Fig. 2.
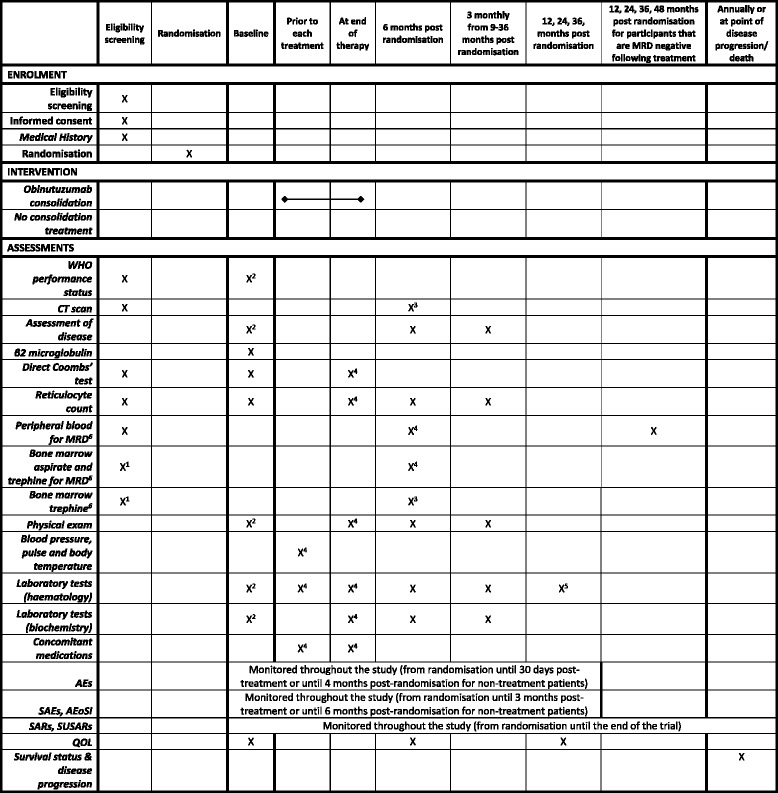
Schedule of enrolment, interventions and assessments for participants who are suitable for randomisation. MRD Minimal residual disease, PFS Progression-free survival. 1To be performed after the analysis of peripheral blood and only in participants whose peripheral blood is MRD positive. 2 To be performed within 4 weeks of randomisation and before treatment is started. 3Only for participants randomised to obinutuzumab and if appropriate clinically. 4Only required for participants randomised to treatment with obinutuzumab. 5Serum immunoglobulins and electrophoresis only. 6Tested Centrally
