Abstract
Background
Mixing patterns of human populations play a crucial role in shaping the spreading paths of infectious diseases. The diffusion of mobile and wearable devices able to record close proximity interactions represents a great opportunity for gathering detailed data on social interactions and mixing patterns in human populations. The aim of this study is to investigate how social interactions are affected by the onset of symptomatic conditions and to what extent the heterogeneity in human behavior can reflect a different risk of infection.
Methods
We study the relation between individuals’ social behavior and the onset of different symptoms, by making use of data collected in 2009 among students sharing a dormitory in a North America university campus. The dataset combines Bluetooth proximity records between study participants with self-reported daily records on their health state. Specifically, we investigate whether individuals’ social activity significantly changes during different symptomatic conditions, including those defining Influenza-like illness, and highlight to what extent possible heterogeneities in social behaviors among individuals with similar age and daily routines may be responsible for a different risk of infection for influenza.
Results
Our results suggest that symptoms associated with Influenza-like illness can be responsible of a reduction of about 40% in the average duration of contacts and of 30% in the daily time spent in social interactions, possibly driven by the onset of fever. However, differences in the number of daily contacts were found to be not statistically significant. In addition, we found that individuals who experienced clinical influenza during the study period were characterized by a significantly higher social activity. In particular, both the number of person-to-person contacts and the time spent in social interactions emerged as significant risk factors for influenza infection.
Conclusions
Our findings highlight that Influenza-like illness can remarkably reduce the social activity of individuals and strengthen the idea that the heterogeneity in social habits among individuals can significantly contribute in shaping differences among the individuals’ risk of infection.
Electronic supplementary material
The online version of this article (doi:10.1186/s12879-017-2623-2) contains supplementary material, which is available to authorized users.
Keywords: Mixing patterns, Time-varying networks, Risk factor, Bluetooth proximity, Influenza-like illness
Background
Mixing patterns of human populations play a crucial role in shaping the spreading paths of infectious diseases [1–3]. Technological advances and the diffusion of mobile and wearable devices, such as smartphones and radio-frequency identification (RFID) sensor systems, are enhancing our ability of gathering high-resolution data on proximity and face-to-face interactions [4–9]. Recently, the usage of wireless sensor network technologies and, more in general, of devices capable of sensing spatial proximity over different scales have been proposed to investigate how the community structure of contacts can affect the transmission dynamics of infectious diseases [10]. This type of data has also been used to simulate the potential spread of the infection within small but strongly connected communities, such as schools [6, 11], during a conference [12] and within hospitals [13–16].
Contact patterns in human populations are characterized by a high variability driven by working and schooling activities, individuals’ age and socio-demographic characteristics such as the household composition and the site of residence. Nonetheless, remarkable changes in individual social behavior may occur over time and social activity levels may be different among individuals characterized by similar age and daily routines. The proposed analysis focuses on a relatively small cluster of individuals representing a strongly connected community (e.g. hanging out and sleeping at the same places). The aim of this study is to investigate how social interactions are affected by the onset of symptomatic conditions and to what extent the heterogeneity in human behavior can reflect a different risk of infection. To do this, we make use of records about the presence of specific symptoms combined with proximity data collected between January and March 2009 among undergraduate students that share a dormitory in a campus of a major university in North America [17]. We define different measures of individuals’ social activity level and a dynamic contact network between study participants, based on Bluetooth proximity records. We perform a statistical analysis on whether or not the individuals’ time spent in social interactions, and the number and duration of daily contacts, significantly changes while experiencing different types of symptomatic conditions, including those defining Influenza-like illness. Finally, we investigate to what extent possible heterogeneities in individuals’ social activity level might be responsible of a significantly different risk of infection, providing some insights on features of social behavior that can be considered as risk factors for the onset of clinical influenza.
Methods
Proximity data and symptoms records
The proposed analysis is based on a dataset collected as part of a longitudinal study carried out between the 10th of January and the 10th of March 2009 including 74 undergraduate students uniformly distributed among four academic years and representing the 80% of the total residents of a dormitory at the campus of a major university in North America [17]. Study participants were asked to daily self report the presence of specific symptoms, and were equipped with a smartphone incorporating a mobile sensing software designed for recording physical proximity between involved individuals during the study period. The software scanned for Bluetooth wireless devices in proximity every 6 min, a compromise between sensing short-term social interactions and battery life [5]. During the same period of time, study participants daily reported whether or not they were experiencing the following symptoms: i) fever, ii) sore throat and cough, iii) runny nose, congestion or sneezing, iv) nausea, vomiting or diarrhea. The study had IRB approval by the Massachusetts Institute of Technology Committee on the Use of Humans as Experimental Subjects (MIT COUHES) and written informed consent from participants. More details are available in [17]. The datasets used in this article can be found in the Additional file 1: S1 and in [18].
Individual social activity and dynamic proximity network
Connections between two individuals were defined as potential interactions between two study participants (e.g. verbal or physical contacts) that may have occurred as a consequence of their physical proximity. No cut-off values on the time spent in physical proximity were considered to define potential individuals’ interactions. A time-varying network between study participants was obtained by computing time dependent adjacency matrices C(t), in which each entry C j,i(t) defines the amount of time spent by individual i in physical proximity with individual j at each time step t. Connections reported in the study are undirected, therefore resulting adjacency matrices are symmetrical (i.e., C i,j = C j,j for any t). The intensity of social activity within the network considered was investigated by aggregating the time spent in proximity interactions between study participants within the same day. Specifically, the latter was computed as the product of the duration and counts of time slots in which physical proximity between individuals was recorded by the Bluetooth devices. Such aggregation has been shown to be a good approximation to analyze the temporal variation of a dynamic network characterizing interactions within a given population [12]. Proximity records defining the relative intensity of connections between study participants over time are therefore used to derive a set of proxy measures of the number and duration of contacts between individuals and of the overall time spent in social interactions. The network defined by proximity records associated with study participants is considered as a representative case study of the heterogeneity of social interactions occurring within a small but strongly connected cluster of individuals.
The relationship between symptoms and human behavior
Possible changes in human behavior triggered by symptomatic episodes were assessed by considering individuals who experienced a given symptom and by comparing their number and duration of social interactions during days in which the symptom was reported with respect to the other days. In order to highlight which are the features of social behavior that may influence the individual risk of infection we analyze the social activity level, characterizing individuals who reported symptoms compliant with an Influenza-like illness with respect to who did not. In this case, a Wilcoxon test was performed to assess the statistical significance (p-value <0.05) of differences detected across these two mutually exclusive groups of individuals. Finally, we investigate the potential transmission events occurred among study participants who could have experienced clinical influenza, by modeling contacts between individuals as driven by the dynamic network defined by Bluetooth proximity records. Possible epidemiological links were identified by considering the onset of symptoms associated with Influenza-like illness [19–21] and on the basis of estimates on the duration of the incubation period and the serial interval coming from the analysis of the 2009 H1N1 pandemic in UK (see Fig. 1) [22]. More details on this analysis can be found in the Additional file 2: S2.
Fig. 1.
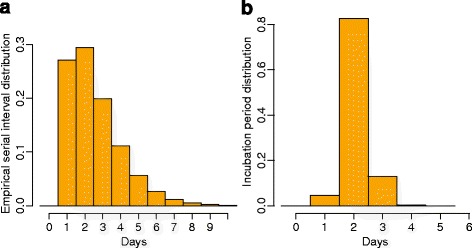
Probability density functions associated with the empirical serial interval (a) and the duration of the incubation period (b) of influenza, as estimated in [22]
Results
Individual social activity
The network of contacts defined by all connections between the study participants recorded from January 10th to March 10th can be classified as a connected mesh, where all pairs of nodes x, y in the network are connected through a path defined by social interactions between study participants that leads from x to y. Each study participant was found at least once in physical proximity with at least one other study participant. Furthermore, about 90% of study participants recorded contacts with more than 40 individuals, corresponding to more than 50% of the considered individuals (see Fig. 2a). However, when the dynamic proximity network is considered, it emerges that, on average, a study participant had contact with 10 other individuals per day (sd 6.12, see Fig. 2b), spending on average 5.18 min per contact per day (sd 2.98 see Fig. 2c) and 1.29 h per day in social interactions with individuals sleeping at the dormitory (sd 0.91 see Fig. 2d). The daily number of contacts resulted strongly correlated with both the daily time spent in social interactions (Pearson correlation coefficient results 0.89 with p-value <0.0001) and the average duration of contacts (Pearson correlation coefficient results 0.56 with p-value <0.0001). This confirms, as already observed by Cattuto et al. [9], the association between number and intensity of connections suggesting that both these measures can be considered as representative of individual social behaviors defining super-connectors and, in turn, potential super-spreaders.
Fig. 2.
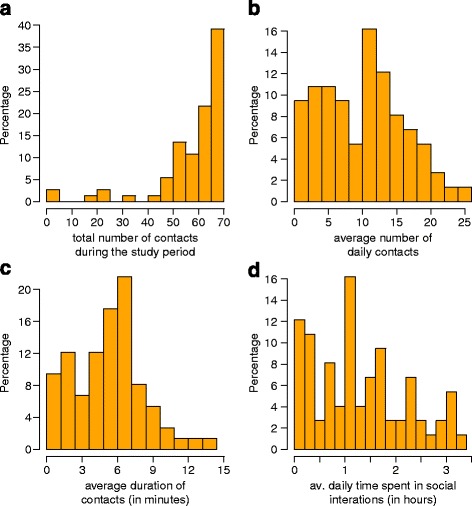
Distribution of a the total number of contacts during the study period, b the average number of contacts per day, c the average duration of contacts, d the average time spent in social interaction per day, according to physical proximity records collected during the study period
Symptomatic episodes and influenza-like illness
Among the 74 considered individuals, 45 reported sore throat and cough, 18 fever, 56 running nose, congestion or sneezing, 28 nausea, vomiting or diarrhea. 15 individuals reported symptoms that can be classified as Influenza-like illness (ILI) according with definitions proposed by the World Health Organization (WHO), the European Centre for Disease Prevention and Control (ECDC), and the Centers for Disease Prevention and Control (CDC) [19–21]. The first ILI case was reported on January 13th, while the last one was recorded on March 4th. The number of consecutive days during which individuals reported an ILI ranges between 1 and 4 days resulting in an average duration of the symptomatic period of 1.73 days (sd 1.22).
Human behavioral changes triggered by symptoms
Our analysis reveals that changes in the individual average number of daily contacts during days characterized by symptomatic conditions is not statistically significant for any of the symptom considered (see Fig. 3a). However, we found that Influenza-like illness may be responsible of a significant decrease in both the average duration of contacts and the daily time spent in social interactions (see Fig. 3b and c). Specifically, our results show that, during symptomatic conditions associated with Influenza-like illness, the duration of contacts is reduced on average by 39% (Wilcoxon test, p-value = 0.01), and the average daily time spent in social interactions is reduced by 31% on average (Wilcoxon test, p-value = 0.012). Our results suggest that this is mainly driven by the presence of fever, since individual activities significantly changes during days in which fever was recorded, while no remarkable differences can be detected neither when considering symptomatic events such as sore throat and cough, nor when considering runny nose, congestion and sneezing as potential triggers of a behavioral change. Specifically, our results suggest that the average daily time spent in social interactions is reduced on average by 36% as a consequence of fever only (Wilcoxon test, p-value < 0.01), along with a reduction of 29% in the mean duration of contacts (Wilcoxon test, p-value = 0.06). Finally, nausea, vomiting and diarrhea were also found to reduce the time spent in social interaction by 17% (p-value = 0.045), although such symptoms do not have a significant influence neither on the duration of each contact nor on the average number of daily contacts.
Fig. 3.
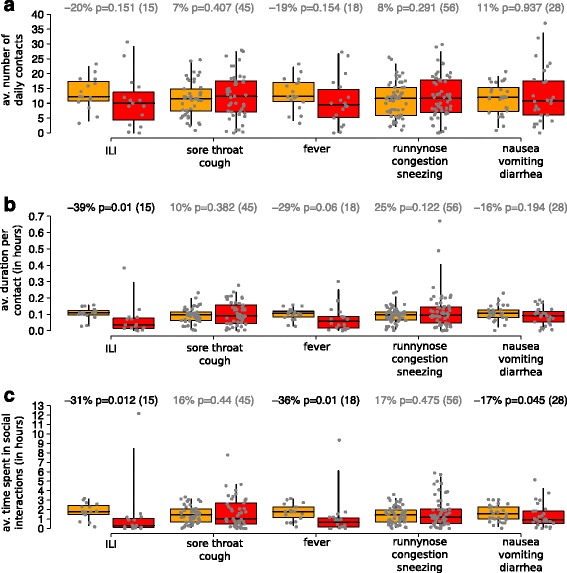
Distribution (2.5, 25, 50, 75, 97.5%) of a the average number of contacts per day of individuals who reported symptoms during days in which symptoms were reported (in red) and during days in which symptoms were not reported (orange); b as a) but for the average duration of contacts; c as a) but for the average daily time spent in social interaction. Percentage of change in social behavior triggered by different symptoms are shown on the top of each panel, along with the p-value corresponding to the performed paired Wilcoxon test and sample sizes associated with each symptomatic condition
Individual social interactions and potential risk factors for influenza
In order to give some insights on how social behaviors can impact the risk of infection, we investigate differences in behavior between the study participants who reported an Influenza-like illness with respect to those who did not reported symptoms compliant with clinical influenza. As shown in Fig. 4, our results suggest that the former group of individuals spent on average 60% more time in social interactions (Wilcoxon test, p-value = 0.006), had 15% more connections with other individuals during the whole study period (Wilcoxon test, p-value = 0.002), had 46% larger number of contacts per day (Wilcoxon test, p-value = 0.016) lasting on average 30% longer (Wilcoxon test, p-value < 0.027).
Fig. 4.
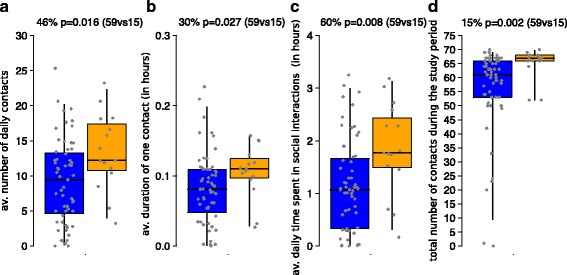
Distribution (2.5, 25, 50, 75, 97.5%) of a the average number of contacts per day recorded for individuals who reported symptoms associated with Influenza-like illness (in orange) and for individuals who did not (blue); b as a) but for the average duration of contacts; c as a) but for the daily time spent in social interactions; d as a) but for total number of contacts during the whole study period. Differences in social behavior associated with ILI cases are shown on the top of each panel, along with the p-value corresponding to the performed Wilcoxon test and the sample of the two mutually exclusive groups considered
Epidemiological links associated with influenza-like illness
The investigation on possible influenza transmission events occurred among study participants shows that, among 15 individuals reporting symptoms compliant with clinical influenza, 7 could be classified as isolated symptomatic cases. More specifically, by considering potential transmission events caused by individuals’ physical proximity with observed clinical influenza cases, at least 7 separate chains of transmission were identified. This means that the time series of symptoms reported by study participants is not compliant with the hypothesis that a unique epidemic occurred within the network of study participants, exclusively sustained by symptomatic infections. Such inconsistency does not emerge when a fully connected network is considered. More details can be found in the Additional file 2: S2.
Discussion
Our results show that symptoms associated with influenza can be responsible of a statistically significant reduction of both the average duration per contact (about 40% less) and the daily time spent in social interactions (about 30% less), possibly driven by the presence of fever. Changes in the daily number of contacts, triggered by symptoms associated with Influenza-like illness, were instead found not statistically significant. These change in the type of individuals’ interactions are particularly of interest given that the duration of contacts has been suggested to play a crucial role in shaping the transmission dynamics of infectious diseases [12, 14].
Our analysis reveals that a remarkable heterogeneity in terms of social behaviors can characterize individuals with similar age and daily routines. Interestingly, we found that individuals who experienced Influenza-like illness were characterized by a significantly higher social activity with respect to study participants who did not. In particular, a high number of person-to-person contacts and a large amount of time spent in proximity interactions can be considered as representative measures of human behavior defining super-connectors and super-spreaders and therefore suggested as potential risk factors for influenza infection.
Given that the onset of specific symptoms can reduce individuals’ social interactions, silent transmission though asymptomatic (and more active) individuals may contribute to spread the infection in the population. The presence of influenza viral shedding in patients with influenza who have very few or no symptoms has been recently confirmed [23]. Estimates on the prevalence of influenza asymptomatic carriers (i.e. infected individuals showing no symptoms) and influenza subclinical cases (i.e. symptomatic cases that do not meet the criteria for Influenza-like illness) ranges respectively from 5.2 to 35.5% and from 25.4 to 61.8% [24]. Nonetheless, it has been suggested that “silent spreaders” (i.e., individuals who are infectious while asymptomatic or pre-symptomatic) may be less important in the spread of influenza epidemics than previously thought [25].
The main limitation of this work relies on the small number of study participants considered, and on the highly specific network defined by sampling close proximity every six minutes. Indeed, in our analysis, social interactions of shorter duration and contacts occurred outside the student network were not accounted for. Relevant academic and extra-curricular activities might have not been covered by the study, either because the mobile phones could not be permanently on (e.g. during classes), or because of contacts with people not taking part to the study. As a consequence, any expectation that symptomatic cases of a given infection should be linked in a single chain of transmission may result inappropriate. In particular, the performed analysis on possible epidemiological links associated with Influenza-like illness recorded among study participants during the follow-up period suggests that less than 50% of observed cases can be ascribed to symptomatic infections occurred among study participants, and either asymptomatic cases or contacts with people not taking part to the study might have been important sources of infection. In particular, since no wash out of study participants was observed during the period under study, the spread of influenza among the considered individuals may have occurred either through infections generated outside the considered network of contacts or through transmission from asymptomatic infectious cases. This critical aspect does not emerge when a fully connected network is considered therefore strengthening the idea that, as suggested by Isella et al. [26] and Stehlé et al. [12], static aggregated networks are not suitable for the investigation of transmission chains within small groups of individuals, and that the assumption of homogeneous mixing within strongly connected communities could lead to erroneous epidemiological interpretations of the underlying transmission paths.
Although social connections recorded during the study period provide only partial information on individuals’ social behavior in the underlying community, the dormitory may still represent the preferential place where students live, cook and sleep [17] and it is likely that individuals who reported a significant higher social activity level with other study participants have been characterized by similar social behaviors in the general community.
Conclusions
In this work we used data of 74 undergraduate students, collected in a dormitory of a North America campus during the 2009. The data include both physical proximity records obtained by Bluetooth sensors on individuals’ smartphones and self-reported surveys about the presence of specific symptoms. The carried out analysis shows that symptomatic conditions associated with Influenza-like illness can remarkably reduce the social activity of individuals. In particular, our findings suggest that changes in contact patterns caused by influenza are mainly driven by the onset of fever and mainly affect the duration of individuals’ contacts, rather than the number of individuals with whom they have social interactions. Obtained results highlight that, as already observed by Obadia et al. [12] and Smieszek et al. [27], the heterogeneity in social habits among individuals can significantly contribute in shaping differences among the individuals’ risk of infection. Interestingly, we found that, beyond the well-known role played by age in shaping individuals’ contact patterns [1, 11, 14], remarkable differences in social behavior can also emerge among individuals characterized by similar daily routines. Finally, our study suggests that transmission by asymptomatic cases can be crucial to sustain the spread of the infection in small groups and may contribute to a significant fraction of cases in the general population. The potential role played by silent influenza transmission claims for new studies aimed at investigating the probability of developing symptoms and the differential infectiousness between asymptomatic and symptomatic cases.
Additional files
Data on proximity and symptoms records. (TXT 21495 kb)
Supplementary Text. (TXT 82 kb)
Acknowledgments
The authors would like to thank Wen Dong (SUNY Buffalo) for his help and constructive feedback on the dataset used in the study.
Funding
The research leading to these results has received funding from the European Research Council under the European Union’s Seventh Framework Programme (FP7/2007–2013)/ERC Grant agreement 283,955 (DECIDE) to PP. The work of SM was funded partly by the EU Cimplex Grant agreement n. 641,191 under the H2020 Framework programme. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Availability of data and materials
The datasets used in this article can be found in the Additional Files and in [18].
Abbreviations
- CDC
Centers for disease control and prevention
- ECDC
European centre for disease control and prevention
- H1N1
Influenza A (H1N1) virus
- ILI
Influenza-like Illness
- RFID
Radio-frequency Identification
- UK
United Kingdom
- WHO
World Health Organization
Authors’ contributions
PP, BL, SM participated in the conception and design of the study. PP and RV performed the statistical analysis and drafted the first version of the manuscript. PP, RV, BL and SM contributed to the analysis and interpretation of obtained results and critically revised the manuscript draft. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The study had IRB approval by the Massachusetts Institute of Technology Committee on the Use of Humans as Experimental Subjects (MIT COUHES) and written informed consent from participants. More details are available in [17].
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Footnotes
Electronic supplementary material
The online version of this article (doi:10.1186/s12879-017-2623-2) contains supplementary material, which is available to authorized users.
References
- 1.Mossong J, Hens N, Jit M, Beutels P, Auranen K, Mikolajczyk R, Massari M, Salmaso S, Scalia Tomba G, Wallinga J, Heijne J, Sadkowska-Todys M, Rosinska M, Edmunds WJ. Social contacts and mixing patterns relevant to the spread of infectious diseases. PLoS Med. 2008;5(3):e74. doi: 10.1371/journal.pmed.0050074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Merler M, Ajelli M. The role of population heterogeneity and human mobility in the spread of pandemic influenza. Proc R Soc B. 2010;277(1681):557–565. doi: 10.1098/rspb.2009.1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ajelli M, Poletti P, Melegaro A, Merler S. The role of different social contexts in shaping influenza transmission during the 2009 pandemic. Sci Rep. 2014;4:7218. doi: 10.1038/srep07218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mastrandrea R, Fournet J, Barrat A. Contact patterns in a high school: a comparison between data collected using wearable sensors, contact diaries and friendship surveys. PLoS One. 2015;10(9):e0136497. doi: 10.1371/journal.pone.0136497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eagle N, Pentland A, Lazer D. Inferring friendship network by using mobile phone data. Proc Natl Acad Sci. 2009;106(36):15274–15278. doi: 10.1073/pnas.0900282106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Salathé M, Kazandjieva M, Woo Lee J, Levis P, Feldman MW, Jones JH. A high-resolution human contact network for infectious disease transmission. Proc Natl Acad Sci. 2010;107(51):22020–22025. doi: 10.1073/pnas.1009094108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Onnela JP, Waber B, Pentland A, Schnorf S, Lazer D. Using Sociometers to quantify social interaction patterns. Sci Rep. 2014;4:5604. doi: 10.1038/srep05604. [DOI] [Google Scholar]
- 8.Salathé M, Bengtsson L, Bodnar TJ, Brewer DD, Brownstein JS, Buchee C, Campbell M, Cattuto C, Khandelwal S, Mabry PL, Vespignani A. Digital Epidemiology. PLoS Comput Biol. 2012;8(7):e1002616. doi: 10.1371/journal.pcbi.1002616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cattuto C, Van den Broeck W, Barrat A, Colizza V, Pinton JF, Vespignani A. Dynamics of person-to-person interactions from distributed RFID sensor networks. PLoS One. 2010;5(7):e11596. doi: 10.1371/journal.pone.0011596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Salathé M, Jones JH. Dynamics and control of diseases in networks with community structure. PLoS Comput Biol. 2010;6(4):e1000736. doi: 10.1371/journal.pcbi.1000736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stehlé J, Voirin N, Barrat A, Cattuto C, Isella L, Pinton JF, Quaggiotto M, Van den Broeck W, Régis C, Lina B, Vanhems P. High resolution measurements of face-to-face contact patterns in a primary school. PLoS One. 2011;6(8):e23176. doi: 10.1371/journal.pone.0023176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stehlé J, Voirin N, Barrat A, Cattuto C, Colizza V, Isella L, Régis C, Pinton JF, Khanafer N, Van den Broeck W, Vanhems P. Simulation of an SEIR infectious disease model on the dynamic contact network of conference attendees. BMC Med. 2011;9:87. doi: 10.1186/1741-7015-9-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Isella L, Romano M, Barrat A, Cattuto C, Colizza V, Van den Broeck W, Gesualdo F, Pandolfi E, Rvà L, Rizzo C, Tozzi AE. Close encounters in a pediatric ward: measuring face-to-face proximity and mixing patterns with wearable sensors. PLoS One. 2011;6(2):e17144. doi: 10.1371/journal.pone.0017144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Machens A, Gesualdo F, Rizzo C, Tozzi AE, Barrat A, Cattuto C. An infectious disease model on empirical networks of human contact: bridging the gap between dynamic network data and contact matrices. BMC Infect Dis. 2013;13:185. doi: 10.1186/1471-2334-13-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vanhems P, Barrat A, Cattuto C, Pinton JF, Khanafer N, Régis C, Kim B, Comte B, Voirin N. Estimating potential infection transmission routes in hospital wards using wearable proximity sensors. PLoS One. 2013;8(9):e73970. doi: 10.1371/journal.pone.0073970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Obadia T, Silhol R, Opatowski L, Temime L, Legrand J, Thiébaut ACM, Herrmann JL, Fleury E, Guillemot D, Boelle PY, I-Bird Study Group Detailed contact data and the dissemination of staphylococcus aureus in hospitals. PLoS Comput Biol. 2015;11(3):e1004170. doi: 10.1371/journal.pcbi.1004170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Madan A, Cebrian M, Moturu S, Farrahi K, Pentland A. Sensing the “health state” of a community. IEEE Pervasive Comput. 2012;11(4):36–45. doi: 10.1109/MPRV.2011.79. [DOI] [Google Scholar]
- 18.Madan A, Chronis I, Pentland A. Social evolution dataset. 2012. [Google Scholar]
- 19.World Health Organization (WHO). WHO surveillance case definitions for ILI and SARI. 2014. Available: http://www.who.int/influenza/surveillance_monitoring/ili_sari_surveillance_case_definition/en/.
- 20.European Center for Disease Prevention and Control. Influenza case definitions. Available: https://ecdc.europa.eu/en/infectious-diseases-public-health/surveillance-and-disease-data/eu-case-definitions.
- 21.Influenzanet. ILI case definitions. Available: https://www.influenzanet.eu/results/?page=help.
- 22.Ghani A, Baguelin M, Griffin J, Flashe S, van Hoek AJ, Cauchemez S, Donnelly C, Robertson C, White M, Truscott J, et al. The early transmission dynamics of H1N1pdm influenza in the United Kingdom. Plos Curr. 2009;1:RRN1130. doi: 10.1371/currents.RRN1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ip DKM, Lau LLH, Leung NHL, Fang VJ, Chan KH, Chu DKW, Leung GM, Peiris SM, Uyeki TM, Cowling BJ. Viral shedding and transmission potential of asymptomatic and Paucisymptomatic influenza virus infections in the community. Clin Infect Dis. 2017;64(6):736–742. doi: 10.1093/cid/ciw841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Furuya-Kanamori L, Cox M, Milinovich GJ, Magalhaes RJS, Mackay IM, Yakob L. Heterogeneous and dynamic prevalence of asymptomatic influenza virus infections. Emerg Infect Dis. 2016;22(6):1052–1056. doi: 10.3201/eid2206.151080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lau LLH, Cowling BJ, Fang VJ, Chan KH, Lau EHY, Lipsitch M, Cheng CKY, Houck M, Uyeki TM, Peiris JSM, Leung GM. Viral shedding and clinical illness in naturally acquired influenza virus infections. J Infect Dis. 2010;201(10):1509–1516. doi: 10.1086/652241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Isella L, Stehlé J, Barrat A, Cattuto C, Pinton JF, Van den Broeck W. What’s in a crowd? Analysis of face-to-face behavioral networks. J Theor Biol. 2011;271(1):166–180. doi: 10.1016/j.jtbi.2010.11.033. [DOI] [PubMed] [Google Scholar]
- 27.Smieszek T, Salathé M. A low-cost method to assess the epidemiological importance of individuals in controlling infectious disease outbreaks. BMC Med. 2013;11(1):35. doi: 10.1186/1741-7015-11-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data on proximity and symptoms records. (TXT 21495 kb)
Supplementary Text. (TXT 82 kb)
Data Availability Statement
The datasets used in this article can be found in the Additional Files and in [18].


