Dear Sir,
Primary sclerosing cholangitis (PSC) is a liver disease often associated with IBD.1 Dysbiosis of the gut microbiota is implicated in PSC aetiology in adults,2–4 but less is known about paediatric-onset PSC.
We analysed the faecal microbiota of 27 Japanese patients with paediatric-onset PSC as well as 16 age-matched patients with UC and 23 healthy controls (HCs) (see online supplementary table S1) with pyrosequencing data of 16S rRNA gene V1-V2 region (accession #DRA004773). We assessed the influence of medications on the gut microbiota and found that salazosulfapyridine (SASP) treatment affected the microbiota structure with significant changes in the abundance of six major genera between the treated and untreated patients with PSC, perhaps due to its bactericidal property (see online supplementary figure S1). We thus report the analysis of 13 patients with PSC and 15 patients with UC and 23 HCs, all SASP untreated (see online supplementary table S2).
gutjnl-2016-312533supp001.pdf (141.2KB, pdf)
gutjnl-2016-312533supp002.pdf (192.7KB, pdf)
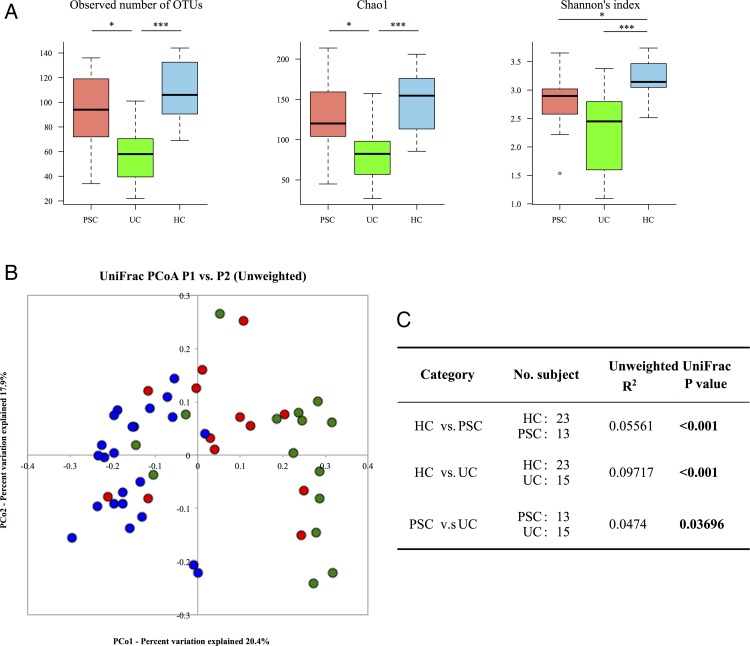
Clustering analysis of the 16S reads revealed that the microbiota in the PSC and HC groups had significantly high species richness compared with the UC group, and the species richness of PSC samples was lower than that of HCs. The PSC group also exhibited an intermediate trend in the Shannon's index between the UC and HC groups (figure 1A). The unweighted UniFrac metric revealed a significant difference in the overall microbiota structure among the three groups, in which the PSC samples tended to aggregate between the HC and UC samples (figure 1B, C). Collectively, the data suggested that the patients with paediatric-onset PSC had gut microbial dysbiosis, the degree of which was less than that of the patients with UC. Dysbiosis in PSC appears to be common regardless of age and the host's genetic background.2 3
Figure 1.
Comparison of the overall faecal microbiota structure among the primary sclerosing cholangitis (PSC), UC and healthy control (HC) groups. (A) Microbial richness and α-diversity in the faecal microbiota of 13 PSC and 15 UC patients without salazosulfapyridine (SASP) treatment and 23 HCs. Richness was evaluated by the observed and the Chao 1-estimated Operational Taxonomic Unit (OTU) numbers generated from clustering of 3000 16S reads per sample, and diversity is based on Shannon's index. Kruskal-Wallis test followed by Steel-Dwass test was used for multiple comparisons (*p<0.05, **p<0.01, ***p<0.001). (B) Principal coordinate analysis (PCoA) based on the unweighted UniFrac analysis of bacterial community structures of the PSC (red), UC (green), and HC (blue) groups. (C) Evaluation of dissimilarity between two groups by permutational multivariate analysis of variance (PERMANOVA). R2 indicates the coefficient of determination. Significant p values are in bold.
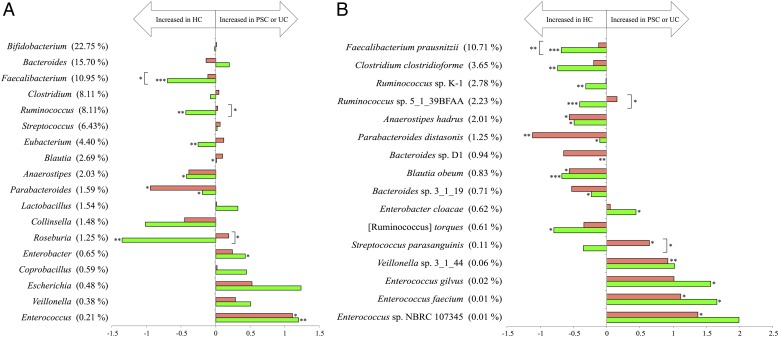
The comparison of microbial abundance identified nine genera showing significant changes among the three groups (figure 2A). The abundance of Parabacteroides and Enterococcus was significantly decreased and increased in the PSC and UC groups compared with HCs, respectively. Over-representation of the Enterococcus genus in adult PSC was also reported.3 The other seven genera showed significant changes in abundance only between the UC and HC groups. However, the abundance of Faecalibacterium, Ruminococcus and Roseburia was significantly higher in the PSC than UC group. Since these three genera include many species producing an anti-inflammatory butyrate, there might be a different degree of inflammatory states between PSC and UC.1 At the species level, we identified 16 species showing significant changes in abundance among the three groups (figure 2B). The significant reduction of Anaerostipes hadrus, Parabacteroides distasonis and Blautia obeum was observed in both the PSC and UC groups, whereas the other seven species were significantly reduced only in the UC group. Regarding the increased species in the PSC group, the abundance of Streptococcus parasanguinis, Veillonella sp. 3_1_44, Enterococcus faecium, and Enterococcus sp. NBRC 107345 was significantly increased in the PSC compared with HCs. Of them, only S. parasanguinis showed a significant enrichment in the PSC compared with UC group. It was also reported that the Streptococcus genus tended to be more abundant in PSC than HC.3 The significant enrichment of Veillonella sp. 3_1_44 in the present PSC group is similar to that of the previous report on adult PSC.2 A concurrent proliferation of the Velillonella and Streptococcus species might involve immunomodulation mediated by symbiotic interactions between these two species in PSC.5 Overall, our data suggest that several distinct species belonging to the genus Enterococcus, Streptococcus and Veillonella are associated with the pathogenicity of paediatric-onset PSC.
Figure 2.
Comparison of the microbial abundance among the primary sclerosing cholangitis (PSC), UC and healthy control (HC) groups. (A) The fold changes of PSC/HC and UC/HC of 18 dominant genera showing >0.5% mean abundance in the three groups, accounting for 90.3% of the total reads. (B) The fold changes of PSC/HC and UC/HC of 16 significant species showing >0.2% mean abundance in the three groups, accounting for 20.6% of the total reads. The fold change was calculated from dividing the mean relative abundance of each genus and species in the PSC and UC groups by that in the HCs, respectively. The mean abundance (%) in the HCs is shown in parentheses. Horizontal axis: the fold change displayed in log10. Since the abundance of Bacteroides sp. D1 in the UC group was ‘zero’, the fold change was not shown. Red and green bars indicate the fold change of PSC/HC and UC/HC, respectively. Significance was determined by the Kruskal-Wallis test followed by the Steel-Dwass test for multiple comparisons (*p<0.05, **p<0.01, ***p<0.001).
Further studies may confirm the PSC-associated bacteria observed here, and the influence of differences in medications as described here, disease duration, nationalities and methodologies on the gut microbiota should be considered.
Acknowledgments
We thank Dr K. Oshima, Dr K. Takanashi, Y. Hattori, E. Iioka, M. Kiuchi, K. Komiya, R. Kurokawa, C. Shindo, R. Takayasu, N. Yamashita (The University of Tokyo), Dr M. Takahata (BIOBANK, Ltd.), Y. Noguchi (Azabu University) and I. Mimura (Okayama University) for their technical support.
Footnotes
Joint last coauthorship: TS and MH contributed equally.
Contributors: KI, WS, HM, TS and MH planned the study. KI, TT, MO-K, SU, AI, TF and TS contributed to the collection of samples and clinical data. KI, WS, HM and MH contributed to the collection and analysis of the primary sequence data of faecal samples. KI and WS intensely contributed to the statistical analysis of the data. KI wrote the first draft, and WS, TS and MH contributed to the completion of the manuscript. All authors read, critically revised for important intellectual content and approved the final manuscript.
Funding: This study was supported by Health Labor Science Research Grants from Research on Measures for Intractable Diseases; the Intractable Hepato-Biliary Diseases Study Group in Japan; the Global COE Project of the ‘Genome Information Big Bang’ from the Ministry of Education, Culture, Sports, Science and Technology (MEXT) of Japan to M.H; and an administration grant from the University of Tokyo to the Laboratory of Metagenomics.
Competing interests: TS reports personal fees from Ootsuka Pharmaceutical outside the submitted work. AI and TF report personal fees from Astellas Pharma and MSD outside the submitted work.
Ethics approval: This study was approved by the ethical committees of Saiseikai Yokohamashi Tobu Hospital, Azabu University, Waseda University, and the University of Tokyo, and signed informed consent was obtained from all subjects who provided specimens.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Hirschfield GM, Karlsen TH, Lindor KD, et al. Primary sclerosing cholangitis. Lancet 2013;382:1587–99. 10.1016/S0140-6736(13)60096-3 [DOI] [PubMed] [Google Scholar]
- 2. Kummen M, Holm K, Anmarkrud JA, et al. The gut microbial profile in patients with primary sclerosing cholangitis is distinct from patients with ulcerative colitis without biliary disease and healthy controls. Gut Published Online First: 17 Feb 2016. doi: 10.1136/gutjnl-2015-310500 10.1136/gutjnl-2015-310500 [DOI] [PubMed] [Google Scholar]
- 3. Sabino J, Vieira-Silva S, Machiels K, et al. Primary sclerosing cholangitis is characterised by intestinal dysbiosis independent from IBD. Gut 2016;65:1681–9. 10.1136/gutjnl-2015-311004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ruhlemann MC, Heinsen FA, Zenouzi R, et al. Faecal microbiota profiles as diagnostic biomarkers in primary sclerosing cholangitis. Gut Published Online First: 23 May 2016. doi: 10.1136/gutjnl-2016-312180 10.1136/gutjnl-2016-312180 [DOI] [PubMed] [Google Scholar]
- 5. van den Bogert B, Meijerink M, Zoetendal EG, et al. Immunomodulatory properties of Streptococcus and Veillonella isolates from the human small intestine microbiota. PLoS ONE 2014;9:e114277 10.1371/journal.pone.0114277 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
gutjnl-2016-312533supp001.pdf (141.2KB, pdf)
gutjnl-2016-312533supp002.pdf (192.7KB, pdf)