Abstract
During negative energy balance, the concentration of different fatty acids, especially of oleic acid (OA) increases in the follicular fluid of cattle. Previously, we showed that OA induced morphological, physiological and molecular changes in cultured bovine granulosa cells. In our present study we analyzed effects of OA on the expression of markers for granulosa and Sertoli cell identity, FOXL2 and SOX9, respectively, in addition to effects on the FOXL2 regulated genes ESR2, FST, PTGS2 and PPARG. The results showed that OA down-regulated FOXL2, ESR2, FST and PPARG but up-regulated PTGS2 and SOX9. From these data we conclude that OA can compromise granulosa cell functionality and may initiate trans-differentiation processes in bovine granulosa cells. This novel mechanism may be causally involved in postpartum fertility problems of lactating dairy cows.
Keywords: Negative energy balance, PTGS2, ESR2, Cell identity
Introduction
High-yielding dairy cows suffer from negative energy balance after parturition [1, 2]. To meet their energy requirements in spite of low blood glucose levels lactating dairy cows mobilize fat from adipose tissue. Consequently, levels of free fatty acids like palmitic acid (PA, 16:0), stearic acid (SA, 18:0), oleic acid (OA, 18:1) and of β-hydroxybutyric acid increase in the plasma and follicular fluid [3, 4], which can cause decreased milk yield and increased vulnerability to infections, metabolic diseases and sub-fertility. Short-term fasting of cattle also increases the levels of different fatty acids in the follicular fluid and especially of OA [5]. In our previous study, we showed that OA at physiological concentrations remarkably affected the cell morphology, reduced the abundance of functionally important transcripts as FSHR, LHCGR, STAR, CYP11A1, HSD3B1 and CYP19A, and decreased the production of 17-beta estradiol (E2) in cultured bovine granulosa cells (GC). In contrast, the expression of CD36 and SLC27A1, encoding fatty-acid transporters was increased [6]. Sahmi et al. [7] showed that the transcription factor and GC identity marker FOXL2 is vital for higher activity of the CYP19A1 promoter P2 in bovine GC. So we hypothesized that OA may affect the expression of FOXL2 and thus the GC identity. FOXL2 is involved in the regulation of the Sertoli cell marker SOX9 in the ovary at early developmental and adult stages [8–12]. In adult mice the deletion of FOXL2 in GC increases the expression of SOX9 and development of seminiferous tubule-like structures in the ovary [11]. Also the deletion of estrogen receptors or low levels of estrogen resulted in a similar phenotype of GC [12–14], thus demonstrating that the expression of SOX9 in ovaries is normally suppressed via ESR2 (estrogen receptor 2) and E2 along with FOXL2. FOXL2 also positively regulates the expression of ESR2, PPARG and FST, whereas it negatively regulates the expression of PTGS2 [12, 15, 16]. These genes are essentially involved in normal GC function [17–21]. SOX9 is a very early and permanently expressed marker of Sertoli cells [22]. Loss- and gain-of-function studies revealed that SOX9 is required for Sertoli cell differentiation. Deletion of SOX9 in XY gonads leads to male-to-female sex reversal and misexpression in XX mice to female-to-male sex reversal [23, 24], thus demonstrating that SOX9 plays an essential role during testis differentiation processes [25–28]. On the other hand, the loss of the SRY-dependent SOX9 inducibility in bipotential gonadal cells is the earliest sign of pre-granulosa cell differentiation in XX gonads [29].
During the present study we analysed effects of OA in cultured bovine GC on FOXL2 and SOX9, which are essentially involved in the maintenance of granulosa and Sertoli cell identity, respectively. In addition, we also studied effects of OA on other functionally important genes that are regulated by FOXL2 like ESR2, PPARG, FST and PTGS2.
Materials and methods
Serum free bovine granulosa cell culture
As an experimental model GC were aspirated from small to medium sized follicles (2–6 mm) from slaughterhouse material, plated on collagen coated 24-well plates with 1.25 × 105 viable cells and cultured in serum-free α-MEM containing L-Glutamin (2 mM), sodium bicarbonate (0.084%), BSA (0.1%), HEPES (20 mM), sodium selenite (4 ng/ml), transferrin (5 μg/ml), insulin (10 ng/ml), non-essential amino acids (1 mM), penicillin (100 IU) and streptomycin (0.1 mg/ml; Biochrom, Berlin, Germany) with FSH and IGF1 stimulation and androstenedione (2 μM) supplementation (Sigma Aldrich, Steinheim, Germany) at 37 °C in a 5% CO2 atmosphere according to our previous studies [6, 30, 31]. Media were replaced with fresh media including all respective supplements every other day. OA was added from the first change of media (i.e. after 2 days in culture) to the end of culture after 8 days. The amount of added OA (dissolved in ethanol as described previously [6]) in all the experiments was 400 μM, which is in the range of physiological concentrations after fasting [5] and reproducibly affected hormone production and gene expression of cultured GC [6]. As a vehicle control, cells were treated with equal volumes of ethanol corresponding to OA treatment.
RNA isolation, cDNA synthesis and real- time PCR
RNA was isolated with the Nucleo Spin® RNA II Kit according to the manufacturer’s instructions and quantified with a NanoDrop1000 Spectrophotometer (Thermo Scientific, Bonn, Germany). The cDNA was prepared by using the SensiFAST cDNA Synthesis Kit (Bioline, Luckenwalde, Germany) from 200 ng RNA as per the manufacture’s protocol. Transcript abundance of FOXL2, SOX9, ESR2, FST, PPARG and PTGS2 was analyzed as described in our previous studies [6, 31] by real time PCR with SensiFast SYBR No-ROX (Bioline) and gene-specific primers (listed in Table 1) in a Light Cycler 96 instrument (Roche,Mannheim, Germany). The abundance of all transcripts was calculated relative to transcripts of the TATA-binding protein (TBP) as an appropriate housekeeping gene [32].
Table 1.
List of primers used for transcript quantification by qPCR
| Name | Sequence | Size (bp) | NCBI accession no. |
|---|---|---|---|
| ESR2 Forward | GGTCAATCCATCCTACCCCT | 264 | NM_174051.3 |
| ESR2 Reverse | TTCACGCCAAGGACTCTTTT | ||
| FOXL2 Forward | AGCCAAGTTCCCGTTCTACG | 140 | NM_001031750.1 |
| FOXL2 Reverse | GGTCCAGCGTCCAGTAGTTG | ||
| FST Forward | GCACTGGCCGCCTGAGCACCT | 191 | NM_175801 |
| FST Reverse | TGGGGCACAGACGCAGCGGG | ||
| PPARG forward | TATCCCCGGCTTTGTGAACC | 288 | NM_181024.2 |
| PPARG Reverse | GGGCGGTCTCCACTGAGAAT | ||
| PTGS2 Forward | TACAGCACTTGAGTGGCTATCAC | 317 | NM_174445 |
| PTGS2 Reverse | CTGGTCAATTGAAGCCTTTGATAC | ||
| SOX9 Forward | ACCTGGAACTTCAGTGGCG | 147 | XM_010816647.1 |
| SOX9 Reverse | CCAAGTAGGGGAAGGCGAAT | ||
| TBP Forward | GCCTTGTGCTTACCCACCAACAGTTC | 200 | NM_001075742.1 |
| TBP Reverse | TGTCTTCCTGAAACCCTTCAGAATAGGG |
Isolation of soluble cell extracts and immunoblotting
Isolation of soluble cell extracts and immunoblotting for detection of FOXL2 and SOX9 proteins, and of GAPDH as a loading control, was done as described previously [31]. Primary antibodies were from Thermo Fisher Scientific, Dreieich, Germany (FOXL2, Cat# PA1-31950) or Cell Signaling Technology, Frankfurt/main, Germany (SOX9, Cat# CST 7074, GAPDH, Cat# CST 3683), secondary antibodies from Cell Signaling Technology (anti-rabbit IgG, HRP-linked Antibody, Cat# CST) or Santa Cruz, Heidelberg, Germany (donkey anti-goat IgG-HRP, Cat# sc-2020).
Statistical analysis
Data of all experiments were analysed by paired t-test using the GraphPad prism 5.0 software. All experiments were conducted three times independently.
Results
The results showed that OA clearly down regulated the transcript abundance and protein levels of FOXL2, but up regulated those of SOX9 (Fig. 1). As OA inhibited FOXL2 mRNA and protein levels, we analyzed the effects of OA on the expression of genes that are involved in different functions of GC and are regulated by FOXL2 as FST, ESR2, PTGS2 and PPARG [12]. The results showed that OA significantly down regulated the expression of ESR2, PPARG and FST as compared with vehicle treated controls, whereas PTGS2 was increased (Fig. 2).
Fig. 1.
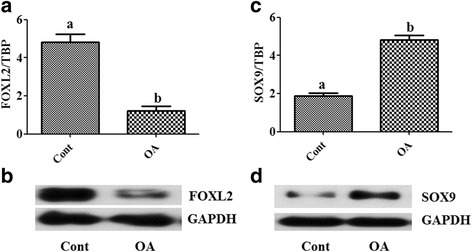
Effects of oleic acid on the expression of the granulosa cell marker FOXL2 and the Sertoli cell marker SOX9 in cultured granulosa cells. Oleic acid (OA) induced down-regulation of FOXL2 mRNA and protein abundance (a and b), respectively, and up-regulation of SOX9 mRNA and protein, respectively (c and d). GAPDH protein is shown as a loading control. Different letters indicate significant differences between treatment groups (mean transcript abundance relative to TBP ± standard error; different letters indicate significantly different means if P < 0.05, unpaired t-test from three independent experiments). Cont denotes vehicle control and OA denotes oleic acid treatment
Fig. 2.
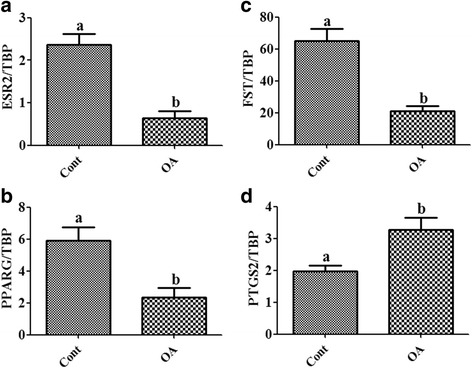
Effect of oleic acid on the expression of FOXL2 regulated genes. (a, b, c and d) OA induced regulation of ESR2, PPARG, FST and PTGS2 transcript abundance levels. Different letters indicate significant differences between treatment groups (mean transcript abundance relative to TBP ± standard error; different letters indicate significantly different means if P < 0.05, unpaired t-test from three independent experiments). Cont denotes vehicle control and OA denotes oleic acid treatment
Discussion
In the present study OA markedly reduced the levels of the transcription factors FOXL2 and ESR2, and increased the expression of SOX9. It is a very well established fact that FOXL2 is involved in maintaining the identity of GC [12]. The deletion of FOXL2 in GC of adult mice resulted in the development of seminiferous tubule-like structures along with the expression of Sertoli-cell marker genes in the ovary [11]. Georges et al. [12] showed that knockdown of FOXL2 or ESR2 leads to a rise of SOX9 levels and in case of a knockdown of both genes, SOX9 levels became even more effectively up-regulated. The same study also showed that knockdown of ESR1 has no clear effect on SOX9 repression following E2 treatment, but ESR2 knockdown resulted in an increase of SOX9 expression, whereas SOX9 expression was markedly increased in the absence of FOXL2. Thus, FOXL2 and ESR2/E2 repress SOX9 expression in follicular GC independent of each other [12]. In addition to this, it has been shown that FOXL2 regulates many genes like FST, PTGES2 and PPARG [12]. Our results showed that OA down regulated the expression of FST and PPARG along with that of FOXL2. The expression of FST in the ovary and GCs is regulated by FOXL2 [8, 16]. Knockdown of FOXL2 leads to decreased expression of PTGS2 and PPARG in murine primary GC [12]. Our results showed that OA induced down-regulation of PPARG along with FOXL2 but not of PTGS2, which was increased by OA treatment. Kim et al. [15] showed that FOXL2 represses the expression of PTGS2, which would be in line with our data. This suggests that OA may up-regulate SOX9 expression by reducing the levels of FOXL2, ESR2 and of E2. The data of our present study demonstrate that the expression of FOXL2, ESR2, PPARG, and FST was clearly decreased. In contrast, the expression of PTGS2 and in particular of SOX9 was increased by OA. Accordingly, we propose that OA induces the Sertoli cell marker SOX9 in GC eventually leading to a partial loss of GC identity, which may severely compromise GC’s functionality.
Conclusions
Our data suggest that (i) OA treatment reduced the expression of FOXL2. (ii) The inhibition of FOXL2 expression in turn may lead to the down-regulation of ESR2, FST and PPARG. (iii) The low levels of FOXL2, ESR2 and of E2 may overcome transcriptional suppression of the Sertoli cell marker SOX9 and thus induce trans-differentiation-like processes, which would severely compromise GC functionality. This novel observation could partly explain the negative effects of increased OA concentrations on post-partum fertility in dairy cows.
Acknowledgments
We thank Veronica Schreiter, Maren Anders, GesineKrüger, Swanhild Rodewald, Ursula Antkewitz and Christian Plinski for excellent technical support during experiments. The publication of this article was funded by the Open Access Fund of the Leibniz Association and the Open Access Fund of the Leibniz Institute for Farm Animal Biology (FBN).
Funding
This study was supported by the core budget of the Leibniz Institute for Farm Animals (FBN).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- CYP19A1
Cytochrome P450, family 19, subfamily A, polypeptide 1
- E2
17-beta estradiol
- ESR2
Estrogen receptor 2
- FOXL2
Forkhead box protein L2
- FSH
Follicle-stimulating hormone
- FST
Follistatin
- GAPDH
Glyceraldehyde-3-phosphate dehydrogenase
- GC
Granulosa cells
- IGF-1
Insulin-like growth factor 1
- PPARG
Peroxisome proliferator-activated receptor gamma
- PTGES2
Prostaglandin E synthase 2
- SOX9
SRY (sex determining region Y)-box 9
- TBP
TATA-binding protein
Authors’ contributions
VRY and JV conceived and designed the experiments, and wrote the paper. VRY performed the experiments. Both authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Contributor Information
Vengala Rao Yenuganti, Email: vengal.ndri@gmail.com.
Jens Vanselow, Email: vanselow@fbn-dummerstorf.de.
References
- 1.Beam SW, Butler WR. Effects of energy balance on follicular development and first ovulation in postpartum dairy cows. J Reprod Fertil Suppl. 1999;54:411–424. [PubMed] [Google Scholar]
- 2.Opsomer G, Grohn YT, Hertl J, Coryn M. Deluyker H, de KA: risk factors for post partum ovarian dysfunction in high producing dairy cows in Belgium: a field study. Theriogenology. 2000;53:841–857. doi: 10.1016/S0093-691X(00)00234-X. [DOI] [PubMed] [Google Scholar]
- 3.Leroy JL, Vanholder T, Mateusen B, Christophe A, Opsomer G, De KA, Genicot G, Van SA. Non-esterified fatty acids in follicular fluid of dairy cows and their effect on developmental capacity of bovine oocytes in vitro. Reproduction. 2005;130:485–495. doi: 10.1530/rep.1.00735. [DOI] [PubMed] [Google Scholar]
- 4.Rukkwamsuk T, Geelen MJ, Kruip TA, Wensing T. Interrelation of fatty acid composition in adipose tissue, serum, and liver of dairy cows during the development of fatty liver postpartum. J Dairy Sci. 2000;83:52–59. doi: 10.3168/jds.S0022-0302(00)74854-5. [DOI] [PubMed] [Google Scholar]
- 5.Aardema H, Lolicato F, van de Lest CH, Brouwers JF, Vaandrager AB, van Tol HT, Roelen BA, Vos PL, Helms JB, Gadella BM. Bovine cumulus cells protect maturing oocytes from increased fatty acid levels by massive intracellular lipid storage. Biol Reprod. 2013;88:164. doi: 10.1095/biolreprod.112.106062. [DOI] [PubMed] [Google Scholar]
- 6.Yenuganti VR, Viergutz T, Vanselow J. Oleic acid induces specific alterations in the morphology, gene expression and steroid hormone production of cultured bovine granulosa cells. Gen Comp Endocrinol. 2016;232:134–144. doi: 10.1016/j.ygcen.2016.04.020. [DOI] [PubMed] [Google Scholar]
- 7.Sahmi F, Nicola ES, Zamberlam GO, Goncalves PD, Vanselow J, Price CA. Factors regulating the bovine, caprine, rat and human ovarian aromatase promoters in a bovine granulosa cell model. Gen Comp Endocrinol. 2014;200:10–17. doi: 10.1016/j.ygcen.2014.02.008. [DOI] [PubMed] [Google Scholar]
- 8.Ottolenghi C, Omari S, Garcia-Ortiz JE, Uda M, Crisponi L, Forabosco A, Pilia G, Schlessinger D. Foxl2 is required for commitment to ovary differentiation. Hum Mol Genet. 2005;14:2053–2062. doi: 10.1093/hmg/ddi210. [DOI] [PubMed] [Google Scholar]
- 9.Ottolenghi C, Pelosi E, Tran J, Colombino M, Douglass E, Nedorezov T, Cao A, Forabosco A, Schlessinger D. Loss of Wnt4 and Foxl2 leads to female-to-male sex reversal extending to germ cells. Hum Mol Genet. 2007;16:2795–2804. doi: 10.1093/hmg/ddm235. [DOI] [PubMed] [Google Scholar]
- 10.Garcia-Ortiz JE, Pelosi E, Omari S, Nedorezov T, Piao Y, Karmazin J, Uda M, Cao A, Cole SW, Forabosco A, et al. Foxl2 functions in sex determination and histogenesis throughout mouse ovary development. BMC Dev Biol. 2009;9:36. doi: 10.1186/1471-213X-9-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Uhlenhaut NH, Jakob S, Anlag K, Eisenberger T, Sekido R, Kress J, Treier AC, Klugmann C, Klasen C, Holter NI, et al. Somatic sex reprogramming of adult ovaries to testes by FOXL2 ablation. Cell. 2009;139:1130–1142. doi: 10.1016/j.cell.2009.11.021. [DOI] [PubMed] [Google Scholar]
- 12.Georges A, L'Hote D, Todeschini AL, Auguste A, Legois B, Zider A, Veitia RA. The transcription factor FOXL2 mobilizes estrogen signaling to maintain the identity of ovarian granulosa cells. elife. 2014;3. doi:10.7554/eLife.04207.:10. [DOI] [PMC free article] [PubMed]
- 13.Dupont S, Krust A, Gansmuller A, Dierich A, Chambon P, Mark M. Effect of single and compound knockouts of estrogen receptors alpha (ERalpha) and beta (ERbeta) on mouse reproductive phenotypes. Development. 2000;127:4277–4291. doi: 10.1242/dev.127.19.4277. [DOI] [PubMed] [Google Scholar]
- 14.Fisher CR, Graves KH, Parlow AF, Simpson ER. Characterization of mice deficient in aromatase (ArKO) because of targeted disruption of the cyp19 gene. Proc Natl Acad Sci U S A. 1998;95:6965–6970. doi: 10.1073/pnas.95.12.6965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim SY, Weiss J, Tong M, Laronda MM, Lee EJ, Jameson JL. Foxl2, a forkhead transcription factor, modulates nonclassical activity of the estrogen receptor-alpha. Endocrinology. 2009;150:5085–5093. doi: 10.1210/en.2009-0313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cheng JC, Chang HM, Qiu X, Fang L, Leung PC. FOXL2-induced follistatin attenuates activin A-stimulated cell proliferation in human granulosa cell tumors. Biochem Biophys Res Commun. 2014;443:537–542. doi: 10.1016/j.bbrc.2013.12.010. [DOI] [PubMed] [Google Scholar]
- 17.Sirois J. Induction of prostaglandin endoperoxide synthase-2 by human chorionic gonadotropin in bovine preovulatory follicles in vivo. Endocrinology. 1994;135:841–848. doi: 10.1210/endo.135.3.8070377. [DOI] [PubMed] [Google Scholar]
- 18.Glister C, Satchell L, Knight PG. Granulosal and thecal expression of bone morphogenetic protein- and activin-binding protein mRNA transcripts during bovine follicle development and factors modulating their expression in vitro. Reproduction. 2011;142:581–591. doi: 10.1530/REP-11-0150. [DOI] [PubMed] [Google Scholar]
- 19.Rosenfeld CS, Yuan X, Manikkam M, Calder MD, Garverick HA, Lubahn DB. Cloning, sequencing, and localization of bovine estrogen receptor-beta within the ovarian follicle. Biol Reprod. 1999;60:691–697. doi: 10.1095/biolreprod60.3.691. [DOI] [PubMed] [Google Scholar]
- 20.Froment P, Fabre S, Dupont J, Pisselet C, Chesneau D, Staels B, Monget P. Expression and functional role of peroxisome proliferator-activated receptor-gamma in ovarian folliculogenesis in the sheep. Biol Reprod. 2003;69:1665–1674. doi: 10.1095/biolreprod.103.017244. [DOI] [PubMed] [Google Scholar]
- 21.Zhang H, Li Q, Lin H, Yang Q, Wang H, Zhu C. Role of PPARgamma and its gonadotrophic regulation in rat ovarian granulosa cells in vitro. Neuro Endocrinol Lett. 2007;28:289–294. [PubMed] [Google Scholar]
- 22.Morais da Silva S, Hacker A, Harley V, Goodfellow P, Swain A, Lovell-Badge R: Sox9 expression during gonadal development implies a conserved role for the gene in testis differentiation in mammals and birds. Nat Genet. 1996;14:62-68. [DOI] [PubMed]
- 23.Jakob S, Lovell-Badge R. Sex determination and the control of Sox9 expression in mammals. FEBS J. 2011;278:1002–1009. doi: 10.1111/j.1742-4658.2011.08029.x. [DOI] [PubMed] [Google Scholar]
- 24.Wilhelm D, Palmer S, Koopman P. Sex determination and gonadal development in mammals. Physiol Rev. 2007;87:1–28. doi: 10.1152/physrev.00009.2006. [DOI] [PubMed] [Google Scholar]
- 25.Kent J, Wheatley SC, Andrews JE, Sinclair AH, Koopman P. A male-specific role for SOX9 in vertebrate sex determination. Development. 1996;122:2813–2822. doi: 10.1242/dev.122.9.2813. [DOI] [PubMed] [Google Scholar]
- 26.Chaboissier MC, Kobayashi A, Vidal VI, Lutzkendorf S, van de Kant HJ, Wegner M, De Rooij DG, Behringer RR, Schedl A. Functional analysis of Sox8 and Sox9 during sex determination in the mouse. Development. 2004;131:1891–1901. doi: 10.1242/dev.01087. [DOI] [PubMed] [Google Scholar]
- 27.Barrionuevo F, Bagheri-Fam S, Klattig J, Kist R, Taketo MM, Englert C, Scherer G. Homozygous inactivation of Sox9 causes complete XY sex reversal in mice. Biol Reprod. 2006;74:195–201. doi: 10.1095/biolreprod.105.045930. [DOI] [PubMed] [Google Scholar]
- 28.Vidal VP, Chaboissier MC, De Rooij DG, Schedl A. Sox9 induces testis development in XX transgenic mice. Nat Genet. 2001;28:216–217. doi: 10.1038/90046. [DOI] [PubMed] [Google Scholar]
- 29.Harikae K, Miura K, Shinomura M, Matoba S, Hiramatsu R, Tsunekawa N, Kanai-Azuma M, Kurohmaru M, Morohashi K, Kanai Y. Heterogeneity in sexual bipotentiality and plasticity of granulosa cells in developing mouse ovaries. J Cell Sci. 2013;126:2834–2844. doi: 10.1242/jcs.122663. [DOI] [PubMed] [Google Scholar]
- 30.Baufeld A, Vanselow J. Increasing cell plating density mimics an early post-LH stage in cultured bovine granulosa cells. Cell Tissue Res. 2013;354:869–880. doi: 10.1007/s00441-013-1712-9. [DOI] [PubMed] [Google Scholar]
- 31.Yenuganti VR, Vanselow J. Cultured bovine granulosa cells rapidly lose important features of their identity and functionality, but partially recover under long term culture conditions. Cell & Tissue Research. 2017. [DOI] [PMC free article] [PubMed]
- 32.Baddela VS, Baufeld A, Yenuganti VR, Vanselow J, Singh D. Suitable housekeeping genes for normalization of transcript abundance analysis by real-time RT-PCR in cultured bovine granulosa cells during hypoxia and differential cell plating density. Reprod Biol Endocrinol. 2014;12:118. doi: 10.1186/1477-7827-12-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.


