Abstract
The human body harbors an enormous number of microbiota that influence cancer susceptibility, in part, via their prodigious metabolic capacity and their profound influence on immune cell function. Microbial pathogens drive tumorigenesis in 15–20% of cancer cases. An even larger number of malignancies are associated with an altered composition of commensal microbiota (dysbiosis) based on microbiome studies utilizing metagenomic sequencing. Although association studies cannot distinguish whether changes in microbiota are causes or effects of cancer, a causative role is supported by rigorously controlled pre-clinical studies utilizing gnotobiotic mouse models colonized with one or more specific bacteria. These studies demonstrate that microbiota can alter cancer susceptibility and progression by diverse mechanisms such as modulating inflammation, inducing DNA damage, and producing metabolites involved in oncogenesis or tumor suppression. Evidence is emerging that microbiota can be manipulated for improving cancer treatment. By incorporating probiotics as adjuvants for checkpoint immunotherapy or by designing small molecules that target microbial enzymes, microbiota can be harnessed to improve cancer care.
Introduction
Cancer is a leading cause of morbidity and mortality with ~1.7 million newly diagnosed cancer cases and ~600,000 cancer deaths this year in the USA alone1. In addition to the tremendous suffering it afflicts, cancer is a significant economic burden with healthcare costs exceeding $125 billion per year in the USA2. In spite of a recent, high-impact report that cancer is primarily stochastic or “bad luck” due to the accumulation of spontaneous mutations during DNA replication in tissues where stem cells undergo a relatively large number of cell divisions3, it is widely believed that the environment significantly influences cancer risk4, 5. Numerous epidemiologic and occupational health studies support the importance of lifestyle factors and exposure to known or suspected carcinogens in the development of cancer. In fact, it is estimated that 15–20% of cancer cases are driven by infectious agents6, 20–30% of cancer cases are largely due to tobacco use, and 30–35% cases are associated with diet, physical activity, and/or energy balance (e.g., obesity)7, 8. Ultraviolet (UV) radiation from sunlight, alcohol, and many other substances (e.g., asbestos, benzene, radon) also play a role, both alone and in combination (i.e., mixed exposures), although relative risk is dependent on the dose and duration of each exposure and the genetic background of each individual.
The microbiota that inhabit our gastrointestinal (GI) tract and other anatomical sites can be considered an environmental factor that we are continuously exposed to at high doses throughout life. The vast majority of these microbes are commensal bacteria, which have been difficult to culture, limiting our understanding until recently. However, during the past decade, the advent of metagenomic sequencing approaches that combine next-generation DNA sequencing technologies with the computational analysis of targeted (16S rRNA hypervariable regions) or whole-genome shotgun sequence reads have documented the diversity and abundance of microbes at different body sites in a culture-independent manner9, 10 (Figure 1A). The complexity of microbiota can be described using α and β diversity as two metrics borrowed from environmental microbial ecology. α diversity describes the richness (i.e., number of organisms and evenness of distribution of those organisms) in a given sample, whereas β diversity defines the extent of absolute or relative overlap in shared taxa between samples11. There is a wide range of microbial β diversity that exists in the microbiota that exists between individuals. Some individuals are enriched for a particular organism, which may be minimally represented in others. The overall community structure, or enterotype, varies between individuals to different extents based on genetics, where each person lives, body mass index, diet, and other environmental and lifestyle factors 12.
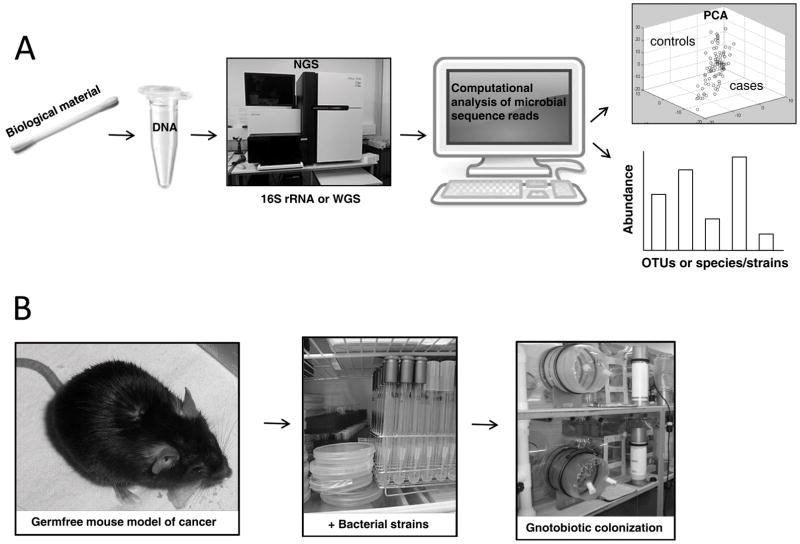
Figure 1. Microbiome research strategy.
(A) Flow chart of metagenomic sequence analysis. Biological material (buccal swabs, fecal samples, tissue biopsies, saliva) are procured from disease cases and healthy controls (panel 1); DNA is prepared from each sample (panel 2); Next-generation DNA sequencing (NGS) is performed to obtain targeted (16S rRNA hypervariable regions) or whole-genome shotgun (WGS) sequence reads (panel 3); Computational assembly and analysis of microbial sequence reads allows the microbial community structure to be assessed for each sample (panel 4); Principal Component Analysis (PCA) is a statistical procedure that compares the degree of relatedness of sequence reads between samples and illustrates the relationship between cases (red circles) and controls (blue circles), which often form distinct clusters with minimal overlap (panel 5 upper). Other computational methods allow the abundance of different microbial taxa to be quantified when compared to databases (panel 5 lower). Analysis of 16S data yields the relative abundance of Operational Taxanomic Units (OTUs) and their phylogenetic relationships. Analysis of WGS data provides greater taxonomic resolution, down to the abundance of specific strains within a single species that vary with respect to gene content including virulence factors and single nucleotide polymorphisms (SNPs), and provides more insight into pathways. WGS provides much more information but is more expensive and computationally intensive with less complete database resources, in part, due to a limited number of reference genomes. Further details can be found in other reviews (e.g., 9, 11). (B) Because a microbiome change between cases and controls can be either a cause or consequence of disease, gnotobiotic mouse models are utilized to evaluate the function of specific microbiota in the host. Germfree mouse models, which were originally obtained via C-section delivery but are now obtained by embryo transfer into germfree surrogate females (panel 1), are colonized by oral gavage (panel 2) with one bacterial strain (monoassociated), a consortium of specific bacteria (polyassociated), or complex microbial communities (e.g., fecal microbiota transplants) while maintained in gnotobiotic isolators (panel 3).
Numerous metagenomic sequencing studies have revealed significant differences in the composition of microbial communities between healthy and diseased individuals (Figure 1A). As a corollary, microbiota have been implicated in causing or preventing a variety of disease states including cancer, and this idea is supported by rigorously controlled experiments utilizing gnotobiotic mouse models colonized with one or more specific bacteria (Figure 1B). There is also emerging evidence that microbiota can be manipulated for the treatment of various disease states including cancer. In this review, we will discuss these topics in the context of cancer prevention and treatment.
The human microbiome
The human body harbors as many microbial cells as all of our somatic and germ cells combined13. Furthermore, the collective genome of our microbiota, referred to as the microbiome, encodes ~100 fold more genes than the human genome14. The vast majority of these microbiota are bacteria that reside within our GI tract, although archaea, viruses, and eukaryotes such as yeast and protozoans are also represented within the GI tract and at other body sites14. Like most other mammals, humans first acquire significant amounts of microbiota from their mother during birth. The composition of microbiota is highly dynamic during the first three years of life and then becomes relatively stable and more adult-like with increased complexity, although many smaller changes constantly occur throughout childhood, adolescence, middle age, and old age.15–19
Host genetics influences the composition of an individual’s microbiome based on twin studies demonstrating that the β diversity of unrelated individuals exceeds that of dizygotic twins, which in turn is more diverse than monozygotic twins20. Not unexpectedly, some taxa are more heritable than others. By considering microbiome composition as a complex trait, genome-wide association studies (GWAS) have begun to map loci in humans and mice21. Some of the human loci associated with microbiome traits are in close proximity to loci having effects on disease risk. Although linkage disequilibrium makes it difficult to distinguish between causative and linked single nucleotide polymorphisms (SNPs), some candidate genes such as the vitamin D receptor are currently being assessed22. However, the overall genetic architecture underlying microbiome traits is complicated with relatively small effects sizes that have been difficult to replicate21. Perhaps this is not surprising considering the large effect that diet and other environmental factors exert, thereby representing “noise” that masks modest genetic effects. To address this constraint, it might be useful to integrate dietary intervention studies and GWAS as exemplified by one recent study demonstrating that only individuals with a specific genotype have a correlation between milk consumption and Bifidobacterium abundance21, 23.
As mentioned above, our diets influence the composition of our microbiota although long-term dietary patterns outweigh short-term changes in diet24, 25. It is not surprising that a particular diet selects for certain microbiota at the expense of others, considering that different taxa of gut microbiota have distinct metabolic capacities. A recent study suggests that certain microbiota can even go extinct26. In this study, mice provided a low-fiber diet underwent microbiome changes that were reversible, consistent with previously published studies. But after providing the low-fiber diet for several successive generations, the maternally transmitted microbiome underwent a progressive loss of diversity, with some taxa becoming undetectable. This finding identifies a trans-generational mechanism mediated by the microbiota, rather than epigenetics, and may be relevant for families that consume much less fiber than is recommended, which is not uncommon in the USA and other industrialized countries. A plethora of other factors affect the microbiome including international travel, infections, and pharmaceuticals27. Subsequent to such changes, or after an infection is resolved, most, but not all, commensal microbiota return to their baseline levels. This type of incomplete recovery complicates risk assessment because a transient event may affect a subset of microbiota in a long-term manner that influences disease risk later in life.
Changes in lifestyles and societal norms influence the microbiome at each stage of life. Vaginal versus cesarean section methods of delivery and breast milk versus formula feeding significantly affects the infant microbiota28. Some of these microbiota differences persist beyond infancy and into adulthood although most do not. Nevertheless, even transient differences in the infant are potentially important because infancy represents a developmental window of susceptibility for a variety of disease states, in part because various cell types (e.g., neurons, lymphocytes) are still developing. This idea is supported by the finding that compositional differences in the microbiota of three-month-old infants were associated with development of asthma later in life22. Based on animal studies, infants and children may be particularly sensitive to low doses of antibiotics in the food supply that can induce obesity via alterations in the microbiota29. These examples of asthma and obesity are related to the hygiene hypothesis, which posits that diminished exposure to microbiota during early childhood impairs immune tolerance, predisposing individuals to allergies and other chronic disease states. Much later in life, the microbiome of the elderly is influenced by lifestyle with individuals living at long-term residential care centers having less diversity than individuals living independently in the community30. These compositional differences are correlated with dietary differences, increased inflammation, and frailty of individuals at long-term residential care centers, but the issue of causation versus correlation has not been addressed.
Despite the preponderance of microbial cells in the human body, they have small, mitochondria-like dimensions and collectively account for only several pounds of each person’s body weight, corresponding to 2–7% of an individual’s biomass excluding water weight. However, our microbiota exert an outsized effect on human biology because of their prodigious metabolic capacity and profound effects on the immune system. The relationship between commensal microbiota and the human host is a complicated one that is largely beneficial but sometimes detrimental to human health. On the one hand, our gut microbiota increase our ability to absorb nutrients and extract calories from our diets. For example, the gut microbiome is highly enriched for genes involved in carbohydrate metabolism including ≥115 families of glycoside hydrolases and ≥21 families of polysaccharide lyases31, 32. There is a dearth of corresponding genes in the human genome due to a lack of selective pressure because mammals (and all animals) and their genomes co-evolved with gut microbiota and the microbiome. Commensal gut microbiota also play a crucial role in the development and homeostasis of the innate and adaptive immune systems. These beneficial functions are contingent on eubiosis, wherein microbiota remain either commensal or symbiotic with their hosts. However, it is difficult to define a standardized, ideal eubiosis due to the enormous population variation, and what is optimal eubiosis in one individual may differ in another. Changes in diet, antibiotic administration, and invasion of pathogens cause variable changes in microbiota composition among different individuals. Nevertheless, an individual’s microbiota remains largely resilient to perturbation and can return to baseline levels over time33. In contrast to eubiosis, there is an altered community structure in various disease states that is referred to as dysbiosis. For example, obesity is associated with an altered ratio of the two dominant phyla of GI bacteria, Bacteroidetes and Firmicutes, and this taxonomic shift increases calorie extraction and adiposity in mice34, 35. Dysbiosis can increase the representation of deleterious microbiota that produce harmful metabolites and antigens leading to maladaptive immune responses. These disturbances are particularly relevant to oncology, considering that deregulated metabolism and inflammation are recognized as hallmarks of cancer36.
Microbial pathogens drive certain cancers
Perhaps the best evidence that microbiota are not passengers or bystanders comes from Helicobacter pylori,, and several oncogenic viruses that drive cancer (Table 1). H. pylori infections are strongly linked to gastric adenocarcinoma, and this is mediated by inflammation with H. pylori–induced gastritis considered a precursor of cancer37. In work that led to the 2005 Nobel Prize in Physiology or Medicine, Dr. Barry Marshall infected himself with H. pylori to fulfill Koch’s postulates and demonstrate that H. pylori is an etiologic agent of gastritis and gastric ulcers37. For this reason, H. pylori is in the process of being exterminated from human populations throughout the world. However, H. pylori protects against Barrett’s esophagus and esophageal adenocarcinoma, possibly by affecting stomach pH and ameliorating acid reflux38, 39. This demonstrates that the relationship between so-called pathogenic microbes and the human host can be considerably more complicated than initially assumed. This is particularly so with bacterial drivers of carcinogenesis. Unlike viruses, which express constitutively active viral mimics of cellular proto-oncogenes40, tumor initiation and progression associated with microbial dysbiosis is a multifactorial event and arises following “multiple hits”. Not all individuals infected with oncogenic microorganisms develop cancer. Genetic heterogeneity in the microbe as well as the host, in addition to environmental factors, determines cancer prevalence and severity. For example, only H. pylori strains containing the cagA virulence factor efficiently trigger gastritis and gastric cancer. Host genetics, which influence the immune response, are another important determinant of whether an infected individual develops cancer. Furthermore, diet and lifestyle factors such as alcohol, tobacco use, and obesity play important roles, and chronic inflammation is believed to be a particularly critical risk factor.
Table 1.
Microbes designated as Class 1 (carcinogens) by the International Agency for Research on Cancer (IARC)6
| Microbe | Site of cancer |
|---|---|
| Helicobacter pylori | Stomach |
| Hepatitis B virus (HBV) Hepatits C virus (HCV) Opisthorchis viverrini Clonorchis sinensis |
Liver |
| Human Papillomavirus (HPV) | Cervix Vagina Vulva Anus Penis Oropharynx |
| Epstein-Barr virus (EBV) | Nasopharynx Non-Hodgkin lymphoma Hodgkin’s lymphoma |
| Kaposi’s sarcoma associated herpesvirus (KSHV or HHV8) | Kaposi’s sarcoma Primary effusion lymphoma |
| Human T-cell lymphotropic virus type 1 (HTLV-1) | Adult T-cell lymphoma |
| Schistosoma haematobium | Bladder |
Metagenomic sequencing studies reveal associations between commensal bacteria and cancer incidence
Microbial pathogens are the etiologic agents for 15–20% of cancer cases, but commensal microbiota have a more widespread influence on the initiation and progression of tumorigenesis. Metagenomic sequencing studies have detected significant differences in the composition of microbial communities in numerous human cancer cases compared to controls (Figure 1A). Many of these studies analyzed fecal samples obtained from colorectal cancer cases and controls, although biopsied tissues, saliva, and other biological materials have been analyzed for multiple types of cancer. Table 2 lists some of the studies that have been published along with cancer type, sampling site, and observed microbiome changes. A central theme arising from these studies is that cancer cases are associated with a dysbiosis that includes a marked decrease in both microbial diversity and community stability. Yet, the observed microbiome differences vary on a case-by-case basis, and usually involve relatively modest quantitative differences in the abundance of specific taxa of bacteria. Although the combined effects in aggregate are believed to be more robust, the relationship between dysbiosis and cancer is nuanced when compared to H. pylori and oncogenic viruses that drive cancer in a highly penetrant manner as discussed in the last section.
Table 2.
A sample of published metagenomic studies analyzing cases and controls
| Type of cancer | Sampling material and site | Conclusion | Findings | Ref | ||
|---|---|---|---|---|---|---|
| Enriched in Cases | Reduced in Cases | Enriched in Controls | ||||
| Colorectal Adenoma | Mucosal adherent bacteria | Higher diversity and richness in cases compared to controls. | Proteobacteria, Dorea spp., Faecalibacterium spp. | Bacteroidetes, Coprococcus spp. | 54 | |
| Colorectal Adenoma | Mucosal adherent bacteria | Higher diversity and richness in cases compared to controls. Similar evenness. | 30 genera including: Acidovorax, Aquabacterium, Cloacibacterium, Helicobacter, Lactococcus, Lactobacillus, Pseudomonas | Streptococcus | 53 | |
| Colorectal Adenoma | Pre-neoplastic colon polyps from African American patients | No statistically significant differences | Slight increases in Proteobacteria (K. pneumoniae, E. coli), Verrucomicrobia, Firmicutes | Bacteroides | Slightly higher abundance of Oscillospira guillermondii, Subdoligranulum | 148 |
| Colorectal Adenoma | Adenomatous tissues | Bifidobacterium sp, Eubacteria | 149 | |||
| Colorectal Tubular Adenoma, Adenocarcinoma | Mucosal adherent bacteria | Dysbiosis in cases compared to healthy controls | Fusobacterium nucleatum, Enterobacteriaceae, Methanobrevibacter (Archaea, Methanobacteriales) | 150 | ||
| Colorectal Adenoma, Carcinoma | Feces | Progressive dysbiosis concurrent with progressive disease | Adenoma: Blautia, Ruminococcus, Clostridium, Lachnospiraceae Carcinoma: Fusobacterium, Bacteroides, Phascolarctobacterium, Porphyromonas. | 151 | ||
| Colorectal Adenoma | Feces | No significant differences; underpowered study confounded by antibiotics treatment | Proteobacteria, TM7 | 152 | ||
| Colorectal Carcinoma | Mucosal tissues | Increased abundance of Fusobacterium | F. nucleatum, F. mortiferum, F. necrophorum | Bacteroidetes, Firmicutes | 43 | |
| Colorectal Adenoma | Feces | Compositional shifts occur in adenomatous tissues that correlate with alterations in bacterial metabolism | Bilophila, Desulfovibrio, Mogibacterium, Bacteroidetes. Streptococcus, Veillonella, Mogibacterium and Sutterella predict presence of adenomatous polyps. | 156 | ||
| Colorectal polyps, carcinoma | Fecal and mucosal samples, from tumor and tumor adjacent regions | Mucosal microbiota differs in cases and controls, particularly if lesion is proximal or distal. Fecal and mucosal microbiota differ in CRC; analyses suggest that microbiota shifts are not secondary to the cancer | Bacteroides, Roseburia, Ruminococcus, Oscillibacter, and oral pathogens: Porphyromonas, Peptostreptococcus, Parvimonas, Fusobacterium. Clusters of coabundance groups: Bacteroidetes cluster 2, Firmictues cluster 2, Pathogen cluster, Prevotella cluster | On mucosa: Clusters of coabundance groups: Bacteroidestes Cluster 1, Firmicutes Cluster 1. In feces: Lachnospiraceae incertae sedis and Coprococcus. | 155 | |
| Breast cancer | Tumor and adjacent normal breast tissue; healthy tissue from controls | Compositional differences between healthy controls and tumor-adjacent tissue from patients. Similar compositional profiles between tumor and tumor-adjacent normal tissue within the same patient. Strains isolated from tumors induced DNA double strand breaks in vitro. | Bacillus, Enterobacteriaceae, Staphylococcus, Comamonadaceae, unclassified Bacteroidetes | Prevotella, Lactococcus, Corynebacterium, Streptococcus, Micrococcus | 153 | |
| Breast cancer | Nipple aspirate fluid (NAF) of survivors and healthy controls | No compositional differences on areolar skin. Ductal microbiota are significantly different between survivors and healthy controls. Microbiota profiles are similar for paired areolar and NAF from the same individual | Alistepes | unclassified Sphingomonadaceae family member | 154 | |
Gut dysbiosis primarily involves shifts in the abundance of commensal bacteria including some that function as opportunistic pathogens. For example, in several studies that compared colorectal tumors to normal adjacent colonic tissues from the same individuals53, 54, the tumor samples had an underrepresentation of the two dominant phyla, Bacteroidetes and Firmicutes, but an overrepresentation of Fusobacterium sp.41–44. Fusobacterium is an invasive anaerobe previously associated with periodontitis and appendicitis, but not cancer. Despite the consistent results that were observed, the overall microbial communities of a tumor and a matched non-cancerous colon sample from one individual were more similar to each other than were tumors or non-cancerous samples from different individuals. This highlights one of the challenges of this approach and supports the idea that the microbiome will be an important factor in precision medicine.
Metagenomic sequencing studies have limitations, however. They are association studies and cannot determine whether a particular microbiota change is a cause or a consequence of cancer. Very few studies are longitudinal and sample the microbiota at different stages of tumorigenesis. In fact, most studies are conducted at a relatively late stage after immune cell infiltration, altered tumor cell metabolism (including hypoxia, and lower pH), and other changes have occurred that increase the likelihood of microbiome changes being secondary to tumorigenesis. In addition, many studies analyze the fecal microbiome, which is different than the mucosal-associated microbiome and less likely to be relevant to disease45. Metagenomic sequencing also does not provide insight into the spatial distribution of microbes including the organization of microbial communities into biofilms, which might be just as important as the composition of the community. For example, colonoscopies have demonstrated that biofilms are present in nearly all right-sided (proximal) colorectal cancer cases compared to 15% of healthy controls 46. Finally, current 16S rRNA-based techniques lack the resolution to detect strain-level differences, including the ability to distinguish between commensal and pathogenic isolates. However, whole-genome shotgun sequencing, coupled with rapidly evolving bioinformatics approaches, can now resolve this limitation47, 48.
Gnotobiotic mouse models demonstrate causality and provide mechanistic insights
To demonstrate the functional importance of microbiota in carcinogenesis, mouse models of cancer maintained germfree (i.e., devoid of all microbiota) in gnotobiotic isolators are colonized with one or more specific bacteria (Figure 1B). For example, human Escherichia coli strains harboring the pks (polyketide synthase) pathogenicity island are enriched in the colonic mucosa of colorectal cancer patients with an incidence of 67% compared to 21% in healthy controls49, 50. To demonstrate that pks plays a causal role in tumorigenesis, IL-10 knockout mice were monoassociated with two strains of E. coli that were either pks+ or Δpks (containing and deleted of pks, respectively) and treated with the pro-carcinogen azoxymethane (AOM) to induce colorectal tumors49. Although both E. coli strains stimulated inflammation to a similar extent, there was a significant difference in tumor progression with all of the tumors in the pks+ group becoming malignant while all of the tumors in the Δpks group remained benign. It was demonstrated that pks, which encodes a genotoxin called colibactin, induces DNA damage in colonocytes based on the γH2AX marker49.
Microbiota can be either oncogenic, as described above, or tumor suppressive as described below. A number of metagenomic sequencing studies have identified a significant enrichment of butyrate-producing bacteria in healthy controls compared to colorectal cancer cases51. Butyrate is a short-chain fatty acid produced by bacterial fermentation of fiber in the colon and has tumor-suppressive properties in colorectal cancer cell lines51. To demonstrate that butyrate is tumor suppressive in vivo, gnotobiotic mice were colonized with a consortium of 4–5 commensal bacteria including the presence or absence of Butyrivibrio fibrisolvens, a prodigious butyrate producer, then provided high- or low-fiber diets, and treated with AOM to induce colorectal tumors52. Only the combination of a high-fiber diet and B. fibrisolvens yielded high levels of butyrate in the lumen and reduced tumor burden, and neither intervention was individually effective. Tumor suppression was attenuated when a mutant B. fibrisolvens strain with diminished butyrate production was introduced. Furthermore, the protective effects of high fiber and B. fibrisolvens were recapitulated by directly providing the mice with a butyrate-fortified diet, confirming this is a bacterial-derived, tumor-suppressive metabolite. Furthermore, Warburg metabolism drove the intratumoral accumulation of butyrate, which functions as a histone deacetylase (HDAC) inhibitor, thus epigenetically regulating genes involved in cell proliferation and apoptosis52. The findings have translational potential by hypothesizing that the conflicting results from prospective-cohort studies that investigate fiber in colorectal prevention could be resolved by evaluating microbiome differences among the participants.
Gnotobiotic mouse models have limitations, as well. Germfree mouse models of cancer can be colonized with complex microbiota (e.g., fecal microbiota transplants from human cases versus controls), but it is often necessary for them to be monoassociated or polyassociated with specific microbiota to identify which microbes influence tumor initiation and progression in the host. Utilization of genetically modified bacterial strains, as described above for E. coli and B. fibrisolvens, is particularly useful for elucidating molecular mechanisms. However, although this reductionist approach is necessary for basic mechanistic studies, the lack of microbial diversity in monoassociated and polyassociated mouse models limits their translational relevance. Gnotobiotic mouse models also do not receive the diverse and varied diets consumed by humans. Furthermore, many gut microbiota are obligate anaerobes that have not yet been cultured, which limits the repertoire of specific bacterial isolates that can be studied. Most human gut bacteria have long been considered unculturable, even under anaerobic conditions, but recent reports suggest that this is not the case and that many previously “unculturable” taxa can, in fact, be cultured55. The prospect of culturing diverse bacteria and modifying their functional outpus using CRISPR-mediated gene editing56 will undoubtedly increase the utility of gnotobiotic mouse cancer models in the future.
Microbial mechanisms of oncogenesis and tumor suppression
Our commensal bacteria influence cancer largely through their metabolic capacity, and their effects on immune cells and inflammation. Therefore, it is not surprising that the gastrointestinal tract has received the most attention and is particularly important. The GI tract is where the vast majority of commensal bacteria reside, and is the primary site of metabolism and nutrient absorption. The GI tract also harbors more immune cells than all other mucosal and lymphoid tissues and is crucial for immune cell development and function. A number of microbial-mediated mechanisms have been elucidated that either promote or inhibit tumorigenesis as depicted in Figures 2 and 3, and described below in the following subsections.
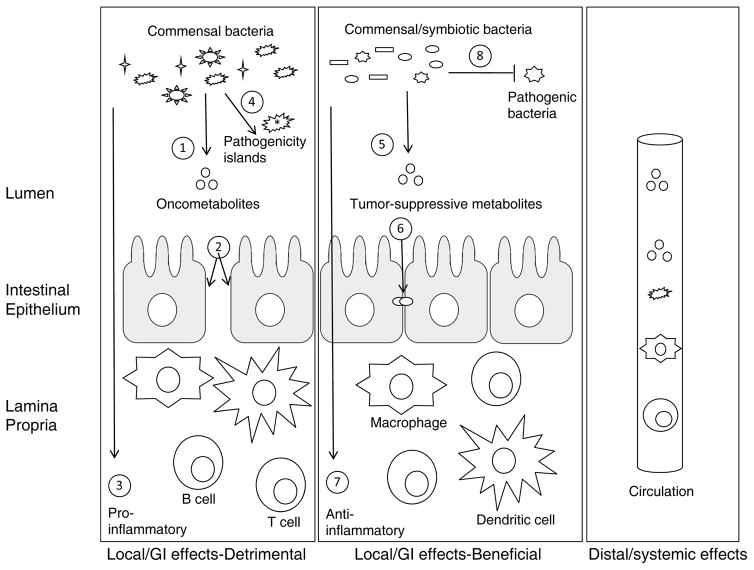
Figure 2. Gut microbiota have differential effects on tumorigenesis in the GI tract and at distant sites.
The colon is depicted with a single layer of intestinal epithelial cells (yellow) separating commensal bacteria (black shapes) in the lumen above from immune cells (4 different colors) in the underlying lamina propria. The bacteria can have local effects that are either oncogenic (left box) or tumor suppressive (center box) for colorectal cancer, or they can have distal effects mediated by the circulation that are oncogenic or tumor suppressive for cancer at other anatomical sites (right box). Some of the general effects that gut microbiota can have on tumorigenesis are numbered. Left box: 1, Production of putative oncometabolites such as hydrogen sulfide; 2, Impairment of barrier function, which increases the exposure of immune cells to bacterial endotoxins (e.g., LPS) and antigens; 3, Direct effects of bacterial metabolites and antigens on immune cells to stimulate inflammation by altering immune cell subsets (e.g., the effect of segmented filamentous bacteria or SFB on TH17 cells) and hyperactivating immune cell responses via pro-inflammatory cytokines (e.g., IL-6); 4, The presence of virulence factors including pathogenicity islands, which distinguish pathogens from commensals such as E. coli pks, can exert multiple effects including the induction of DNA damage and aberrant Wnt signaling. Center box: 5, Production of putative tumor-suppressive metabolites such as butyrate, which functions via multiple mechanisms; 6, Maintenance of barrier function; 7, Direct effects on immune cells to prevent inflammation by altering immune cells subsets (e.g. the ability of butyrate to induce TReg cells) and dampening the immune cell response via immunosuppressive cytokines (e.g., IL-10); 8, Competitive exclusion of pathogenic bacteria similar to the prevention of lethal C. difficile infections. Right box: Gut microbiota can also have oncogenic or tumor-suppressive effects at distal sites in the body via circulation of microbiota, microbial metabolites, activated or suppressed immune cells, and cytokines.
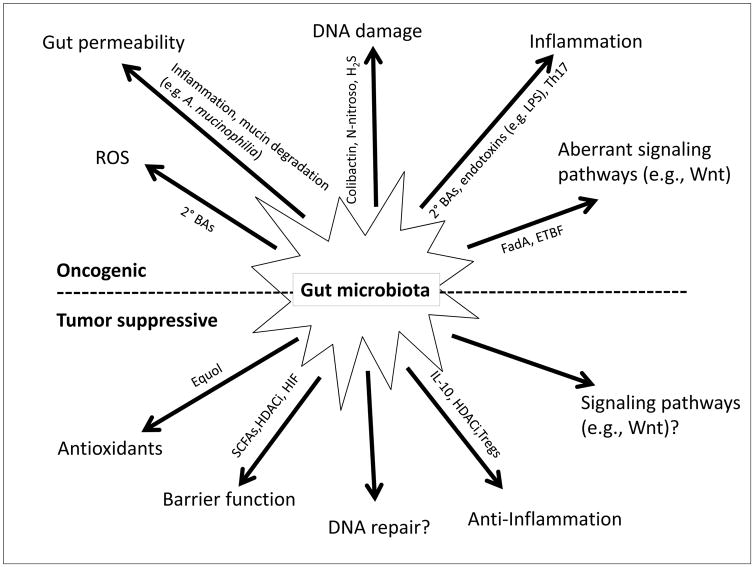
Figure 3. Microbial mechanisms of oncogenesis and tumor suppression.
Microbiota can contribute to oncogenesis (top, black) or tumor suppression (bottom, white) by a variety of molecular mechanisms that are listed at the end of each line. The mechanisms are listed from left to right in a symmetrical manner (top-bottom) to make it easier to appreciate that some are diametrically opposed. The mechanisms are carried out by a variety of microbial gene products, metabolites, and immune modulators, some of which are indicated in smaller font along each arrow. See text for details. Question marks indicate speculative mechanisms that have not yet been characterized.
1. Immune system and inflammation
The association between inflammation and cancer is particularly strong for colorectal cancer (CRC). Inflammatory bowel disease (IBD) patients with chronic colonic inflammation have a 2- to 10-fold increased risk of CRC57, while aspirin and other NSAIDs (non-steroidal anti-inflammatory drugs) have a stronger protective effect for CRC than other cancers58, 59. The association between inflammation and CRC mediated by gut microbiota is supported by pre-clinical research utilizing mouse models. IL-10 knockout mice have healthy colons when maintained in a germfree environment, but develop colitis shortly after conventionalizing by receiving fecal microbiota transplants from specific pathogen-free (SPF) mice60. This finding supports the idea that IL-10 is an immune-suppressive cytokine that prevents inappropriate immune responses directed against commensal gut microbiota. The inflammatory phenotype of IL-10 knockout mice maintained with conventional microbiota significantly increases the penetrance and multiplicity of colonic tumors in response to AOM treatment, compared to wild-type mice61. To demonstrate that the extent of inflammation correlates with tumor burden, IL-10 knockout mice monoassociated with a mildly colitogenic strain of Bacteroides vulgatus have an intermediate AOM-induced tumor phenotype. The NF-κB pathway, critical for mediating the innate immune response, links microbiota-induced inflammation and CRC. Toll-like receptors (TLRs) detect bacterial antigens including endotoxins (e.g., LPS, flagellin) and signal through the MyD88 adaptor and NF-κB transcription factors to trigger an inflammatory response. A MyD88 knockout prevents colonic tumors in AOM-treated, IL-10 knockout mice maintained with microbiota in a SPF facility61.
It is important to distinguish chronic, widespread inflammation, which is generally tumor promoting, from a local immune response where inflammation is restricted to the tumor microenvironment, which can be tumor-suppressive. Proinflammatory TH17 cells are dependent on microbiota since they are absent in germfree mice and are induced by certain subsets of GI microbiota such as segmented filamentous bacteria (SFB)62. TH17 cells have an unsettled role with respect to tumor immunity, as reports indicate their ability to infiltrate and eradicate some tumors, while also being correlated with poor prognosis in other instances of cancer63. Enterotoxigenic Bacteroides fragilis (ETBF) encodes a pathogenic toxin that can trigger TH17-mediated colitis, with concurrent colon-specific STAT3 activation and tumor induction in susceptible ApcMin (Multiple Intestinal Neoplasia) mice, which is reversed by IL-17 antibody blockade64.
Microbial-derived butyrate can induce naïve T cells and dendritic cells into a TReg cell fate65–67. Butyrate-mediated histone deacetylase (HDAC) inhibition can epigenetically activate the FOXP3 master regulator, while signaling through G protein coupled receptors (GPRs) such as GPR43 and GPR109a can expand the pool of TReg cells. TReg cells have an ambiguous role in cancer68. On the one hand, their anti-inflammatory function may mitigate inflammation-driven tumorigenesis, and on the other, being immunosuppressive, TReg infiltration into the tumor microenvironment may attenuate anti-tumor responses.
Intestinal microbiota alter gut barrier function, thus indirectly altering immune cell responses. The colonic epithelium is a single cell layer that separates myriad microbiota in the lumen from intraepithelial lymphocytes (IEL) and cells of the innate and adaptive immune system in the lamina propria. A thick (~100 micron) layer of mucus, which is produced by goblet cells, covers the colonic epithelium and prevents most microbes from coming into direct contact with the epithelium and breaching the barrier. A breach is not even required to activate IEL, which do not require priming like other T cells, and secrete pro-inflammatory cytokines in immediate response to encountering antigens. Diet and gut microbiota were recently shown to maintain mucus and barrier function in a mouse model69. A fiber-free diet resulted in dysbiosis with diminished fiber-fermenting bacteria including butyrate producers and increased representation of two mucus-degrading bacteria (Akkermansia muciniphilia and Bacteroides caccae). Mucus degradation led to increased susceptibility to a mucosal pathogen, Citrobacter rodentium, which resulted in a ‘leaky gut’ condition and colitis, which is a risk factor for CRC. The depletion of butyrate-producing bacteria is also likely to be important, as described in the next section, based on their ability to promote barrier function by upregulating claudins and occludins that comprise tight junctions between epithelial cells. A number of other beneficial microbiota including Lactobacillus and Bifidobacterium used as probiotics have been reported to improve barrier function and diminish permeability70.
2. Diet and microbial metabolites
Many dietary and digestive components are metabolized by bacteria in the GI tract, yielding putative oncometabolites and tumor-suppressive metabolites71. Excessive consumption of red meat is a risk factor for colorectal cancer and several other cancers by a variety of mechanisms, including some that are dependent on gut bacteria. High levels of protein intake can lead to increased protein levels in the colon where many types of bacteria, including some Firmicutes and Bacteroides sp., ferment amino acids into N-nitroso compounds that induce DNA alkylation and mutations in the host72. Proteobacteria encode nitroreductases and nitrate reductases that play a role in this process, and they are also strongly associated with inflammation73. Charred meat is a particular concern because it gives rise to carcinogenic heterocyclic amines that are metabolized by colonic bacteria, yielding electrophilic metabolites that are suspected of inflicting DNA damage74.
To digest saturated fat associated with red meat consumption, bile acids are produced in the liver, conjugated to taurine or glycine, and secreted into the GI tract. Approximately 5% of these primary bile acids escape enterohepatic circulation and reach the colon where they are converted by bacteria into secondary bile acids. This is carried out in two steps with deconjugation of the taurine or glycine moieties followed by a dehydrogenation or dehydroxylation reaction. For example, primary cholic acid is converted by certain bacteria including Clostridium scindens into secondary deoxycholic acid (DCA). DCA functions as a tumor promoter by perturbing cell membranes to release arachidonic acid, which is converted by COX-2 and lipooxygenase into prostaglandins and reactive oxygen species (ROS) that trigger inflammation and DNA damage75. Taurine also functions as a tumor promoter by generating genotoxic hydrogen sulfide, while also stimulating the growth of certain inflammatory bacteria such as Bilophilia wadsworthia75. F. nucleatum, enriched in human colorectal cancer cases as described above, produces hydrogen sulfide in response to red meat consumption76, 77
GI bacteria metabolize other dietary factors into putative tumor-suppressive metabolites. Dietary fibers are fermented by certain clades of colonic bacteria such as Clostridium clusters IV and XIVa into short-chain fatty acids (SCFAs). Butyrate, among the 3 most abundant SCFAs, serves as the primary energy source of colonocytes, and has been implicated in colorectal cancer prevention based on human metagenomic sequencing studies and gnotobiotic mouse models as discussed in previous sections. A pleiotropic molecule, butyrate likely exerts its tumor-suppressive properties by multiple mechanisms. As a HDAC inhibitor, butyrate epigenetically regulates the expression of genes involved in cell proliferation and apoptosis 52. Butyrate is also a ligand for certain GPRs also been implicated in tumor suppression78. Both of these mechanisms are believed to be important for butyrate’s ability to induce TReg cells, as discussed above. Finally, butyrate helps maintain epithelial barrier function, which is also important for preventing inflammation, and this too may involve dual mechanisms. Multiple studies have shown that butyrate upregulates the expression of tight junction genes including claudins and zonula occludens via HDAC inhibition79, while another study demonstrated that butyrate is oxidized as an energy source to such an extent that it triggers a HIF-1α-based mechanism to maintain barrier function80. Other examples of whole foods and dietary components converted by gut microbiota into metabolites with potential tumor-suppressive functions include: Daidzein in soy-based products is converted to equol, which functions as an antioxidant; glucosinolates in cruciferous vegetables such as broccoli are converted to sulforaphane and other isothiocyantes that function as HDAC inhibitors with anti-inflammatory effects; ellagic acid in certain berries is metabolized to urolithins which alter estrogens and inhibit COX-2 and inflammation81, 82. Finally, it should be emphasized that most commensal bacteria are neither “good” nor “bad” per se; rather, our diets dictate whether microbiota produce metabolites that exacerbate or ameliorate tumor progression. For example, Clostridium scindens produces secondary bile acids in response to dietary fat, but it is also a member of Clostridium cluster XIVa that produces butyrate in response to fiber.
3. Cell signaling pathways
The APC tumor-suppressor gene is mutated in CRC more frequently than any other gene83, 84. Many familial and sporadic CRC cases are initiated by homozygous, loss-of-function APC mutations that result in nuclear β-catenin accumulation, aberrant Wnt signaling, and altered expression of downstream target genes such as c-MYC to increase cell proliferation. The Wnt pathway is also perturbed in several mouse models of CRC including AOM-induced tumors. Furthermore, Wnt signaling can also be deregulated by epigenetic silencing of APC (e.g., DNA hypermethylation of the APC promoter) or by perturbation by an opportunistic pathogen. For example, F. nucleatum encodes FadA, an adhesin that binds to lectins and E-cadherin on the surface of host epithelial cells and activates β-catenin signaling85. ETBF, an opportunistic pathogen enriched in CRC, secretes a zinc-dependent metalloprotease that cleaves and degrades the extracellular domains of E-cadherin, facilitating the intracellular release of β-catenin that is normally inactivated via binding to intracellular E-cadherins. Nuclear translocation of β-catenin leads to activation of downstream target genes such as c-MYC, which promote proliferation86. Some Salmonella typhi strains secrete AvrA to activate β-catenin and are associated with hepatobilliary cancers87, 88.
JAK-STAT is another important signaling pathway that is inappropriately activated in colorectal cancer and other cancers. ETBF constitutively activates STAT3 via phosphorylation and nuclear translocation in colorectal tumors64. It is also possible for cellular signaling pathways to modify bacterial virulence factors. For example, the H. pylori cagA is an important virulence factor that is widely phosphorylated by cellular Src and Abl kinases. Unphosphorylated and phosphorylated CagA have different interactions with a broad repertoire of cellular signaling proteins, many of which are involved in regulating cellular proliferation pathways89.
4. DNA damage
DNA damage is a major driver of carcinogenesis. Genotoxins are damaging either by forming adducts or causing double-stranded breaks in DNA which, when unresolved by normal DNA repair processes, can introduce point mutations, insertions, deletions, or chromosomal rearrangements such as inversions and translocations. Microbial genotoxins can directly damage host cell DNA. Colibactin is expressed by a number of Enterobacteriaceae in addition to E. coli90 and induces double-strand breaks in host DNA49, 91. Similar DNA damage induction has been observed for the cytolethal distending toxin (CDT) produced by certain Proteobacteria92.
Bacterial metabolites can also be indirectly genotoxic, by producing free radicals and affecting reactive oxygen species (ROS). For example, Enterococcus faecalis is a commensal strain known to produce large amounts of extracellular superoxide (O2−) at the luminal side of the colonic mucosa93. H2O2 resulting from the rapid O2− degradation can broadly damage eukaryotic cellular DNA by forming DNA-protein crosslinks, DNA breaks, and point mutations. The ETBF Bacteroides fragilis toxin (BFT) is a virulence factor that upregulates bacterial polyamine catabolism pathways, generating reactive oxygen species that can also damage host DNA, leading to colon tumors94.
Bile production increases in individuals consuming an excessively fatty diet. A number of studies indicate that bile acids rapidly induce both ROS and reactive nitrogen species (RNS), collectively, which can damage host cell DNA (reviewed in 95). Furthermore, diets enriched in fats induce blooms of B. wadsworthia, a sulfite-reducing bacterium that is frequently associated with inflammatory bowel disease (IBD)96.
In contrast to the deleterious effects of ROS, the repair of injured intestinal mucosa relies upon redox signaling. Formylated peptides produced and excreted by microbiota activate colonic epithelial formyl peptide receptors (FPR), which induce localized ROS generation that activates redox signaling pathways and migration-associated proteins, thereby facilitating mucosal epithelial wound healing97. Symbiotic Lactobacilli are particularly adept at stimulating ROS generation via NADPH oxidase 1, thus enhancing epithelial cell proliferation98.
5. Distant sites
Gut microbiota, metabolites, and immune cells can exit the gut via the circulation and influence tumorigenesis at distant sites in the body (Figure 2, right panel). They reach the liver via the enterohepatic circulation and hepatic portal vein before entering the systemic circulation. This is noteworthy because the liver serves as the primary site for the recognition of potentially harmful endobiotic and xenobiotic compounds, which are excreted after detoxification by hepatic enzymes. A range of endogenous chemicals, including hormones, bile acids, and cholesterol metabolites, as well as ingested or inhaled toxins are first functionalized by Phase I cytochrome P450s and then often conjugated with glucuronic acid or sulfate by Phase II UDP-glucuronosyltransferases (UGTs) or sulfotransferases, respectively. Although numerous detoxified compounds are filtered through the kidneys, many are eliminated via the bile duct into the GI tract, where they are substrates for a variety of microbial enzyme systems that convert them back into chemicals that can be reabsorbed, circulated systemically to influence distant sites, and then returned to the liver for reprocessing and re-elimination. Such enterohepatic recirculation often involves both mammalian and microbial pathways, and plays important roles in normal systemic physiology as well as intestinal and extra-intestinal states of disease.
To demonstrate the impact of the microbiome on circulating metabolite levels, a metabolomics study compared serum from germfree and conventional mice and reported that microbiota affect the abundance of 10% of the metabolites by a magnitude of ≥50%99. Some of these metabolites influence tumorigenesis at various sites in the body. For example, the secondary bile acid DCA promotes a condition similar to nonalcoholic steatohepatitis (NASH) and obesity-associated hepatocellular carcinoma in a mouse model100. Other gut microbiota-derived metabolites implicated in cancer prevention such as equol have been detected in a variety of tissues (e.g., breast) and biological fluids such as blood, urine and prostatic fluid82. Gut bacteria participate in the metabolism of endogenous estrogens, potentially affecting breast cancer81, 82. Gut inflammatory responses can also affect breast cancer progression, based on studies where Helicobacter hepaticus in the GI tract promoted mammary carcinoma in mouse models, via a TNFα-dependent mechanism101, 102. In mice bearing mutant K-ras and p53, commensal bacteria induce TLR5 and NF-κB signaling to promote systemic inflammation to enhance tumor growth at multiple distant sites103. These results are consistent with a TLR5 SNP in >7% of humans which abrogates the immune response to flagellin in the gut, and is correlated with long-term ovarian cancer survival103.
Finally, it should be highlighted that each of the above mechanisms undoubtedly works in combination rather than in isolation. For example, while the E. coli pks pathogenicity island induces DNA damage, it is enabled by chronic inflammation as demonstrated by the lack of difference between pks+ and Δpks strains in tumor progression on a wild-type genetic background49. In other words, the chronic inflammation of IL-10 knockout mice apparently increases pks oncogenesis. Combinatorial mechanisms may potentiate oncogenesis following an initiating event that may be insufficient to drive transformation in isolation.
Cancer treatment
Recent preclinical studies utilizing cell culture and animal models, human clinical studies, as well as meta analyses of clinical studies has revealed that gut microbiota alter the host response to a variety of anticancer drugs, with immunomodulation emerging as one of the central mechanisms facilitating these differential responses. Dysbiosis is not only the consequence, but often also the cause for differential responses to therapy. As a prime example, increased intestinal diversity was predictive of decreased mortality in patients receiving allogeneic hematopoetic stem cell transplants (allo-HSCT) for the treatment of hematopoietic malignancies104. That immune modulation resulting from enhanced microbial diversity governs the intensity of graft versus host disease is an important consideration for patients beginning allo-HSCT. Moreover, compositional shifts resulting from treatment may themselves be responsible for some side effects of chemotherapy.
1. Immunotherapy
The adaptive immune system plays a vital role in the detection and clearance of cancer cells, and T lymphocytes are the central regulator of this response. T cell activation occurs in a series of steps and relies on the presence of a second costimulatory or coinhibitory signal that is provided by additional surface molecules on antigen presenting cells. Coinhibitory molecules such as Programmed cell death-1 (PD-1), PD-L1 (PD-1 ligand), and cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) serve as immune checkpoints that dampen the immune response to prevent autoimmune diseases. However, coinhibitory ligands and receptors are often overexpressed in cancer cells and stromal cells within the tumor microenvironment and help the cancer evade immune-mediated destruction. Monoclonal antibodies against CTLA-4 (ipilimumab), PD-1, (nivolumab), and PD-L1 (pembrolizumab) are FDA-approved immune checkpoint inhibitors that unleash the patient’s own immune responses against tumors. They have proven highly effective for treating melanomas, Hodgkin’s lymphoma, lung, kidney, and bladder cancer.
Similar to other cancer therapies, there is considerable inter-individual variation in patients’ responses to checkpoint inhibitors105–107. Interestingly, the efficacy of checkpoint inhibitors appears to be dependent on the patient’s gut microbiome, which itself closely interacts with the immune system. Therefore it is not unexpected that interaction between the gut microbiota and immune checkpoint inhibitors may explain the observed variation in clinical responses. Two independent studies recently demonstrated that gut microbiota reconcile different responses to immune checkpoint inhibitors in mouse models of melanoma. Sivan and colleagues noted that tumor growth varied depending on whether the mice were obtained from The Jackson Laboratory (JAX) or Taconic vendors108. These mice were on the same genetic background (C57BL/6) but had distinct microbial compositions. Tumors grew slower and responded more robustly to anti-PD-L1 immunotherapy in JAX mice compared to Taconic mice. Fecal microbiota transplants from JAX donors into Taconic recipients enhanced the anti-PD-L1 antitumor efficacy. The authors identified Bifidobacterium as crucial, and ‘therapeutic feeding’ (i.e., probiotics) of Bifidobacterium alone was able to mediate anti-PD-L1 efficacy by altering dendritic cell activity that enhanced CD8+ T cell responses to eradicate tumors.
In the other study, Vetizou et al. observed a rapid shift in the microbiome upon anti-CTLA-4 administration, characterized by a reduction in Bacteroidales and Burkholderiales and an increase in the abundance in Clostridiales105. Anti-CTLA-4 immunotherapy failed to reduce tumor burden in a germfree state, but this defect was overcome by introducing Bacteroides fragilis and/or B. thetaiotaomicron. Overall, introduction of these bacteria enhanced tumor specificity by triggering dendritic cell maturation and modulating IL-12-dependent TH1 responses. Although the two studies identified different microbiota and used different checkpoint blockades, their mechanism of action was quite similar with dendritic cell maturation/activation and improved function of tumor-infiltrating effector T cells.
The utility of immune checkpoint inhibitors comes at the price of gastrointestinal and hepatic complications109. Hepatitis, diarrhea, and enterocolitis are characteristic side effects of immune checkpoint inhibitiors, which result from a complex interplay of host genetics, immune responses, environment, and the microbiota. Patients who develop new-onset, immune-mediated colitis resulting from anti-CTLA4 monoclonal antibody therapy have a reduced abundance of Bacteroidetes compared to colitis-free individuals also receiving ipilimumab110. Microbial modules associated with polyamine transport and vitamin B (B1, B2, and B5) synthesis conferred protection, as their relative abundance was highly associated with colitis-free individuals.
Synthetic CpG oligonucleotides (CpG-ON) are ligands for TLR9 on immune cells, and enhance immune responses. When combined with peptide vaccines, CpG-ON and inhibitory IL-10 receptor antibodies confer a therapeutic benefit, with reduced tumor volume and extended survival time in humans111. When CpG-ON and IL-10R antibodies are injected into mouse tumors, they diminish tumor burden via proinflammatory cytokines. They are ineffectual when mice are treated with antibiotics, or rendered germfree111, 112.
2. Chemotherapy
Not unexpectedly, chemotherapy alters the composition of microbial communities in patients, although the significance of the altered microbiome with respect to prognosis is unclear113–117. Perhaps more importantly, the specific composition of microbiota can influence the anticancer response of a variety of conventional chemotherapeutics based on work conducted in mouse models. The platinum chemotherapeutic, oxaliplatin, exerts its tumor retardation effects in a microbiota-dependent manner. Eliminating microbiota with a regimen of broad-spectrum antibiotics significantly altered host gene expression: genes promoting cancer metabolism and cancer development were upregulated with a concomitant downregulation of inflammatory, phagocytic, and antigen-presenting pathways. Moreover, antibiotic treatment decreased the recruitment of immune cells important for mediating tumor regression with a corresponding decrease in their proinflammatory potential. Oxaliplatin efficacy was dependent on the intratumoral production of reactive oxygen species (ROS), which is attenuated in germfree mice, and reduced ROS generation corresponded with diminished intratumoral DNA damage112. This finding suggests that immunomodulatory effects mediated by the microbiota in response to chemotherapeutic compounds blurs the distinction between chemotherapy and immunotherapy.
Cyclophosphamide (CP) is an alkylating agent commonly used for chemotherapy that reduces small intestinal villus height and disrupts the intestinal barrier, causing translocation of commensals to secondary lymphoid organs along with accumulation of inflammatory cells. Viaud and colleagues discovered that CP’s antitumor effects are attenuated in mice raised to be germfree or made so using antibiotics118. In the latter case, antibiotics selectively targeting Gram-positive bacteria, compared to Gram-negative targeted antibiotics, significantly reduced CP efficacy. Thus, specific Gram-positive bacteria (Lactobacillus johnsonii, L. murinus, Enterococcus hirae and segmented filamentous bacteria) were found to be essential to mediate CP’s anti-tumor response in a mouse model of nonmetastasizing sarcoma. A follow-up study from the same group reported that E. hirae translocation increased the intratumoral CD8/TReg ratio112. Furthermore, the Gram-negative Barnesiella intestihominis was found to be an important effector of CP’s antitumor effects via increased infiltration of Interferon-γ producing T cells within cancer lesions119. Interestingly, advanced lung and ovarian cancer patients with E. hirae and B. intestinihominis (but not other bacteria) specific TH1 cell memory responses were predicted to have lengthened progression-free survival. Collectively, these studies are the onus to incorporate particular species of Enterococcus and Barnesiella into an optimized microbiota cocktail to be administered concurrently with CP and possibly other alkylating agents. In the future, these bacteria or their specific immunomodulatory products/metabolites may be incorporated as adjuvants to improve the efficacy of existing chemotherapeutics.
3. Microbial drug targets in oncology
Currently, the pharmaceutical and biotechnology industries focus on cellular targets for developing chemotherapies and targeted therapies. However, in the not-distant future, microbiota might also be drug targets. Microbial drug targets also have the potential to ameliorate the damaging side effects that many chemotherapeutics have on the GI tract. Some side effects, such as those resulting from irinotecan (camptothecin), are serious enough that they limit the dose or duration of therapy. Irinotecan is a topoisomerase I inhibitor that blocks DNA replication preferentially in rapidly dividing cells and is used to treat colorectal and pancreatic cancer. Administered as a pro-drug, irinotecan is metabolized into the active chemotherapeutic agent (SN38), and it is subsequently glucuronidated in the liver to form the inactive SN38-G and is excreted via the GI tract. Microbiota express β-glucuronidase enzymes that hydrolyze the glucuronic acid moiety, which bacteria scavenge as an energy source, thereby reactivating SN38 in the GI lumen. Increased SN38 levels in the intestines causes severe and sometimes life-threatening diarrhea, often requiring dose de-escalation and frequent dose adjustment.
Germfree mice exhibit less GI damage and tolerate higher doses of irinotecan compared to conventional mice with intact microbiota120. A clinical trial noted a slight clinical benefit of administering neomycin concurrent with irinotecan to reduce side effects121. However, administering broad-spectrum antibiotics can indiscriminately kill a wide number of GI commensals and open up niches for pathogens such as Clostridium difficile (C. diff). As an alternative, small molecule inhibitors targeting bacterial β-glucuronidases have been developed that do not cross-react with human β-glucuronidases, and are non-toxic to either mammalian cells or bacteria122–124. In preclinical studies, mice receiving concurrent treatment with β-glucuronidase inhibitors are protected from irinotecan-induced diarrhea124. Other chemotherapeutic agents also have adverse effects in the GI tract. For example, doxorubicin is similar to irinotecan in that GI damage requires microbiota125. These findings suggest that targeting microbiota may diminish the toxicity of multiple chemotherapeutics.
Future directions
As the adage goes, an ounce of prevention is better than a pound of cure. Numerous studies have demonstrated that short-chain fatty acids synthesized during bacterial fermentation of plant-based fibers broadly protects against the development of cancer. Incorporating fiber-rich, prebiotic foods in the diet early in life as well as limiting red meat consumption and decreasing the incidence of obesity should help to reduce global tumor burden in the long run. Moreover, burgeoning gene-editing technologies using CRISPR-Cas9126–129 should allow engineering of probiotic bacteria with specific capabilities (e.g. expression of superoxide dismutase to counteract superoxide producing ETBF), or conversely, to delete pathogenic components of bacterial genomes (e.g. pks pathogenicity island deletion in E. coli).
Dysbiosis appears to be a harbinger of tumorigenesis, and not only precedes disease onset, but also propagates throughout the course of tumor progression. Maintaining eubiosis, or an optimal microbiota composition, is key to preventing events that may initiate disease. Therefore, there is clearly an onus to develop more specific, narrow-range antibiotics that selectively target pathogens or pathobionts while preserving eubiosis.
Randomized clinical trials strongly demonstrate the utility of fecal microbiota transplants (FMTs) in resolving recurrent and refractory C. difficile infections130. Instances of improved clinical outcomes following FMTs have also been reported for celiac disease131 and irritable bowel syndrome132, and preclinical studies suggest FMTs protect against colitis130. However, these positive findings have been mixed with negative results. Therefore, randomized clinical trials are necessary to establish therapeutic efficacy for each disease state. Continual efforts should be made to develop capsule-based synthetic FMTs that contain rationally selected consortia of cultured bacteria. In addition to infinitely increased palatability, this approach should allow for regular, even daily consumption, which may be necessary for disease states where reconstruction of the microbial community takes precedence over pathogen exclusion, as in the case of C. difficile infection. Synthetic FMTs may also prevent certain drawbacks associated with traditional FMTs, such as the potential acquisition of unwanted phenotypes, antibiotic-resistant bacteria or viruses that evade screening protocols133.
Metabolic syndrome is increasingly associated with cancer development, and resulting mortality134. Insulin resistance is the linchpin in the development of metabolic syndrome and has been observed in many different forms of cancer such as prostate, breast and colorectum135–137. Gut microbiota can regulate various metabolic features such as nutrient harvesting138, hepatic metabolism of lipids and cholesterol139, and fat storage140 and can also compromise the intestinal mucus barrier when diets low in dietary fiber are introduced69. Intermittent fasting, or caloric restriction, is known to improve insulin sensitivity along with reduction of other vital markers such as blood pressure and inflammation 141. In mouse models, cycles of starvation, alternating with a variety of chemotherapeutic agents, results in long-term cancer-free survival, compared to either modality alone142. Whether the microbiota can mediate the enhanced response to chemotherapeutics during cycles of nutrient deprivation remains to be determined.
A number of recent sophisticated cell culture systems feature the in vitro propagation of organoids derived from wild type, diseased, or genetically recombined tissues143–145. Coupling these advancements with genetic screens that utilize transposon systems provide the ability to distinguish between factors that either cause (“drive”) or minimally influence (“passenger”) genetic or epigenetic alterations in host cells146. Co-culture of microbes and microbial derivatives with colonoids will provide mechanistic insight into host-microbe interactions147.
Precision medicine promises medical treatments that are optimized to account for individual patients’ genetic makeup and differences in lifestyle and environment. Given the broad range of effects that microbiota exert on human health, compositional differences between patients should also factor into deciding who would benefit from a particular treatment modality. As mentioned in earlier sections, the presence or absence of specific bacterial community members, or even their metabolites, can alter the prevalence, severity and treatment of cancer, and may serve as prognostic biomarkers. For example, patients receiving immunotherapy treatments may benefit from B. intestinihominis or E. hirae species to improve efficacy118; patients slated to receive irinotecan treatment may benefit from bacterial β-glucuronidase-targeting drugs124. Translating these cutting-edge innovations into clinical interventions will benefit from reduced costs for whole genome and transcriptome sequencing, as will simplified inquiry and interpretation by developing standardized bioinformatics analysis pipelines. Furthermore, increasing the access to centralized, cloud-based repositories for whole genome and transcriptome sequencing databases will facilitate data mining approaches by computational scientists. In the future, it is likely that combining pharmacogenomics information with custom microbial organisms or their specific metabolites will allow for precise dosing, symptom management, and improved therapeutic responses.
Acknowledgments
Funding Sources: NIH R01-0D-02057 (SJB), USDA 055336 (SJB). NIH CA098468 (MRR), CA207416 (MRR, SJB), T32DK007737(APB)
The authors thank Dr. Marc Weinberg for critical reading of the manuscript, and members of the Redinbo and Bultman laboratories for helpful discussions.
Footnotes
Conflict of Interest: One author (MRR) has inventions licensed to and equity ownership in Symberix, Inc. a pharmaceutical company creating microbiome-targeted therapeutics.
Author Contributions: Each of the 3 authors contributed content and helped write the manuscript.
References
We sincerely apologize to our colleagues whose work we were unable to include due to space constraints.
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.Mariotto AB, Yabroff KR, Shao Y, Feuer EJ, Brown ML. Projections of the cost of cancer care in the United States: 2010–2020. J Natl Cancer Inst. 2011;103:117–128. doi: 10.1093/jnci/djq495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tomasetti C, Vogelstein B. Cancer etiology. Variation in cancer risk among tissues can be explained by the number of stem cell divisions. Science. 2015;347:78–81. doi: 10.1126/science.1260825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ashford NA, Bauman P, Brown HS, et al. Cancer risk: role of environment. Science. 2015;347:727. doi: 10.1126/science.aaa6246. [DOI] [PubMed] [Google Scholar]
- 5.Harris CC. Editorial. Carcinogenesis. 2016;37:1. doi: 10.1093/carcin/bgv246. [DOI] [PubMed] [Google Scholar]
- 6.Humans IWGotEoCRt. Biological agents. Volume 100 B. A review of human carcinogens. IARC Monogr Eval Carcinog Risks Hum. 2012;100:1–441. [PMC free article] [PubMed] [Google Scholar]
- 7.Anand P, Kunnumakkara AB, Sundaram C, et al. Cancer is a preventable disease that requires major lifestyle changes. Pharm Res. 2008;25:2097–2116. doi: 10.1007/s11095-008-9661-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Willett WC. Diet and cancer. Oncologist. 2000;5:393–404. doi: 10.1634/theoncologist.5-5-393. [DOI] [PubMed] [Google Scholar]
- 9.Goodrich JK, Di Rienzi SC, Poole AC, et al. Conducting a microbiome study. Cell. 2014;158:250–262. doi: 10.1016/j.cell.2014.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weinstock GM. Genomic approaches to studying the human microbiota. Nature. 2012;489:250–256. doi: 10.1038/nature11553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morgan XC, Huttenhower C. Chapter 12: Human microbiome analysis. PLoS Comput Biol. 2012;8:e1002808. doi: 10.1371/journal.pcbi.1002808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arumugam M, Raes J, Pelletier E, et al. Enterotypes of the human gut microbiome. Nature. 2011;473:174–180. doi: 10.1038/nature09944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sender R, Fuchs S, Milo R. Are We Really Vastly Outnumbered? Revisiting the Ratio of Bacterial to Host Cells in Humans. Cell. 2016;164:337–340. doi: 10.1016/j.cell.2016.01.013. [DOI] [PubMed] [Google Scholar]
- 14.Savage DC. Microbial ecology of the gastrointestinal tract. Annu Rev Microbiol. 1977;31:107–133. doi: 10.1146/annurev.mi.31.100177.000543. [DOI] [PubMed] [Google Scholar]
- 15.Dominguez-Bello MG, Blaser MJ, Ley RE, Knight R. Development of the human gastrointestinal microbiota and insights from high-throughput sequencing. Gastroenterology. 2011;140:1713–1719. doi: 10.1053/j.gastro.2011.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Palmer C, Bik EM, DiGiulio DB, Relman DA, Brown PO. Development of the human infant intestinal microbiota. PLoS Biol. 2007;5:e177. doi: 10.1371/journal.pbio.0050177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dominguez-Bello MG, Costello EK, Contreras M, et al. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc Natl Acad Sci U S A. 2010;107:11971–11975. doi: 10.1073/pnas.1002601107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Claesson MJ, Cusack S, O’Sullivan O, et al. Composition, variability, and temporal stability of the intestinal microbiota of the elderly. Proc Natl Acad Sci U S A. 2011;108(Suppl 1):4586–4591. doi: 10.1073/pnas.1000097107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Biagi E, Nylund L, Candela M, et al. Through ageing, and beyond: gut microbiota and inflammatory status in seniors and centenarians. PLoS One. 2010;5:e10667. doi: 10.1371/journal.pone.0010667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goodrich JK, Waters JL, Poole AC, et al. Human genetics shape the gut microbiome. Cell. 2014;159:789–799. doi: 10.1016/j.cell.2014.09.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Benson AK. The gut microbiome-an emerging complex trait. Nat Genet. 2016;48:1301–1302. doi: 10.1038/ng.3707. [DOI] [PubMed] [Google Scholar]
- 22.Wang J, Thingholm LB, Skieceviciene J, et al. Genome-wide association analysis identifies variation in vitamin D receptor and other host factors influencing the gut microbiota. Nat Genet. 2016;48:1396–1406. doi: 10.1038/ng.3695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bonder MJ, Kurilshikov A, Tigchelaar EF, et al. The effect of host genetics on the gut microbiome. Nat Genet. 2016;48:1407–1412. doi: 10.1038/ng.3663. [DOI] [PubMed] [Google Scholar]
- 24.David LA, Maurice CF, Carmody RN, et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2014;505:559–563. doi: 10.1038/nature12820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu Z, Knight R. Dietary effects on human gut microbiome diversity. Br J Nutr. 2015;113(Suppl):S1–5. doi: 10.1017/S0007114514004127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sonnenburg ED, Smits SA, Tikhonov M, Higginbottom SK, Wingreen NS, Sonnenburg JL. Diet-induced extinctions in the gut microbiota compound over generations. Nature. 2016;529:212–215. doi: 10.1038/nature16504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.David LA, Materna AC, Friedman J, et al. Host lifestyle affects human microbiota on daily timescales. Genome Biol. 2014;15:R89. doi: 10.1186/gb-2014-15-7-r89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mueller NT, Bakacs E, Combellick J, Grigoryan Z, Dominguez-Bello MG. The infant microbiome development: mom matters. Trends Mol Med. 2015;21:109–117. doi: 10.1016/j.molmed.2014.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cox LM, Blaser MJ. Antibiotics in early life and obesity. Nat Rev Endocrinol. 2015;11:182–190. doi: 10.1038/nrendo.2014.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Claesson MJ, Jeffery IB, Conde S, et al. Gut microbiota composition correlates with diet and health in the elderly. Nature. 2012;488:178–184. doi: 10.1038/nature11319. [DOI] [PubMed] [Google Scholar]
- 31.Gill SR, Pop M, Deboy RT, et al. Metagenomic analysis of the human distal gut microbiome. Science. 2006;312:1355–1359. doi: 10.1126/science.1124234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Microbial ecology: human gut microbes associated with obesity. Nature. 2006;444:1022–1023. doi: 10.1038/4441022a. [DOI] [PubMed] [Google Scholar]
- 33.Lozupone CA, Stombaugh JI, Gordon JI, Jansson JK, Knight R. Diversity, stability and resilience of the human gut microbiota. Nature. 2012;489:220–230. doi: 10.1038/nature11550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Turnbaugh PJ, Backhed F, Fulton L, Gordon JI. Diet-induced obesity is linked to marked but reversible alterations in the mouse distal gut microbiome. Cell Host Microbe. 2008;3:213–223. doi: 10.1016/j.chom.2008.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhao L. The gut microbiota and obesity: from correlation to causality. Nat Rev Microbiol. 2013;11:639–647. doi: 10.1038/nrmicro3089. [DOI] [PubMed] [Google Scholar]
- 36.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 37.Marshall BJ, Warren JR. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet. 1984;1:1311–1315. doi: 10.1016/s0140-6736(84)91816-6. [DOI] [PubMed] [Google Scholar]
- 38.Vaezi MF, Falk GW, Peek RM, et al. CagA-positive strains of Helicobacter pylori may protect against Barrett’s esophagus. Am J Gastroenterol. 2000;95:2206–2211. doi: 10.1111/j.1572-0241.2000.02305.x. [DOI] [PubMed] [Google Scholar]
- 39.Wang F, Xia P, Wu F, et al. Helicobacter pylori VacA disrupts apical membrane-cytoskeletal interactions in gastric parietal cells. J Biol Chem. 2008;283:26714–26725. doi: 10.1074/jbc.M800527200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bhatt AP, Damania B. AKTivation of PI3K/AKT/mTOR signaling pathway by KSHV. Front Immunol. 2012;3:401. doi: 10.3389/fimmu.2012.00401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Castellarin M, Warren RL, Freeman JD, et al. Fusobacterium nucleatum infection is prevalent in human colorectal carcinoma. Genome Res. 2012;22:299–306. doi: 10.1101/gr.126516.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Repass J, Maherali N, Owen K. Reproducibility Project: Cancer B, Reproducibility Project Cancer B. Registered report: Fusobacterium nucleatum infection is prevalent in human colorectal carcinoma. Elife. 2016:5. doi: 10.7554/eLife.10012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kostic AD, Gevers D, Pedamallu CS, et al. Genomic analysis identifies association of Fusobacterium with colorectal carcinoma. Genome Res. 2012;22:292–298. doi: 10.1101/gr.126573.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McCoy AN, Araujo-Perez F, Azcarate-Peril A, Yeh JJ, Sandler RS, Keku TO. Fusobacterium is associated with colorectal adenomas. PLoS One. 2013;8:e53653. doi: 10.1371/journal.pone.0053653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Araujo-Perez F, McCoy AN, Okechukwu C, et al. Differences in microbial signatures between rectal mucosal biopsies and rectal swabs. Gut Microbes. 2012;3:530–535. doi: 10.4161/gmic.22157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dejea CM, Wick EC, Hechenbleikner EM, et al. Microbiota organization is a distinct feature of proximal colorectal cancers. Proc Natl Acad Sci U S A. 2014;111:18321–18326. doi: 10.1073/pnas.1406199111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zolfo M, Tett A, Jousson O, Donati C, Segata N. MetaMLST: multi-locus strain-level bacterial typing from metagenomic samples. Nucleic Acids Res. 2016 doi: 10.1093/nar/gkw837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ward DV, Scholz M, Zolfo M, et al. Metagenomic Sequencing with Strain-Level Resolution Implicates Uropathogenic E. coli in Necrotizing Enterocolitis and Mortality in Preterm Infants. Cell Rep. 2016;14:2912–2924. doi: 10.1016/j.celrep.2016.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Arthur JC, Perez-Chanona E, Muhlbauer M, et al. Intestinal inflammation targets cancer-inducing activity of the microbiota. Science. 2012;338:120–123. doi: 10.1126/science.1224820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Buc E, Dubois D, Sauvanet P, et al. High prevalence of mucosa-associated E. coli producing cyclomodulin and genotoxin in colon cancer. PLoS One. 2013;8:e56964. doi: 10.1371/journal.pone.0056964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bultman SJ. Emerging roles of the microbiome in cancer. Carcinogenesis. 2014;35:249–255. doi: 10.1093/carcin/bgt392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Donohoe DR, Holley D, Collins LB, et al. A gnotobiotic mouse model demonstrates that dietary fiber protects against colorectal tumorigenesis in a microbiota- and butyrate-dependent manner. Cancer Discov. 2014;4:1387–1397. doi: 10.1158/2159-8290.CD-14-0501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sanapareddy N, Legge RM, Jovov B, et al. Increased rectal microbial richness is associated with the presence of colorectal adenomas in humans. ISME J. 2012;6:1858–1868. doi: 10.1038/ismej.2012.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shen XJ, Rawls JF, Randall T, et al. Molecular characterization of mucosal adherent bacteria and associations with colorectal adenomas. Gut Microbes. 2010;1:138–147. doi: 10.4161/gmic.1.3.12360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Browne HP, Forster SC, Anonye BO, et al. Culturing of ‘unculturable’ human microbiota reveals novel taxa and extensive sporulation. Nature. 2016;533:543–546. doi: 10.1038/nature17645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Selle K, Klaenhammer TR, Barrangou R. CRISPR-based screening of genomic island excision events in bacteria. Proc Natl Acad Sci U S A. 2015;112:8076–8081. doi: 10.1073/pnas.1508525112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Itzkowitz SH, Harpaz N. Diagnosis and management of dysplasia in patients with inflammatory bowel diseases. Gastroenterology. 2004;126:1634–1648. doi: 10.1053/j.gastro.2004.03.025. [DOI] [PubMed] [Google Scholar]
- 58.Cuzick J, Otto F, Baron JA, et al. Aspirin and non-steroidal anti-inflammatory drugs for cancer prevention: an international consensus statement. Lancet Oncol. 2009;10:501–507. doi: 10.1016/S1470-2045(09)70035-X. [DOI] [PubMed] [Google Scholar]
- 59.Cole BF, Logan RF, Halabi S, et al. Aspirin for the chemoprevention of colorectal adenomas: meta-analysis of the randomized trials. J Natl Cancer Inst. 2009;101:256–266. doi: 10.1093/jnci/djn485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sellon RK, Tonkonogy S, Schultz M, et al. Resident enteric bacteria are necessary for development of spontaneous colitis and immune system activation in interleukin-10-deficient mice. Infect Immun. 1998;66:5224–5231. doi: 10.1128/iai.66.11.5224-5231.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Uronis JM, Muhlbauer M, Herfarth HH, Rubinas TC, Jones GS, Jobin C. Modulation of the intestinal microbiota alters colitis-associated colorectal cancer susceptibility. PLoS One. 2009;4:e6026. doi: 10.1371/journal.pone.0006026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ivanov II, Atarashi K, Manel N, et al. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell. 2009;139:485–498. doi: 10.1016/j.cell.2009.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bailey SR, Nelson MH, Himes RA, Li Z, Mehrotra S, Paulos CM. Th17 cells in cancer: the ultimate identity crisis. Front Immunol. 2014;5:276. doi: 10.3389/fimmu.2014.00276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wu S, Rhee KJ, Albesiano E, et al. A human colonic commensal promotes colon tumorigenesis via activation of T helper type 17 T cell responses. Nat Med. 2009;15:1016–1022. doi: 10.1038/nm.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Arpaia N, Campbell C, Fan X, et al. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature. 2013;504:451–455. doi: 10.1038/nature12726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Smith PM, Howitt MR, Panikov N, et al. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science. 2013;341:569–573. doi: 10.1126/science.1241165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Furusawa Y, Obata Y, Fukuda S, et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature. 2013;504:446–450. doi: 10.1038/nature12721. [DOI] [PubMed] [Google Scholar]
- 68.Wolf D, Sopper S, Pircher A, Gastl G, Wolf AM. Treg(s) in Cancer: Friends or Foe? J Cell Physiol. 2015;230:2598–2605. doi: 10.1002/jcp.25016. [DOI] [PubMed] [Google Scholar]
- 69.Desai MS, Seekatz AM, Koropatkin NM, et al. A Dietary Fiber-Deprived Gut Microbiota Degrades the Colonic Mucus Barrier and Enhances Pathogen Susceptibility. Cell. 2016;167:1339–1353. e1321. doi: 10.1016/j.cell.2016.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kelly JR, Kennedy PJ, Cryan JF, Dinan TG, Clarke G, Hyland NP. Breaking down the barriers: the gut microbiome, intestinal permeability and stress-related psychiatric disorders. Front Cell Neurosci. 2015;9:392. doi: 10.3389/fncel.2015.00392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Louis P, Hold GL, Flint HJ. The gut microbiota, bacterial metabolites and colorectal cancer. Nat Rev Microbiol. 2014;12:661–672. doi: 10.1038/nrmicro3344. [DOI] [PubMed] [Google Scholar]
- 72.Gill CI, Rowland IR. Diet and cancer: assessing the risk. Br J Nutr. 2002;88(Suppl 1):S73–87. doi: 10.1079/BJN2002632. [DOI] [PubMed] [Google Scholar]
- 73.Shin NR, Whon TW, Bae JW. Proteobacteria: microbial signature of dysbiosis in gut microbiota. Trends Biotechnol. 2015;33:496–503. doi: 10.1016/j.tibtech.2015.06.011. [DOI] [PubMed] [Google Scholar]
- 74.Huycke MM, Gaskins HR. Commensal bacteria, redox stress, and colorectal cancer: mechanisms and models. Exp Biol Med (Maywood) 2004;229:586–597. doi: 10.1177/153537020422900702. [DOI] [PubMed] [Google Scholar]
- 75.Ridlon JM, Wolf PG, Gaskins HR. Taurocholic acid metabolism by gut microbes and colon cancer. Gut Microbes. 2016;7:201–215. doi: 10.1080/19490976.2016.1150414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Claesson R, Edlund MB, Persson S, Carlsson J. Production of volatile sulfur compounds by various Fusobacterium species. Oral Microbiol Immunol. 1990;5:137–142. doi: 10.1111/j.1399-302x.1990.tb00411.x. [DOI] [PubMed] [Google Scholar]
- 77.Fukamachi H, Nakano Y, Yoshimura M, Koga T. Cloning and characterization of the L-cysteine desulfhydrase gene of Fusobacterium nucleatum. FEMS Microbiol Lett. 2002;215:75–80. doi: 10.1111/j.1574-6968.2002.tb11373.x. [DOI] [PubMed] [Google Scholar]
- 78.Singh N, Gurav A, Sivaprakasam S, et al. Activation of Gpr109a, receptor for niacin and the commensal metabolite butyrate, suppresses colonic inflammation and carcinogenesis. Immunity. 2014;40:128–139. doi: 10.1016/j.immuni.2013.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ploger S, Stumpff F, Penner GB, et al. Microbial butyrate and its role for barrier function in the gastrointestinal tract. Ann N Y Acad Sci. 2012;1258:52–59. doi: 10.1111/j.1749-6632.2012.06553.x. [DOI] [PubMed] [Google Scholar]
- 80.Kelly CJ, Zheng L, Campbell EL, et al. Crosstalk between Microbiota-Derived Short-Chain Fatty Acids and Intestinal Epithelial HIF Augments Tissue Barrier Function. Cell Host Microbe. 2015;17:662–671. doi: 10.1016/j.chom.2015.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bultman SJ. The microbiome and its potential as a cancer preventive intervention. Semin Oncol. 2016;43:97–106. doi: 10.1053/j.seminoncol.2015.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hullar MA, Burnett-Hartman AN, Lampe JW. Gut microbes, diet, and cancer. Cancer Treat Res. 2014;159:377–399. doi: 10.1007/978-3-642-38007-5_22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Armaghany T, Wilson JD, Chu Q, Mills G. Genetic alterations in colorectal cancer. Gastrointest Cancer Res. 2012;5:19–27. [PMC free article] [PubMed] [Google Scholar]
- 84.Wood LD, Parsons DW, Jones S, et al. The genomic landscapes of human breast and colorectal cancers. Science. 2007;318:1108–1113. doi: 10.1126/science.1145720. [DOI] [PubMed] [Google Scholar]
- 85.Rubinstein MR, Wang X, Liu W, Hao Y, Cai G, Han YW. Fusobacterium nucleatum promotes colorectal carcinogenesis by modulating E-cadherin/beta-catenin signaling via its FadA adhesin. Cell Host Microbe. 2013;14:195–206. doi: 10.1016/j.chom.2013.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wu S, Lim KC, Huang J, Saidi RF, Sears CL. Bacteroides fragilis enterotoxin cleaves the zonula adherens protein, E-cadherin. Proc Natl Acad Sci U S A. 1998;95:14979–14984. doi: 10.1073/pnas.95.25.14979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Garrett WS. Cancer and the microbiota. Science. 2015;348:80–86. doi: 10.1126/science.aaa4972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lu R, Wu S, Zhang YG, et al. Enteric bacterial protein AvrA promotes colonic tumorigenesis and activates colonic beta-catenin signaling pathway. Oncogenesis. 2014;3:e105. doi: 10.1038/oncsis.2014.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Backert S, Tegtmeyer N, Selbach M. The versatility of Helicobacter pylori CagA effector protein functions: The master key hypothesis. Helicobacter. 2010;15:163–176. doi: 10.1111/j.1523-5378.2010.00759.x. [DOI] [PubMed] [Google Scholar]
- 90.Putze J, Hennequin C, Nougayrede JP, et al. Genetic structure and distribution of the colibactin genomic island among members of the family Enterobacteriaceae. Infect Immun. 2009;77:4696–4703. doi: 10.1128/IAI.00522-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Nougayrede JP, Homburg S, Taieb F, et al. Escherichia coli induces DNA double-strand breaks in eukaryotic cells. Science. 2006;313:848–851. doi: 10.1126/science.1127059. [DOI] [PubMed] [Google Scholar]
- 92.Thelestam M, Frisan T. Cytolethal distending toxins. Rev Physiol Biochem Pharmacol. 2004;152:111–133. doi: 10.1007/s10254-004-0030-8. [DOI] [PubMed] [Google Scholar]
- 93.Huycke MM, Abrams V, Moore DR. Enterococcus faecalis produces extracellular superoxide and hydrogen peroxide that damages colonic epithelial cell DNA. Carcinogenesis. 2002;23:529–536. doi: 10.1093/carcin/23.3.529. [DOI] [PubMed] [Google Scholar]
- 94.Goodwin AC, Destefano Shields CE, Wu S, et al. Polyamine catabolism contributes to enterotoxigenic Bacteroides fragilis-induced colon tumorigenesis. Proc Natl Acad Sci U S A. 2011;108:15354–15359. doi: 10.1073/pnas.1010203108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bernstein H, Bernstein C, Payne CM, Dvorak K. Bile acids as endogenous etiologic agents in gastrointestinal cancer. World J Gastroenterol. 2009;15:3329–3340. doi: 10.3748/wjg.15.3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Devkota S, Wang Y, Musch MW, et al. Dietary-fat-induced taurocholic acid promotes pathobiont expansion and colitis in Il10−/ − mice. Nature. 2012;487:104–108. doi: 10.1038/nature11225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Jones RM, Mercante JW, Neish AS. Reactive oxygen production induced by the gut microbiota: pharmacotherapeutic implications. Curr Med Chem. 2012;19:1519–1529. doi: 10.2174/092986712799828283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Jones RM, Luo L, Ardita CS, et al. Symbiotic lactobacilli stimulate gut epithelial proliferation via Nox-mediated generation of reactive oxygen species. EMBO J. 2013;32:3017–3028. doi: 10.1038/emboj.2013.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wikoff WR, Anfora AT, Liu J, et al. Metabolomics analysis reveals large effects of gut microflora on mammalian blood metabolites. Proc Natl Acad Sci U S A. 2009;106:3698–3703. doi: 10.1073/pnas.0812874106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Yoshimoto S, Loo TM, Atarashi K, et al. Obesity-induced gut microbial metabolite promotes liver cancer through senescence secretome. Nature. 2013;499:97–101. doi: 10.1038/nature12347. [DOI] [PubMed] [Google Scholar]
- 101.Fox JG, Ge Z, Whary MT, Erdman SE, Horwitz BH. Helicobacter hepaticus infection in mice: models for understanding lower bowel inflammation and cancer. Mucosal Immunol. 2011;4:22–30. doi: 10.1038/mi.2010.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lakritz JR, Poutahidis T, Mirabal S, et al. Gut bacteria require neutrophils to promote mammary tumorigenesis. Oncotarget. 2015;6:9387–9396. doi: 10.18632/oncotarget.3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Rutkowski MR, Stephen TL, Svoronos N, et al. Microbially driven TLR5-dependent signaling governs distal malignant progression through tumor-promoting inflammation. Cancer Cell. 2015;27:27–40. doi: 10.1016/j.ccell.2014.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Taur Y, Jenq RR, Perales MA, et al. The effects of intestinal tract bacterial diversity on mortality following allogeneic hematopoietic stem cell transplantation. Blood. 2014;124:1174–1182. doi: 10.1182/blood-2014-02-554725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Vetizou M, Pitt JM, Daillere R, et al. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science. 2015;350:1079–1084. doi: 10.1126/science.aad1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Pitt JM, Vetizou M, Waldschmitt N, et al. Fine-Tuning Cancer Immunotherapy: Optimizing the Gut Microbiome. Cancer Res. 2016;76:4602–4607. doi: 10.1158/0008-5472.CAN-16-0448. [DOI] [PubMed] [Google Scholar]
- 107.Pitt JM, Vetizou M, Daillere R, et al. Resistance Mechanisms to Immune-Checkpoint Blockade in Cancer: Tumor-Intrinsic and -Extrinsic Factors. Immunity. 2016;44:1255–1269. doi: 10.1016/j.immuni.2016.06.001. [DOI] [PubMed] [Google Scholar]
- 108.Sivan A, Corrales L, Hubert N, et al. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Science. 2015;350:1084–1089. doi: 10.1126/science.aac4255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Cramer P, Bresalier RS. Gastrointestinal and Hepatic Complications of Immune Checkpoint Inhibitors. Curr Gastroenterol Rep. 2017;19:3. doi: 10.1007/s11894-017-0540-6. [DOI] [PubMed] [Google Scholar]
- 110.Dubin K, Callahan MK, Ren B, et al. Intestinal microbiome analyses identify melanoma patients at risk for checkpoint-blockade-induced colitis. Nat Commun. 2016;7:10391. doi: 10.1038/ncomms10391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Guiducci C, Vicari AP, Sangaletti S, Trinchieri G, Colombo MP. Redirecting in vivo elicited tumor infiltrating macrophages and dendritic cells towards tumor rejection. Cancer Res. 2005;65:3437–3446. doi: 10.1158/0008-5472.CAN-04-4262. [DOI] [PubMed] [Google Scholar]
- 112.Iida N, Dzutsev A, Stewart CA, et al. Commensal bacteria control cancer response to therapy by modulating the tumor microenvironment. Science. 2013;342:967–970. doi: 10.1126/science.1240527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.van Vliet MJ, Tissing WJ, Dun CA, et al. Chemotherapy treatment in pediatric patients with acute myeloid leukemia receiving antimicrobial prophylaxis leads to a relative increase of colonization with potentially pathogenic bacteria in the gut. Clin Infect Dis. 2009;49:262–270. doi: 10.1086/599346. [DOI] [PubMed] [Google Scholar]
- 114.Montassier E, Batard E, Massart S, et al. 16S rRNA gene pyrosequencing reveals shift in patient faecal microbiota during high-dose chemotherapy as conditioning regimen for bone marrow transplantation. Microb Ecol. 2014;67:690–699. doi: 10.1007/s00248-013-0355-4. [DOI] [PubMed] [Google Scholar]
- 115.Montassier E, Gastinne T, Vangay P, et al. Chemotherapy-driven dysbiosis in the intestinal microbiome. Aliment Pharmacol Ther. 2015;42:515–528. doi: 10.1111/apt.13302. [DOI] [PubMed] [Google Scholar]
- 116.Stringer AM, Al-Dasooqi N, Bowen JM, et al. Biomarkers of chemotherapy-induced diarrhoea: a clinical study of intestinal microbiome alterations, inflammation and circulating matrix metalloproteinases. Support Care Cancer. 2013;21:1843–1852. doi: 10.1007/s00520-013-1741-7. [DOI] [PubMed] [Google Scholar]
- 117.Nam YD, Kim HJ, Seo JG, Kang SW, Bae JW. Impact of pelvic radiotherapy on gut microbiota of gynecological cancer patients revealed by massive pyrosequencing. PLoS One. 2013;8:e82659. doi: 10.1371/journal.pone.0082659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Viaud S, Saccheri F, Mignot G, et al. The intestinal microbiota modulates the anticancer immune effects of cyclophosphamide. Science. 2013;342:971–976. doi: 10.1126/science.1240537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Daillere R, Vetizou M, Waldschmitt N, et al. Enterococcus hirae and Barnesiella intestinihominis Facilitate Cyclophosphamide-Induced Therapeutic Immunomodulatory Effects. Immunity. 2016;45:931–943. doi: 10.1016/j.immuni.2016.09.009. [DOI] [PubMed] [Google Scholar]
- 120.Brandi G, Dabard J, Raibaud P, et al. Intestinal microflora and digestive toxicity of irinotecan in mice. Clin Cancer Res. 2006;12:1299–1307. doi: 10.1158/1078-0432.CCR-05-0750. [DOI] [PubMed] [Google Scholar]
- 121.de Jong FA, Kehrer DF, Mathijssen RH, et al. Prophylaxis of irinotecan-induced diarrhea with neomycin and potential role for UGT1A1*28 genotype screening: a double-blind, randomized, placebo-controlled study. Oncologist. 2006;11:944–954. doi: 10.1634/theoncologist.11-8-944. [DOI] [PubMed] [Google Scholar]
- 122.Wallace BD, Roberts AB, Pollet RM, et al. Structure and Inhibition of Microbiome beta-Glucuronidases Essential to the Alleviation of Cancer Drug Toxicity. Chem Biol. 2015;22:1238–1249. doi: 10.1016/j.chembiol.2015.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Roberts AB, Wallace BD, Venkatesh MK, Mani S, Redinbo MR. Molecular insights into microbial beta-glucuronidase inhibition to abrogate CPT-11 toxicity. Mol Pharmacol. 2013;84:208–217. doi: 10.1124/mol.113.085852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wallace BD, Wang H, Lane KT, et al. Alleviating cancer drug toxicity by inhibiting a bacterial enzyme. Science. 2010;330:831–835. doi: 10.1126/science.1191175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Rigby RJ, Carr J, Orgel K, King SL, Lund PK, Dekaney CM. Intestinal bacteria are necessary for doxorubicin-induced intestinal damage but not for doxorubicin-induced apoptosis. Gut Microbes. 2016;7:414–423. doi: 10.1080/19490976.2016.1215806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Eid A, Mahfouz MM. Genome editing: the road of CRISPR/Cas9 from bench to clinic. Exp Mol Med. 2016;48:e265. doi: 10.1038/emm.2016.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Doudna JA, Charpentier E. Genome editing. The new frontier of genome engineering with CRISPR-Cas9. Science. 2014;346:1258096. doi: 10.1126/science.1258096. [DOI] [PubMed] [Google Scholar]
- 128.Hsu PD, Lander ES, Zhang F. Development and applications of CRISPR-Cas9 for genome engineering. Cell. 2014;157:1262–1278. doi: 10.1016/j.cell.2014.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Yuan M, Webb E, Lemoine NR, Wang Y. CRISPR-Cas9 as a Powerful Tool for Efficient Creation of Oncolytic Viruses. Viruses. 2016;8:72. doi: 10.3390/v8030072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Lee CH, Steiner T, Petrof EO, et al. Frozen vs Fresh Fecal Microbiota Transplantation and Clinical Resolution of Diarrhea in Patients With Recurrent Clostridium difficile Infection: A Randomized Clinical Trial. JAMA. 2016;315:142–149. doi: 10.1001/jama.2015.18098. [DOI] [PubMed] [Google Scholar]
- 131.Zoller V, Laguna AL, Prazeres Da Costa O, Buch T, Goke B, Storr M. Fecal microbiota transfer (FMT) in a patient with refractory irritable bowel syndrome. Dtsch Med Wochenschr. 2015;140:1232–1236. doi: 10.1055/s-0041-103798. [DOI] [PubMed] [Google Scholar]
- 132.van Beurden YH, van Gils T, van Gils NA, Kassam Z, Mulder CJ, Aparicio-Pages N. Serendipity in Refractory Celiac Disease: Full Recovery of Duodenal Villi and Clinical Symptoms after Fecal Microbiota Transfer. J Gastrointestin Liver Dis. 2016;25:385–388. doi: 10.15403/jgld.2014.1121.253.cel. [DOI] [PubMed] [Google Scholar]
- 133.Alang N, Kelly CR. Weight gain after fecal microbiota transplantation. Open Forum Infect Dis. 2015;2:ofv004. doi: 10.1093/ofid/ofv004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Uzunlulu M, Telci Caklili O, Oguz A. Association between Metabolic Syndrome and Cancer. Ann Nutr Metab. 2016;68:173–179. doi: 10.1159/000443743. [DOI] [PubMed] [Google Scholar]
- 135.Zadra G, Photopoulos C, Loda M. The fat side of prostate cancer. Biochim Biophys Acta. 2013;1831:1518–1532. doi: 10.1016/j.bbalip.2013.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Esposito K, Chiodini P, Colao A, Lenzi A, Giugliano D. Metabolic syndrome and risk of cancer: a systematic review and meta-analysis. Diabetes Care. 2012;35:2402–2411. doi: 10.2337/dc12-0336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Colangelo LA, Gapstur SM, Gann PH, Dyer AR, Liu K. Colorectal cancer mortality and factors related to the insulin resistance syndrome. Cancer Epidemiol Biomarkers Prev. 2002;11:385–391. [PubMed] [Google Scholar]
- 138.Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444:1027–1031. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- 139.Cho I, Yamanishi S, Cox L, et al. Antibiotics in early life alter the murine colonic microbiome and adiposity. Nature. 2012;488:621–626. doi: 10.1038/nature11400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Backhed F, Ding H, Wang T, et al. The gut microbiota as an environmental factor that regulates fat storage. Proc Natl Acad Sci U S A. 2004;101:15718–15723. doi: 10.1073/pnas.0407076101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Longo VD, Panda S. Fasting, Circadian Rhythms, and Time-Restricted Feeding in Healthy Lifespan. Cell Metab. 2016;23:1048–1059. doi: 10.1016/j.cmet.2016.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Lee C, Raffaghello L, Brandhorst S, et al. Fasting cycles retard growth of tumors and sensitize a range of cancer cell types to chemotherapy. Sci Transl Med. 2012;4:124ra127. doi: 10.1126/scitranslmed.3003293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Attayek PJ, Ahmad AA, Wang Y, et al. In Vitro Polarization of Colonoids to Create an Intestinal Stem Cell Compartment. PLoS One. 2016;11:e0153795. doi: 10.1371/journal.pone.0153795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Fujii M, Shimokawa M, Date S, et al. A Colorectal Tumor Organoid Library Demonstrates Progressive Loss of Niche Factor Requirements during Tumorigenesis. Cell Stem Cell. 2016;18:827–838. doi: 10.1016/j.stem.2016.04.003. [DOI] [PubMed] [Google Scholar]
- 145.Weeber F, van de Wetering M, Hoogstraat M, et al. Preserved genetic diversity in organoids cultured from biopsies of human colorectal cancer metastases. Proc Natl Acad Sci U S A. 2015;112:13308–13311. doi: 10.1073/pnas.1516689112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Chen HJ, Wei Z, Sun J, et al. A recellularized human colon model identifies cancer driver genes. Nat Biotechnol. 2016;34:845–851. doi: 10.1038/nbt.3586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Kaiko GE, Ryu SH, Koues OI, et al. The Colonic Crypt Protects Stem Cells from Microbiota-Derived Metabolites. Cell. 2016;167:1137. doi: 10.1016/j.cell.2016.10.034. [DOI] [PubMed] [Google Scholar]
- 148.Brim H, Yooseph S, Zoetendal EG, et al. Microbiome analysis of stool samples from African Americans with colon polyps. PLoS One. 2013;8:e81352. doi: 10.1371/journal.pone.0081352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Nugent JL, McCoy AN, Addamo CJ, Jia W, Sandler RS, Keku TO. Altered tissue metabolites correlate with microbial dysbiosis in colorectal adenomas. J Proteome Res. 2014;13:1921–1929. doi: 10.1021/pr4009783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Mira-Pascual L, Cabrera-Rubio R, Ocon S, et al. Microbial mucosal colonic shifts associated with the development of colorectal cancer reveal the presence of different bacterial and archaeal biomarkers. J Gastroenterol. 2015;50:167–179. doi: 10.1007/s00535-014-0963-x. [DOI] [PubMed] [Google Scholar]
- 151.Zackular JP, Rogers MA, Ruffin MTt, Schloss PD. The human gut microbiome as a screening tool for colorectal cancer. Cancer Prev Res (Phila) 2014;7:1112–1121. doi: 10.1158/1940-6207.CAPR-14-0129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Goedert JJ, Gong Y, Hua X, et al. Fecal Microbiota Characteristics of Patients with Colorectal Adenoma Detected by Screening: A Population-based Study. EBioMedicine. 2015;2:597–603. doi: 10.1016/j.ebiom.2015.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Urbaniak C, Gloor GB, Brackstone M, Scott L, Tangney M, Reid G. The Microbiota of Breast Tissue and Its Association with Breast Cancer. Appl Environ Microbiol. 2016;82:5039–5048. doi: 10.1128/AEM.01235-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Chan AA, Bashir M, Rivas MN, et al. Characterization of the microbiome of nipple aspirate fluid of breast cancer survivors. Sci Rep. 2016;6:28061. doi: 10.1038/srep28061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Flemer B, Lynch DB, Brown JM, et al. Tumour-associated and non-tumour-associated microbiota in colorectal cancer. Gut. 2016 doi: 10.1136/gutjnl-2015-309595. [DOI] [PMC free article] [PubMed]
- 156.Hale VL, Chen J, Johnson S, et al. Shifts in the fecal microbiota associated with adenomatous polyps. Cancer Epidemiol Biomarkers Prev. 2016 doi: 10.1158/1055-9965.EPI-16-0337. [DOI] [PMC free article] [PubMed] [Google Scholar]