb/g (FIT25). Importantly, addition of the marker Fn to FIT at these lower thresholds still outperformed than stand-alone FIT in classifying CRC (p<0.001 at both thresholds) and advanced adenoma (p=0.002 and p=0.008, respectively). Second, in the meta-analysis by Lee et al, studies using colonoscopy as the reference standard had a lower sensitivity (71%) compared with studies using longitudinal follow-up (87%). Our observed sensitivity of FIT (73.1%) is similar to the pooled estimate of 71% the meta-analysis of studies in which colonoscopy was the reference standard.
One advantage of this study is the use of qPCR to quantitate Fusobacterium as a key marker. This simple approach will enhance the applicability and clinical utility of the finding. As with our previous study9 and other discovery cohorts,11 12 26 exploratory studies often require high throughput sequencing technologies resulting in numerous markers for further validation. Previous CRC metagenomic studies have reported between 11 to 22 markers for classifying CRC from controls.9 11 12 Although feasible technically, it would be inexpedient to evaluate so many markers for clinical use. The high cost may also render the test impractical for countries with poor resources, especially in Asia which harbours 60% of the world's population.27 28 In this study, we have selected three most discriminative in CRC. These markers should have contributed most to the classification model, although further increment in performance is still possible with combination of other microbial markers. Furthermore, the high sensitivities of the combined test for early stage and proximal CRCs (86.4% and 92.9%, respectively) support its potential utility as a screening test, especially in the Asian populations where there is an increasing proportion of proximal lesions.27 29
In addition, the cost of the screening test is an important factor to consider when it is used as a screening modality for population-based programmes. A FIT kit costs an average of US$26.30 While the commercial multitarget stool DNA costs over US$600 and may not be cost-effective for a screening setting,31 the addition of a single marker Fn may substantially reduce the cost. Hence, an incremental cost-utility analysis, taking into account the higher cost yet enhanced performance should be performed, so as to inform clinicians and policy makers. Besides, the affordability and acceptability of patients and physicians will need to be explored in future studies.
In conclusion, this study identifies faecal Fusobacterium as a useful biomarker for detecting CRC and advanced adenoma. Quantification of this marker is key to improving the diagnostic performance of FIT, as further addition of two microbial markers does not further augment the accuracy. As this study uses a retrospective case-control design, more work is necessary to evaluate the effectiveness of this marker in an average risk population of appropriate age, sex and demographics. Furthermore, this study has not evaluated the microbial markers in other non-neoplastic colorectal diseases, such as diverticulosis or IBDs which may affect the microbiota and thus the performance of the markers. Nevertheless, this relatively simple approach to add a single microbial marker will enhance the clinical applicability. This study takes one step further towards a non-invasive, potentially more accurate and affordable diagnosis of advanced colorectal neoplasia.
Footnotes
Contributors: Study concept and design: SHW, WKKW, JJYS, JY; acquisition of data: TNYK, T-CC, AKCL; analysis and interpretation of data: SHW, TNYK, RZWD, GN; drafting of the manuscript: SHW; critical revision of the manuscript for important intellectual content: JJYS, JY, WKKW, JCYW, FKLC, SSMN, MCSW, SCN, TYTL, LZ; statistical analysis: SHW, RZWD, GN.
Funding: This project was supported by the National Basic Research Program of China (973 Program, 2013CB531401), National Key Technology R&D Program (2014BAI09B05), the Shenzhen Virtual University Park Support Scheme, the Shenzhen Science and Technology Programme, and the Croucher Foundation. SHW is supported by the Croucher Foundation. The study sponsor has no role in the study design, data collection, analysis and interpretation of the data.
Competing interests: None declared.
Patient consent: Obtained.
Ethics approval: The Joint Chinese University of Hong Kong—New Territories East Cluster Clinical Research Ethics Committee.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Ferlay J, Shin HR, Bray F, et al. . Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 2010;127:2893–917. 10.1002/ijc.25516 [DOI] [PubMed] [Google Scholar]
- 2. Schoen RE, Pinsky PF, Weissfeld JL, et al. . Colorectal-cancer incidence and mortality with screening flexible sigmoidoscopy. N Engl J Med 2012;366:2345–57. 10.1056/NEJMoa1114635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Atkin WS, Edwards R, Kralj-Hans I, et al. . Once-only flexible sigmoidoscopy screening in prevention of colorectal cancer: a multicentre randomised controlled trial. Lancet 2010;375:1624–33. 10.1016/S0140-6736(10)60551-X [DOI] [PubMed] [Google Scholar]
- 4. Sung JJ, Ng SC, Chan FK, et al. . An updated Asia Pacific Consensus Recommendations on colorectal cancer screening. Gut 2015;64:121–32. 10.1136/gutjnl-2013-306503 [DOI] [PubMed] [Google Scholar]
- 5. Rex DK, Johnson DA, Anderson JC, et al. . American College of Gastroenterology guidelines for colorectal cancer screening 2009. Am J Gastroenterol 2009;104:739–50. 10.1038/ajg.2009.104 [DOI] [PubMed] [Google Scholar]
- 6. Lee JK, Liles EG, Bent S, et al. . Accuracy of fecal immunochemical tests for colorectal cancer: systematic review and meta-analysis. Ann Intern Med 2014;160:171 10.7326/M13-1484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hundt S, Haug U, Brenner H. Comparative evaluation of immunochemical fecal occult blood tests for colorectal adenoma detection. Ann Intern Med 2009;150:162–9. 10.7326/0003-4819-150-3-200902030-00005 [DOI] [PubMed] [Google Scholar]
- 8. Haug U, Hundt S, Brenner H. Quantitative immunochemical fecal occult blood testing for colorectal adenoma detection: evaluation in the target population of screening and comparison with qualitative tests. Am J Gastroenterol 2010;105:682–90. 10.1038/ajg.2009.668 [DOI] [PubMed] [Google Scholar]
- 9. Yu J, Feng Q, Wong SH, et al. . Metagenomic analysis of faecal microbiome as a tool towards targeted non-invasive biomarkers for colorectal cancer. Gut Published Online First: 25 Sep 2015. 10.1136/gutjnl-2015-309800 10.1136/gutjnl-2015-309800 [DOI] [PubMed] [Google Scholar]
- 10. Nakatsu G, Li X, Zhou H, et al. . Gut mucosal microbiome across stages of colorectal carcinogenesis. Nat Commun 2015;6:8727 10.1038/ncomms9727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zeller G, Tap J, Voigt AY, et al. . Potential of fecal microbiota for early-stage detection of colorectal cancer. Mol Syst Biol 2014;10:766 10.15252/msb.20145645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Feng Q, Liang S, Jia H, et al. . Gut microbiome development along the colorectal adenoma-carcinoma sequence. Nat Commun 2015;6:6528 10.1038/ncomms7528 [DOI] [PubMed] [Google Scholar]
- 13. Zackular JP, Rogers MA, Ruffin MTt, et al. . The human gut microbiome as a screening tool for colorectal cancer. Cancer Prev Res (Phila) 2014;7:1112–21. 10.1158/1940-6207.CAPR-14-0129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kostic AD, Chun E, Robertson L, et al. . Fusobacterium nucleatum potentiates intestinal tumorigenesis and modulates the tumor-immune microenvironment. Cell Host Microbe 2013;14:207–15. 10.1016/j.chom.2013.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rubinstein MR, Wang X, Liu W, et al. . Fusobacterium nucleatum promotes colorectal carcinogenesis by modulating E-cadherin/beta-catenin signaling via its FadA adhesin. Cell Host Microb 2013;14:195–206. 10.1016/j.chom.2013.07.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Abed J, Emgård JE, Zamir G, et al. . Fap2 mediates Fusobacterium nucleatum colorectal adenocarcinoma enrichment by binding to tumor-expressed Gal-GalNAc. Cell Host Microbe 2016;20:215–25. 10.1016/j.chom.2016.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wu CW, Ng SC, Dong Y, et al. . Identification of microRNA-135b in stool as a potential noninvasive biomarker for colorectal cancer and adenoma. Clin Cancer Res 2014;20:2994–3002. 10.1158/1078-0432.CCR-13-1750 [DOI] [PubMed] [Google Scholar]
- 18. Rex DK, Petrini JL, Baron TH, et al. . Quality indicators for colonoscopy. Gastrointest Endosc 2006;63:S16–28. 10.1016/j.gie.2006.02.021 [DOI] [PubMed] [Google Scholar]
- 19. Castellarin M, Warren RL, Freeman JD, et al. . Fusobacterium nucleatum infection is prevalent in human colorectal carcinoma. Genome Res 2012;22:299–306. 10.1101/gr.126516.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fraser CG, Allison JE, Young GP, et al. . A standard for Faecal Immunochemical TesTs for haemoglobin evaluation reporting (FITTER). Ann Clin Biochem 2014;51:301–2. 10.1177/0004563213514392 [DOI] [PubMed] [Google Scholar]
- 21. Huang Y, Pepe MS, Feng Z. Logistic regression analysis with standardized markers. Ann Appl Stat 2013;7:1640–62. 10.1214/13-AOAS634SUPP [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mima K, Nishihara R, Qian ZR, et al. . Fusobacterium nucleatum in colorectal carcinoma tissue and patient prognosis. Gut Published Online First: 26 Aug 2015. 10.1136/gutjnl-2015-310101 10.1136/gutjnl-2015-310101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Etzioni R, Kooperberg C, Pepe M, et al. . Combining biomarkers to detect disease with application to prostate cancer. Biostatistics 2003;4:523–38. 10.1093/biostatistics/4.4.523 [DOI] [PubMed] [Google Scholar]
- 24. Imperiale TF, Ransohoff DF, Itzkowitz SH, et al. . Multitarget stool DNA testing for colorectal-cancer screening. N Engl J Med 2014;371:187–8. 10.1056/NEJMc1405215 [DOI] [PubMed] [Google Scholar]
- 25. McCoy AN, Araújo-Pérez F, Azcárate-Peril A, et al. . Fusobacterium is associated with colorectal adenomas. PLoS ONE 2013;8:e53653 10.1371/journal.pone.0053653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Baxter NT, Ruffin MTt, Rogers MA, et al. . Microbiota-based model improves the sensitivity of fecal immunochemical test for detecting colonic lesions. Genome Med 2016;8:37 10.1186/s13073-016-0290-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sung JJ, Lau JY, Goh KL, et al. . Increasing incidence of colorectal cancer in Asia: implications for screening. Lancet Oncol 2005;6:871–6. 10.1016/S1470-2045(05)70422-8 [DOI] [PubMed] [Google Scholar]
- 28. Ng SC, Wong SH. Colorectal cancer screening in Asia. Br Med Bull 2013;105:29–42. 10.1093/bmb/lds040 [DOI] [PubMed] [Google Scholar]
- 29. Sung JJ, Chan FK, Leung WK, et al. . Screening for colorectal cancer in Chinese: comparison of fecal occult blood test, flexible sigmoidoscopy, and colonoscopy. Gastroenterology 2003;124:608–14. 10.1053/gast.2003.50090 [DOI] [PubMed] [Google Scholar]
- 30. Wong MC, Ching JY, Chan VC, et al. . The comparative cost-effectiveness of colorectal cancer screening using faecal immunochemical test vs. colonoscopy. Sci Rep 2015;5:13568 10.1038/srep13568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ladabaum U, Mannalithara A. Comparative effectiveness and cost effectiveness of a multitarget stool DNA test to screen for colorectal neoplasia. Gastroenterology 2016;151:427–39.e6. 10.1053/j.gastro.2016.06.003 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
gutjnl-2016-312766supp001.pdf (483.2KB, pdf)