Abstract
Objective;
Pregabalin (PGB) is an α2β calcium channel subunit ligand that has previously been shown to reduce chronic pain in multiple conditions. Preclinical studies indicate that PGB may down-regulate brain glutamate release while also inhibiting astrocyte induction of glutamatergic synapse formation. Recent clinical research supports PGB modulating glutamatergic activity and functional brain connectivity in order to produce analgesia. However, no studies have examined concurrent changes in brain gray matter volume (GMV) or evoked-pain connectivity in humans receiving PGB.
Methods
Sixteen female fibromyalgia patients completed a randomized double-blind two-period cross-over study of PGB versus placebo (PBO). Before and after each period, patients had high-resolution structural and evoked pressure-pain functional brain imaging. GMV was analyzed using voxel-based morphometry, and functional connectivity during evoked pressure-pain was also assessed.
Results
PGB administration significantly reduced GMV within the posterior insula bilaterally, whereas there were no significant changes in insular GMV following PBO treatment. GMV reductions were also observed when comparing PGB versus PBO treatment in the medial frontal gyrus, which were associated with reduced clinical pain. These reductions in insular GMV were associated with concomitant reductions in connectivity to the default mode network (DMN), which was also associated with reduced clinical pain.
Conclusion
Short-term PGB treatment alters brain structure and evoked-pain connectivity, and these decreases were associated with reduced clinical pain. We speculate that these fairly rapid changes in GMV may be related to brain neuroplasticity. It is unknown if these effects are generalizable to other chronic pain states.
Keywords: Fibromyalgia, Pregabalin, Voxel-Based Morphometry, fMRI, Evoked-pain connectivity, Gray Matter Volume
Fibromyalgia (FM) is a chronic pain syndrome characterized by widespread musculoskeletal pain and multiple concomitant symptoms including fatigue, sleep disturbances, cognitive dysfunction, and depression(1). FM is estimated to affect approximately 2–8% of the U.S. population, depending on criteria used(1). Understanding of the disorder has increased substantially in recent years and research indicates that the primary symptom of FM, chronic widespread pain, is neurogenic in origin(1). Therapeutic options that target the central nervous system have some efficacy in FM, including tricyclic antidepressants(2), selective norepinephrine and serotonin reuptake inhibitors(3) and certain anticonvulsants, such as gabapentin(4) and pregabalin(5).
Pregabalin (PGB), and its related compound gabapentin, were initially developed as antiepileptic drugs(6). However, both substances have been shown to be efficacious in various chronic pain states (4, 7). Pregabalin is indicated for treatment of FM in the U.S., Japan, and other countries(8).
PGB and gabapentin bind to the α2β subunit of voltage-gated calcium channels and reduce calcium influx at nerve terminals, causing a decreased release of neurotransmitters glutamate(9), norepinephrine(10), and substance P(11). Binding of PGB to α2β is the proposed primary mechanism by which this drug acts as an anticonvulsant, anxiolytic, and analgesic (12). Our recent trial in FM subjects demonstrated that PGB reduces insular glutamate levels and the degree of resting (or intrinsic) connectivity between this structure and the Default Mode Network (DMN)(13), a constellation of brain regions known to support self-referential cognition and auto-biographical memory(14). In addition, these reductions were associated with decreased clinical pain. However, it remains unknown whether this compound also alters brain structure and/or functional connectivity during evoked-pain stimuli.
This study builds upon our previous PGB investigation (13) utilizing the same FM patients. To better understand the range of PGB’s effects on the brain, we performed Voxel-Based Morphometry (VBM) and evoked-pain connectivity analyses to quantify levels of regional brain gray matter volume (GMV) and connectivity, respectively, before and after PGB treatment in FM patients. While structural brain imaging has been used to explore brain GMV in chronic pain(15), there is a discrepancy in findings with some studies showing decreases(16) and others reporting increases(17). While still unverified, these findings may point towards two complementary processes underlying the pathophysiology of FM: increases in GMV in pro-nociceptive structures and decreases in anti-nociceptive regions. Decreases in GMV in areas implicated in pain inhibition could reflect impaired descending inhibitory pathways, while increases in other areas could reflect increased pro-nociceptive activity. Given preclinical and clinical findings of PGB reducing glutamatergic neurotransmission, we hypothesized that PGB may actually reduce regional GMV in pro-nociceptive areas. Additionally, since changes seen in chronic pain are thought to be due to neuroplasticity, we further hypothesized that GMV changes would be reversible following PGB treatment(18). We were also interested whether regions of altered GMV displayed changes in evoked-pain connectivity as an effect of PGB. Evoked-pain connectivity is of interest as it allows us to observe alterations in brain networks in response to stimulated pain experiences. Previous studies have shown changes in resting state functional magnetic resonance imaging (fMRI) in FM, particularly in pain processing regions, such as the insula(19). However, these studies did not explore how connectivity patterns during evoked-pain might change within regions showing alterations in GMV. We hypothesized that due to the effect of gabapentin on blocking excitatory synaptogenesis (20), PGB treatment may induce reductions in connectivity between pro-nociceptive and DMN regions during evoked-pain fMRI, and these changes should correlate with reduced clinical pain.
Patients and Methods
Participants
Twenty-three female FM patients (mean age±SD; 38.6±12.2years) from a previously reported trial(13) participated in the current study. All participants gave written informed consent and study protocols were approved by the University of Michigan Institutional Review Board and Pfizer (Groton, CT). Imaging data were stored, validated, analyzed, and assessed for quality at the University of Michigan independently of Pfizer personnel. Clinical data were double-entered, quality checked, and databases were locked before analysis. Full results are reported at clinicaltrials.gov.
FM participants were included if they: 1) met the American College of Rheumatology (1990) criteria(21) for the diagnosis of FM for >1 year; 2) had continued presence of pain >50% of days; 3) were willing to limit the introduction of new medications/treatment modalities for control of FM symptoms during the study; 4) were >18 and up to 70 years old; 5) were female; 6) were right-handed; and 7) were capable of giving written informed consent. Participants were excluded if they: 1) had current or history of opioid/narcotic analgesic use; 2) had a history of substance abuse; 3) had presence of concurrent autoimmune/inflammatory disease (e.g. rheumatoid arthritis, inflammatory bowel disease, etc.) that cause pain; 4) had concurrent participation in other therapeutic trials; 5) were pregnant and nursing mothers; 6) had severe psychiatric illnesses (current schizophrenia, suicidal ideation, substance abuse within two years); or 7) had current major depression.
Treatment
Patients completed a randomized double-blind two-period cross-over study of PGB versus placebo (PBO). During PGB treatment, drug concentration was dose-escalated to 450mg/day over the course of 14 days. During PBO, sugar pills were taken over 14 days to match the PGB period. Before and after each period (PGB/PBO), patients underwent magnetic resonance imaging (MRI), which included high resolution T1 structural and T2* MRI scanning during which patients experienced evoked pressure-pain applied to the thumbnail bed (Supplementary Figure 1).
Behavioral
Clinical pain was assessed using the Visual Analog Scale (VAS)(22) and Short Form McGill Pain Questionnaire (SF-MPQ), which included total, as well as sensory and affective pain sub-domains(23), immediately before and following PGB and PBO scanning as a measure of present pain. We additionally collected demographics, medication use, and anxiety and depression data at baseline(24)(Supplementary Table 1). Pre-post changes in behavioral measures were assessed with paired t-tests.
Experimental pain
Experimental pressure-pain thresholds were determined during the first behavioral visit prior to scanning, and were held constant for subsequent scans. Thresholds were determined similarly to a previously published study(25) using a multiple random staircase method(26) and rated using the Gracely Box Scale (GBS), a 21-box numerical descriptor scale(27).
At each of the four fMRI sessions (pre/post PGB, pre/post PBO), patients underwent two 10-minute evoked-pain functional scans. Pressure-pain was delivered using a pneumatic system, through which air was delivered to a custom-made stimulation device with a 1-cm2 hard, rubber, circular probe pressed against the left thumbnail bed. Software was created to interface with the analogue air controller, using Agilent VEE Pro, which regulated predetermined pressures to be applied. Additionally, the VEE Pro software communicated with E-Prime v1.0 to maintain accurate timing throughout the experiment.
Identical 10-minute evoked-pain runs delivered four different pressure-pain stimulus conditions. Conditions were divided into equal 25-second blocks, and were pseudo-randomized throughout scanning. The first condition was rest, where the probe remained on the thumb for the full 25 seconds but did not elicit pain, and this condition was interleaved between the other three conditions wherein pressure was applied to the thumbnail in a fluctuating manner, applying short stimuli every 2.5 seconds throughout the 25 second block. These conditions were personalized from each patient’s initial behavioral multiple random staircase session and contained: “light touch,” consisting of no more than 0.25kg/cm2; “mild-moderate pain,” eliciting between 7–8 on the GBS; and “slightly-intense pain,” eliciting between 13–14 on the GBS (Supplementary Figure 2). These values allowed for comparison of changes in response to subjective pressure-pain levels. To assess evoked-pain connectivity changes to objective equal stimulus pressure intensity, either “mild-moderate” or “slightly-intense” pain conditions were adjusted to 2.0kg/cm2 for all patients. Immediately following pressure-pain scanning, patients were asked to rate the lowest and highest responses to the pressure-pain stimuli using the GBS.
MRI Protocol
MRI was performed on a 3.0T GE Signa Scanner (LX VH3 release, Neuro-optimized gradients). For structural scanning, T1-weighted gradient echo data set (TR=12.3ms, TE=3.4ms, flip angle=25°, FOV=256×256, yielding 106 sagittal slices with acquired voxel size of 0.94×0.94×1.5mm and resampled to 1×1×1mm) was acquired using an Eclipse 3.0T 94-quadrature head coil. Inspection of individual T1 MR-images revealed no gross morphological abnormality for any participant. Evoked-pain fMRI scans were collected on the same 3T GE magnet. We utilized a reverse-spiral gradient echo T2*-weighted blood oxygenation-level dependent (BOLD) pulse sequence (TR2500ms, TE=30ms, 240 volumes, 48 AC-PC aligned slices, voxel size=3.44×3.44×3.0 mm).
VBM Analyses
SPM5 software (Functional Imaging Laboratories, London, UK) running under Matlab was used to pre-process and analyze structural data(28). Estimation of total gray matter (GM), white matter (WM), and cerebrospinal fluid (CSF) was performed by segmenting the original image into GM, WM and CSF, using the Ibaspm toolbox(29).
Pre-processing of structural images was performed using VBM5.1 (C. Gaser, default settings), which involved image re-angulation and re-orientation, re-alignment within-subjects to the first time point, spatial normalization, segmentation, and spatial smoothing (Gaussian kernel of 12mm full-width at half-maximum for GM images). Un-modulated images were used for statistical analyses. We utilized a flexible factorial model with age as a nuisance variable, and performed two VBM analyses. In analysis 1, we analyzed effects of PGB and PBO treatments independently on regional GMV, and in analysis 2, we looked for significant regional GMV differences between PGB and PBO treatments (treatment*time interaction). Significant differences in GMV values between time points and treatment arms were identified, applying voxel-wise statistics within the general linear model (GLM). Regions of interest (ROIs) detected from the whole brain group analyses were extracted using Marsbar(30). Additional exploratory analyses were performed examining the reversibility of GMV changes throughout the placebo washout period for patients receiving PGB first. Threshold for VBM significance was set at p<0.001 uncorrected voxel-wise, and cluster-level significance corrected for multiple comparisons using a family-wise threshold (FWE) pfwe<0.05. For a priori ROIs involved in pain processing (e.g. insula/thalamus) that did not meet whole brain significance, we utilized a Small Volume Correction (SVC) with an 8mm sphere and pfwe< 0.05 approach based on previously published pain literature coordinates(19, 31)(Supplementary Table 2). Bivariate Spearman correlational analyses were performed between changes in clinical pain and changes in GMV, with significance set at p<0.05. No corrections were made for multiple comparisons.
Functional Connectivity (fcMRI) Analyses
During evoked-pain functional scans, physiological data were collected simultaneously as cardiac and respiratory fluctuations are known to influence fMRI intrinsic connectivity(32). Respiratory volume data were collected by securing a GE magnetic resonance-compatible chest plethysmograph around each subject’s abdomen. Cardiac data were collected using an infrared pulse oximeter (GE) attached to the subject’s right middle finger. Participants’ motion was minimized using foam pads placed around the head. To allow for scanner stabilization and equilibrium, the first 6 volumes of each functional scan were discarded.
Raw data acquired from fMRI were corrected for physiological motion effects using the RETROICOR method(33). Data were preprocessed using standard protocol in SPM8 (Functional Imaging Laboratories, London, UK) through Matlab, including motion correction via realignment to the first volume in the series, normalization to the Montreal Neurological Institute (MNI) template, and smoothing with an 8mm FWHM Gaussian Kernel. Motion during scanning was assessed using three translations and rotations for each scan. Thresholds for translation were set to ±2 mm, and rotation at ±1º, and subjects exceeding these thresholds were excluded from analyses.
Pre-processed data underwent connectivity analyses via the Functional Connectivity Toolbox v2.0 (CONN)(www.nitrc.org/projects/conn) in SPM8. CONN toolbox utilizes a component-based noise correction method (CompCor), which increases sensitivity and selectivity allowing for a high degree of interscan reliability(34). During preprocessing, WM, CSF, and effects of rest and pain were included as confounds within the GLM. A band pass filter (.01–.1 Hz) was used to remove high frequency noise and linear drift artifacts. Regressors of no interest included motion in first-level analyses, and age in second-level analyses. Using CONN, we assessed seed-to-voxel correlations calculated from the entire evoked-pain run (first-level analysis). Evoked-pain connectivity analyses utilized seeds created from clusters identified in VBM analyses, and assessed for connectivity changes between these regions and other areas using a seed to whole brain approach. For analysis 3, we examined differences in connectivity during evoked-pain (mild-moderate and slightly-intense) conditions (pain vs. light touch contrast) fMRI pre and post PGB and PBO treatments independently, while in analysis 4, we contrasted differences between evoked-pain connectivity during PGB and PBO treatments utilizing a flexible factorial model. We applied a voxel-wise p<0.001 uncorrected threshold with cluster-level significance corrected for multiple comparisons, using a pfwe<0.05. For a priori ROIs involved in pain processing (e.g. insula/thalamus) that did not meet whole brain significance, we utilized a SVC with an 8mm sphere and pfwe< 0.05 approach based on previously published pain literature coordinates(19, 31)(Supplementary Table 2). Bivariate Spearman correlational analyses were performed between changes in clinical pain and changes in connectivity values, with significance set at p<0.05. No corrections were made for multiple comparisons.
Proton Magnetic Resonance Spectroscopy
As our previously published study showed decreases in glutamate and glutamine (Glx) following PGB(13), in an exploratory analysis, we also correlated changes in insular Glx with changes in GMV. Glx levels were acquired through 1H-MRS imaging on a GE 3.0T magnetic resonance scanner (GE, Milwaukee, WI) at rest before and after each treatment. Glx was acquired for the right anterior and posterior insula, with single-voxel point resolved spectroscopy spectra acquired for each ROI with the following parameters: TR=3000ms, TE=30ms, 90-degree flip angle, NEX 8, FOX 16, with a voxel size of 2×2×3 cm. In this study, we correlated changes in insula GMV and evoked-pain connectivity with levels of Glx. Significance was determined as p<0.05.
Results
Participants in study
Sixteen (mean age±SD; 37.3±10.5 years) of the 23 FM patients were included in VBM analyses, and a subset of 15 was included in connectivity analyses. Reasons for exclusion of participants were: 4 had excessive motion during MRI scanning, 2 subjects missed study drug at least once, 1 participant had dental work compromising MRI scanning, and 1 participant suffered an injury leading to development of new pain symptoms. One subject with excessive motion during experimental pain runs had usable structural data and was included in VBM analyses.
Changes in Clinical Pain
For the 16 subjects participating in VBM analyses 1&2, as expected given the small sample, there were trends but no significant reductions in clinical pain for PGB (VAS, p=0.114; SF-MPQ, p=0.216) or PBO (VAS, p=0.223; SF-MPQ, p=0.101) treatments (Supplementary Figure 3). There were no significant reductions in clinical pain for the 15 VBM subjects participating in connectivity analyses 3&4, for either PGB (VAS, p=0.183; SF-MPQ, p=0.328) or PBO (VAS, p=0.101; SF-MPQ, p=0.196) treatments.
PGB Effects on Brain Structure and Connectivity during Evoked-Pain
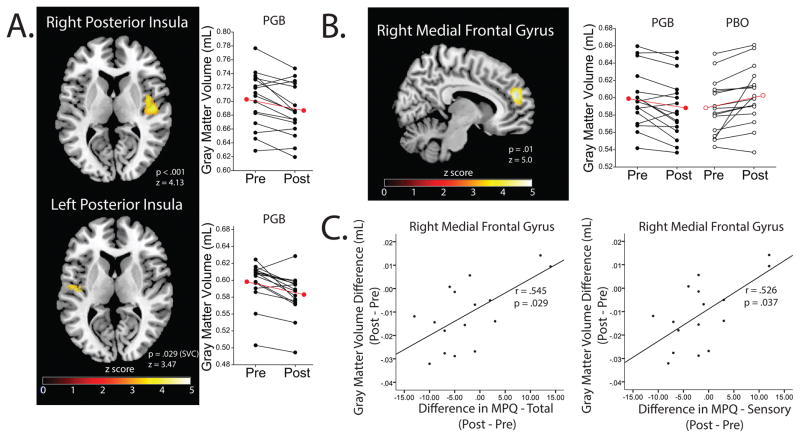
For analysis 1, FM patients displayed significant decreases in volumetric size in GMV following PGB treatment within the following regional brain structures: 1) bilateral posterior insula (right, pfwe<0.001; left, pfwe=0.029 (SVC, x=−39, y=−15, z=1)(31)), 2) medial/superior frontal gyri (pfwe<0.001), and 3) left precentral/postcentral/middle frontal gyri (pfwe=0.046). Following PBO treatment, FM patients had significantly lower GMV in the right superior temporal gyrus (pfwe=0.018)(Table 1, Figure 1). No regions displayed increases in GMV for either PGB or PBO.
Table 1.
Changes in GMV as independent effects of PGB and PBO treatments as well as between treatment modalities
| Brain Region | BA | Cluster Size (# of voxels) | p(FWE) | z-score (peak value) | Peak Voxel Coordinates (MNI) | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| Post PGB < Pre PGB | |||||||
| Right Posterior Insula | 13 | 5813 | < .001 | 4.13 | 48 | −16 | 10 |
| Medial Frontal Gyrus/Superior Frontal Gyrus | 6, 8,9, 32 | 4459 | < .001 | 4.46 | 8 | 50 | 48 |
| Left Precentral/Postcentral/Middle Frontal Gyrus | 4, 6, 8, 9 | 3573 | .046 | 4.09 | −56 | −14 | 44 |
| Left Posterior Insula | 13 | 428 | .029 (SVC) | 3.47 | −46 | −11 | 6 |
| Post PGB > Pre PGB | |||||||
| No significant clusters | |||||||
| Post PBO < Pre PBO | |||||||
| Right Superior Temporal Gyrus | 21, 38 | 1728 | .007 | 3.94 | 43 | 15 | −23 |
| Post PBO > Pre PBO | |||||||
| No significant clusters | |||||||
| Change in PGB (post – pre) < Change in PBO (post – pre) | |||||||
| Right Medial Frontal Gyrus | 9, 10 | 1616 | .010 | 5.05 | 9 | 50 | 22 |
| Left Superior Temporal Gyrus | 22, 42 | 1194 | .039 | 4.41 | −64 | −4 | 6 |
| Cuneus | 7, 19 | 1961 | .004 | 3.90 | −4 | −86 | 32 |
| Left Precentral/Postcentral Gyrus | 2, 3, 4, 6 | 1410 | .019 | 3.89 | −59 | −17 | 40 |
| Superior Frontal Gyrus | 8 | 1440 | .017 | 3.73 | 5 | 34 | 51 |
| Change in PBO (post – pre) > Change in PGB (post – pre) | |||||||
| None | |||||||
Abbreviations: BA= Brodmann Area; MNI= Montreal Neurological Institute; pfwe= family wise error; SVC= Small Volume Correction; PBO= Placebo; PGB= Pregabalin; GMV= Gray Matter Volume.
Figure 1.
Panel A: Acute PGB treatment (14 days) in FM independently decreases insular GMV bilaterally (analysis 1). Panel B: The greatest decreases in GMV between PGB and PBO treatments are seen in the right medial frontal gyrus (analysis 2). Panel C: Decreases in medial frontal gyrus GMV in the PGB cohort is associated with decreases in MPQ total and sensory pain subscale scores (analysis 2). Black lines with filled in circles indicate individual PGB subject changes in GMV. Black lines with empty circles indicate GMV changes during the PBO period. Red lines indicate group means. Abbreviations: FM= Fibromyalgia GMV= Gray Matter Volume; MPQ= McGill Pain Questionnaire; PGB= Pregabalin; PBO= Placebo; SVC= Small Volume Correction.
For the treatment*time interaction in analysis 2, regions displaying reductions in volumetric size of GMV in PGB more than PBO treatment included the: 1) right medial frontal gyrus (MFG)(pfwe=0.010), 2) left superior temporal gyrus (pfwe=0.039), 3) left cuneus (pfwe=0.004), 4) left pre/postcentral gyrus (pfwe=0.019), and 5) superior frontal gyrus (pfwe=0.017). No regions showed significant reductions in PBO more than PGB (Table 1, Figure 1). Furthermore, reductions in right MFG GMV were correlated with decreased SF-MPQ total (r=0.545, p=0.029) and sensory (r=0.526, p=0.037) pain scores (Table 3, Figure 1).
Table 3.
Changes in VBM and evoked-pain connectivity metrics are associated with decreased clinical pain
| Brain Region(s) | Clinical Measure | r - value | p - value |
|---|---|---|---|
| PGB GMV Decreases from Analysis 2 Correlated to Decreases in Clinical Pain | |||
| Medial Frontal Gyrus | Difference in McGill Total Score | .545 | .029** |
| Difference in McGill Sensory Score | .526 | .037** | |
| PGB Evoked-Pain Connectivity fMRI Decreases from Analysis 3 Correlated to Decreases in Clinical Pain | |||
| Right Posterior Insula to Thalamus | Difference in VAS Score | .506 | .065* |
| PGB Evoked-Pain Connectivity fMRI Decreases from Analysis 4 Correlated to Decreases in Clinical Pain | |||
| Left Posterior Insula to Middle/Medial Frontal Gyrus | Difference in McGill Total Score | .603 | .017** |
| Difference in McGill Sensory Score | .601 | .018** | |
| PGB Evoked-Pain Connectivity fMRI Increases from Analysis 4 Correlated to Decreases in Clinical Pain | |||
| Precentral/Postcentral Gyrus to Posterior Cingulate/Precuneus | Difference in VAS Score | −.637 | .019** |
| Left Posterior Insula to Middle Temporal Gyrus | Difference in McGill Affective Score | −.537 | .039** |
Differences in GMV, evoked-pain connectivity metrics, and clinical measures are calculated as post minus pre values; Abbreviations: fMRI= functional Magnetic Resonance Imaging; PGB= Pregabalin; VAS= Visual Analog Scale;
indicates trend (p < .1);
indicates significant correlation (p < .05).
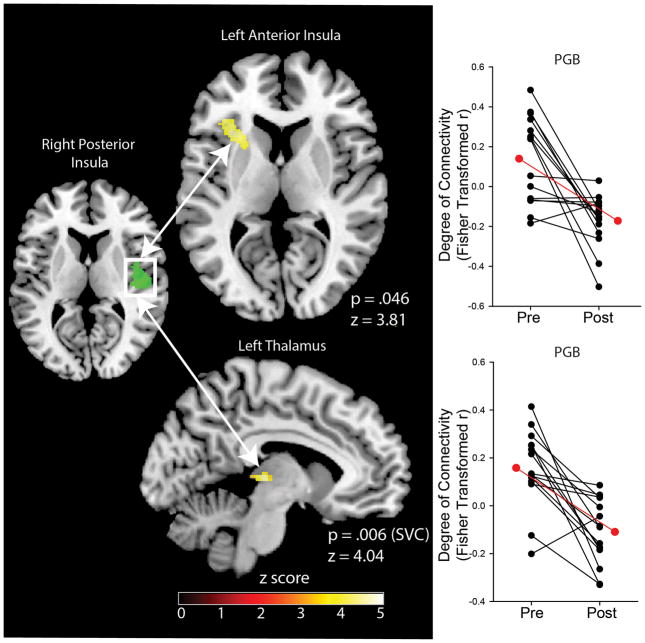
In analysis 3, we observed significant decreases in evoked-pain connectivity as an independent effect of PGB between the right posterior insula (seed) and left anterior insula (pfwe=0.046), and left thalamus (pfwe=0.006 (SVC, x=−10, y=−22, z=6)(19))(Table 2, Figure 2); i.e. the right posterior insula and left anterior insula displayed reduced temporal correlation during evoked pain following PGB. No increases in connectivity between any areas in response to evoked-pain connectivity as an effect of PGB were observed. No decreases or increases in evoked-pain connectivity as a result of PBO were observed. Furthermore, no differences in connectivity as an effect of PGB or PBO independently were found for the remaining VBM seeds, including the MFG and precentral/postcentral gyrus.
Table 2.
Evoked-pain connectivity fMRI differences in regions showing changes in GMV (seeds) following PGB and PBO treatment and between treatment modalities
| Seed | Target Region | BA | Cluster Size (# of voxels) | p(FWE) | z-score (peak value) | Peak Voxel Coordinates (MNI) | ||
|---|---|---|---|---|---|---|---|---|
| (x | y | z) | ||||||
| Evoked-Pain Connectivity Decreases as an Effect of PGB | ||||||||
| Right Posterior Insula | Left Anterior Insula | 13 | 212 | .046 | 3.81 | −30 | 20 | 10 |
| Left Thalamus | N/A | 60 | .006 (SVC) | 4.04 | −4 | −26 | 2 | |
| Evoked-Pain Connectivity Increases as an Effect of PGB | ||||||||
| No significant clusters | ||||||||
| Evoked-Pain Connectivity Decreases as an Effect of PBO | ||||||||
| No significant clusters | ||||||||
| Change in PGB (post – pre) < Change in PBO (post – pre) | ||||||||
| Left Posterior Insula | Right Inferior Parietal Lobule | 40 | 1223 | <.001 | 5.02 | 36 | −44 | 52 |
| Middle/Medial Frontal Gyrus | 10, 11 | 216 | .004 | 4.89 | −26 | 52 | −6 | |
| Right Posterior Insula | Cuneus | 19 | 351 | .004 | 4.88 | −4 | −84 | 26 |
| Left Anterior Insula | 13 | 353 | .004 | 4.62 | −36 | 28 | 8 | |
| Left Precentral/Postcentral Gyrus | Left Postcentral Gyrus | 2, 3 | 195 | .050 | 4.64 | −42 | −22 | 48 |
| Change in PGB (post – pre) > Change in PBO (post – pre) | ||||||||
| Left Posterior Insula | Middle Temporal Gyrus | 37 | 251 | .019 | 4.31 | 50 | −62 | 2 |
| Left Precentral/Postcentral Gyrus | Left Posterior Cingulate/Precuneus | 23, 30, 31 | 392 | .002 | 4.30 | −6 | −58 | 12 |
Abbreviations: BA=Brodmann Area; MNI=Montreal Neurological Institute; pfwe=family wise error; SVC= Small Volume Correction; PBO= Placebo; PGB= Pregabalin; fMRI= functional Magnetic Resonance Imaging.
Figure 2.
A region of the right posterior insula where we observed decreases in GMV showed reduced evoked-pain connectivity to the left anterior insula and left thalamus during the PGB treatment period (analysis 3). Black lines indicate individual seed to target connectivity changes during evoked-pain as an effect of PGB. Red lines indicate group means. Right posterior insula seed was obtained from VBM result. Abbreviations: GMV= Gray Matter Volume; PGB= Pregabalin; SVC= Small Volume Correction; VBM= Voxel-Based Morphometry.
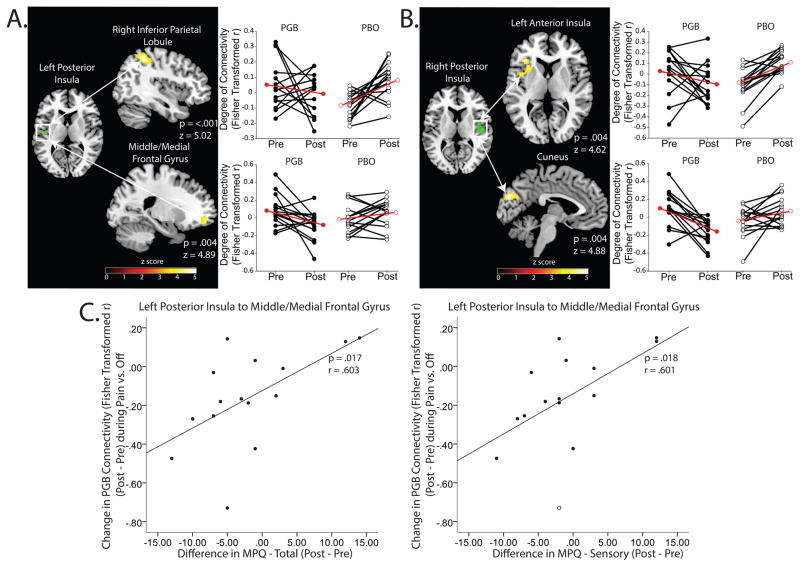
For analysis 4, there was significantly decreased evoked-pain connectivity in the PGB period as compared to PBO for the: 1) left posterior insula(seed)-right inferior parietal lobule (IPL)(pfwe<0.001) as well as a cluster encompassing the middle/medial frontal gyrus (pfwe=0.004), 2) right posterior insula(seed)-left anterior insula (pfwe=0.004) as well as the cuneus (pfwe=0.004)(Table 2, Figure 3A&B), and 3) within the left precentral/postcentral gyrus (pfwe=0.050). Decreases in insula-MFG connectivity were associated with decreases in SF-MPQ total (r=0.603; p=0.017) and sensory (r=0.601; p=0.018) pain scores (Table 3, Figure 3C). We also observed an increase in evoked-pain connectivity in PGB relative to PBO between the: 1) left posterior insula-middle temporal gyrus (pfwe=0.019) and 2) left precentral/postcentral gyrus-left posterior cingulate/precuneus (pfwe=0.002).
Figure 3.
Panel A: A region of the left posterior insula where we observed decreases in GMV showed decreased evoked-pain connectivity to DMN structures (right inferior parietal lobule and middle/medial frontal gyrus) between PGB and PBO treatments (analysis 4). Panel B: Additionally, a region of the right posterior insular where we observed decreases in GMV showed concomitant decreases in evoked-pain connectivity to the left anterior insula and the cuneus (analysis 4). Panel C: Reductions in left posterior insula to middle/medial frontal gyrus evoked-pain connectivity during the PGB period are associated with reductions in MPQ total and sensory pain scores. Right/left posterior insular seeds were obtained from VBM results.
Black lines with filled in circles indicate individual PGB subject changes in evoked-pain connectivity. Black lines with empty circles indicate evoked-pain connectivity changes during PBO. Red lines indicate group means. Abbreviations: GMV= Gray Matter Volume; MPQ= McGill Pain Questionnaire; PGB= Pregabalin; PBO= Placebo.
Relationship between Changes in Brain Structure and Connectivity and Glx
We found no relationship between changes in GMV and changes in Glx in the right posterior insula (p=0.770). There was no relationship between changes in Glx and changes in the degree of evoked-pain connectivity between the right posterior insula and left anterior insula (p=0.211).
Reversal of Gray Matter Volume Changes following PGB Treatment
For the n=7 subjects receiving PGB first, we also explored the reversibility of GMV changes by choosing pain processing regions identified in VBM analyses (left/right insula, analysis 1) and regions showing correlations to clinical pain (MFG, analysis 2). During the washout period between post-PGB (period 1) and pre-PBO treatment (period 2), there were significant increases in GMV for the right posterior insula (p=0.032)(Supplementary Figure 4), the MFG (p=0.020), and a trend for the left posterior insula (p=0.065). Following washout (pre-PBO), differences in GMV relative to baseline (pre-PGB) were not different (right posterior insula, p=0.896; left posterior insula, p=0.703; MFG, p=0.464).
Discussion
The aim of this study was to investigate changes in regional brain morphology as well as seed-based evoked-pain connectivity, associated with changes in GMV, during PGB treatment in FM patients. Our data are the first to show that after acute PGB administration (14 days), regional GMV in the MFG and bilateral insula decreased and then increased within two weeks during washout. We have also shown that these same regions showing reductions in GMV as an effect of PGB treatment also displayed reduced connectivity during evoked-pain stimuli. Perhaps most importantly, changes in GMV and connectivity during evoked-pain were associated with reductions in clinical pain ratings.
Changes in Brain Gray Matter Volume
Both the main effect of PGB (analysis 1) and the comparison between PGB and PBO (analysis 2) revealed significant decreases in regional GMV. PGB alone decreased GMV in the bilateral posterior insula (analysis 1), while the greatest reductions between PGB and PBO treatments were found in the MFG (analysis 2). While chronic pain is typically associated with decreases in GMV(16), increases have also been noted(17) (see below).
Our group has previously shown that FM patients displayed increased levels of glutamine+glutamate (Glx) relative to controls, particularly in the right posterior insula, with levels of Glx being positively correlated with the degree of experimental pain(35). Furthermore, our previous study using the same group of patients as the current analysis revealed that PGB treatment lowered Glx levels in the right posterior insula(13), and reduced connectivity between the insula and the DMN was in turn correlated to reduced clinical pain. Here, we extend these findings to effects on brain structure and associated connectivity during evoked-pain. Based on preclinical data demonstrating that α2β ligands reduce excitatory synapse formation(20), and our data demonstrating that PGB reduces levels of abnormal Glx in FM, we speculate that the decreased GMV seen in our study may be due to decreased glutamate synapse number. While there was no significant correlation between changes in right posterior insular GMV and changes in Glx as an effect of PGB, we note that this may be due to the GMV cluster and spectroscopy voxel not directly overlapping, as well as the small sample size in this pilot study.
The MFG showed the greatest extent of GMV decreases during PGB relative to PBO treatments (analysis 2). While the role of GMV in the MFG in chronic pain is not well understood, this region is part of the Default Mode Network (DMN)(36). Increased MFG connectivity within the DMN in chronic pain has been associated with increased pain rumination, which could impede chronic pain treatment efficacy(37). We have previously reported increased baseline connectivity in FM both within the DMN(38) as well as between the DMN-insula. Specific reductions in DMN-insula connectivity following both non-pharmacological interventions(39) as well as pharmacologic treatment with PGB(13) correlated with reduced clinical pain. Here we extend these findings to structural GMV data showing that DMN GMV reductions as an effect of PGB are also correlated with improved clinical pain.
We acknowledge that it is somewhat difficult to interpret our results of decreasing GMV following successful treatment with PGB as the existing literature of structural imaging in chronic pain shows both increases and decreases in GMV (16, 17). Long-term reductions in GMV, particularly in the setting of chronic pain, have been speculated to involve neuronal atrophy associated with inflammatory processes and/or excitotoxicity (16). However, from a biological perspective, the mechanism of PGB reducing excitatory neurotransmitters is somewhat in agreement with our results here namely: reduced excitatory neurotransmitters (13) are observed in the same region as reduced GMV (i.e. right posterior insula). It is also important to note that our findings originate from an acute within-subject longitudinal treatment study in patients, and not a cross sectional patient versus control analysis. As we did not perform PGB administration in pain free controls, extrapolation of our findings to case-control analyses should be viewed with caution.
That said, Seminowicz et al. performed a non-pharmacological treatment study in chronic low back pain patients and observed increases in cortical thickness in the dorsolateral prefrontal cortex which were associated with improved symptoms(40). This result could be in alignment with our findings as they observed increases in cortical thickness in a largely “anti-nociceptive” region while our findings show reduction in GMV in largely “pro-nociceptive” regions such as the insula. More trials in the same patient population with the same intervention could verify this interpretation, as could neuroimaging experiments in animal models of FM treated with PGB.
Evoked-Pain Connectivity fMRI
We also investigated the effect of PGB on evoked-pain connectivity, using seeds showing decreases in GMV with PGB. Evoked-pain connectivity was of interest since it allowed us to examine effects of PGB treatment on functional networks during a stimulated pain experience. In analysis 3, we found that PGB treatment reduced connectivity between the right posterior insula(seed) and left anterior insula, as well as the thalamus, during evoked-pain. In analysis 4, significant decreases in evoked-pain connectivity in PGB relative to PBO were noted between: 1) the left posterior insula (seed) to right IPL, as well as the MFG, and 2) between the right posterior insula(seed) to left anterior insula, a cluster found to largely overlap that identified in analysis 3.
Prior studies analyzing the effects of PGB have shown that treatment reduced both insular activity(41), and insula-DMN connectivity was related to decreased clinical pain (13). The decreased pro-nociceptive evoked-pain connectivity between the posterior insula–anterior insula in analyses 3 and 4 and posterior insula–thalamus in analysis 3 are consistent with these findings and extend them to connectivity differences during an evoked-pain state. The role of the thalamus(42) and insula(43) in pain processing are well documented, with both displaying abnormal involvement in chronic pain (44–46). Furthermore, the medial dorsal nucleus of the thalamus identified in our study has been shown to display subtle structural alterations in chronic pain(47). The direction of insula-thalamus connectivity changes are consistent with changes in GMV reported in this study, as well as other neural metrics previously published with this FM cohort (see above) (13).
In analysis 4, we noted decreased connectivity between the left posterior insula-MFG, as well as the IPL, another DMN region implicated in chronic pain(48). A main difference between our previous study(13) and the current study findings show altered MFG connectivity during evoked-pain. The MFG, and more generally frontal lobe activity during painful experiences, is related to the attentional processing of pain(49). Here, we show that decreases in insula-MFG connectivity during painful experiences as an effect of PGB are associated with decreases in clinical pain. We speculate that one of the mechanisms by which PGB may impart its analgesic effects is by reducing an abnormally elevated level of attentional pain processing in FM during painful states.
Reversal of GMV changes
Interestingly, we found that GMV decreases did not persist following acute PGB treatment. There are possible explanations for this reversibility. With neural plasticity playing a role in chronic pain(18), it may be plausible that PGB affects synapse formation in a reversible manner, thereby manifesting as plastic changes in brain structure as assessed with MRI. Furthermore, as PGB has been shown to reduce glutamatergic neurotransmission(9), reductions in GMV during treatment and subsequent increases following cessation may be caused by changes in cell volume from PGB-related cell metabolic changes. However, it remains to be seen if longer administration of PGB results in long-term and potentially non-reversible changes in brain GMV. Our findings of acute reversibility strongly suggest that PGB is not leading to either a loss of cell number or brain tissue.
Limitations
This trial has certain limitations. As stated above, while VBM analyses reveal changes in GMV, we do not know the exact causes of these changes. Additionally, functional connectivity was estimated from a heterogeneous state, which included both resting and evoked-pressure and pain periods. While our analyses contrasted these periods, we cannot say that our rest and pain periods were pure. Some of our patients also were taking other medications at the time of scanning, which could have affected our results. This confound may have been mitigated by the fact that the dose of the other medications were held constant over the course of the study. Lastly, PGB failed to significantly reduce clinical pain in our study. Possible contributors to the lack of a significant effect could be the length of PGB administration and relatively small sample size. However, we do not feel that this is a major limitation as PGB has already been shown to be efficacious in reducing FM pain, and analyses have demonstrated that pharmacologic fMRI studies provide greater sensitivity in detecting drug effects than behavioral measures(50).
Conclusion
In the present study, we build upon a previous PGB analysis by showing that drug administration reduces GMV which is associated with seed-based evoked-pain connectivity. Furthermore, we show that PGB-related GMV changes are largely reversible following treatment. It may be the case that overactive synapses in pain processing regions (e.g. insula/DMN) are down-regulated due to PGB treatment reducing glutamate release. Additionally, as pain rumination plays a large role in chronic pain(37), PGB may provide analgesia by ameliorating abnormal neural networks associated with attentional processing during painful stimulation. Revealing drug-associated neural changes correlating with significant improvements in clinical pain has broad implications for FM. Understanding neural targets leading to analgesia in FM can help guide development of future therapeutic interventions, with MRI serving as a useful non-invasive tool of objectively measuring treatment efficacy. However, it remains to be seen if these findings are generalizable to PGB treatment of other chronic pain conditions.
Supplementary Material
Overview of study design. Prior to imaging, subjects underwent a baseline visit for evaluation of inclusion/exclusion criteria. Subjects included in the study were consented and randomized to receive either PGB or PBO treatment first and underwent a baseline MRI session 7 days later, prior to starting treatment regimens. Following baseline imaging, the PGB group underwent dose escalation of Pregabalin to 450 mg/day over the course of 14 days, with a maintained fixed dose of 450 mg/day for the last 3 days. The PBO treatment arm took matching placebo pills over the course of 14 days. Following the first treatment arm, both treatment groups underwent post-treatment imaging, and subsequently underwent a 7-day taper and 8 days of placebo treatment for washout. After washout, participants crossed over to the other study drug during the second treatment arm (the PGB group underwent PBO treatment and vice versa). Imaging during the second treatment arm was identical to the first. Subjects were informed that they would receive placebo or pregabalin at various times, but were not told when they were transferred from one treatment to the other. Additionally, all investigators and members of the research team were blinded to the study. *MRI indicates both structural (VBM) and functional (evoked-pain) imaging. Abbreviations: txt= treatment; MRI: Magnetic Resonance Imaging; PBO= Placebo; PGB= Pregabalin; VBM= Voxel-Based Morphometry.
Evoked-pain paradigm. Diagram representing a portion of the pressure-pain paradigm; stimuli were applied to the thumbnail in fluctuating manner for 25 seconds; “light touch” consisted of a pressure of no more than 0.25 kg/cm2, “mild-moderate pain” consisted of a pressure that elicited between a 7 and 8 on the GBS, and “slightly intense pain” consisted of a pressure that elicited between a 13 and a 14 on the GBS. For each subject, either mild-moderate or slightly intense pain were adjusted to 2 kg/cm2, based on the subject’s pressure pain sensitivity. Abbreviations: GBS= Gracely Box Scale.
No significant changes in clinical pain as a result of PGB or PBO treatment for VBM subjects (n=16). Horizontal bars indicate mean, box indicates interquartile range, and whiskers indicate upper and lower quartiles. Abbreviations: PGB= Pregabalin; PBO= Placebo; VAS= Visual Analog Scale; VBM= Voxel-Based Morphometry.
Changes in right posterior insula GMV are largely reversible following PGB treatment for subjects receiving PGB first (n=7). Black lines indicate subject-specific changes in GMV; Red line indicates mean; Abbreviations: GMV= Gray Matter Volume; PBO= Placebo; PGB= Pregabalin.
Demographics
Sources and coordinates for small volume corrections (SVCs)*
Acknowledgments
This study was funded by Pfizer Inc. The authors thank Keith Newnham, B.A., RT (R)(MR), Functional Magnetic Resonance Technician, University of Michigan, Ann Arbor, Michigan, for expert technical assistance with magnetic resonance imaging. The authors also thank Craig Urwin, B.A., Research Assistant, Department of Anesthesiology, University of Michigan, and Kathy Scott, R.N., Department of Anesthesiology, University of Michigan, for professional treatment and care of our study participants. VN was supported by the following NIH grants (R21-MH103468, P01-AT006663, R01-AT007550; R01-AR064367).
Lynne Pauer is a full-time employee of Pfizer Inc and has stock options with Pfizer Inc.
Daniel J. Clauw has received grant support from and has consulted for Pfizer Inc., Cerephex, Eli Lilly, Merck, Nuvo, Forest, and Cypress Biosciences, and has additionally consulted for Tonix, Theravance, Johnson & Johnson, Pierre Fabre, Wyeth, UCB, Astra Zeneca, Jazz, Abbott, and Iroko
Richard E. Harris has received grant support and has consulted for Pfizer Inc.
References
- 1.Clauw DJ. Fibromyalgia: a clinical review. Jama. 2014;311(15):1547–55. doi: 10.1001/jama.2014.3266. [DOI] [PubMed] [Google Scholar]
- 2.Hauser W, Bernardy K, Uceyler N, Sommer C. Treatment of fibromyalgia syndrome with antidepressants: a meta-analysis. JAMA : the journal of the American Medical Association. 2009;301(2):198–209. doi: 10.1001/jama.2008.944. [DOI] [PubMed] [Google Scholar]
- 3.Arnold LM. Biology and therapy of fibromyalgia. New therapies in fibromyalgia. Arthritis research & therapy. 2006;8(4):212. doi: 10.1186/ar1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moore RA, Wiffen PJ, Derry S, McQuay HJ. Gabapentin for chronic neuropathic pain and fibromyalgia in adults. The Cochrane database of systematic reviews. 2011;(3):CD007938. doi: 10.1002/14651858.CD007938.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Uceyler N, Sommer C, Walitt B, Hauser W. Anticonvulsants for fibromyalgia. The Cochrane database of systematic reviews. 2013;10:CD010782. doi: 10.1002/14651858.CD010782. [DOI] [PubMed] [Google Scholar]
- 6.Dworkin RH, Kirkpatrick P. Pregabalin. Nature reviews Drug discovery. 2005;4(6):455–6. doi: 10.1038/nrd1756. [DOI] [PubMed] [Google Scholar]
- 7.Finnerup NB, Sindrup SH, Jensen TS. The evidence for pharmacological treatment of neuropathic pain. Pain. 2010;150(3):573–81. doi: 10.1016/j.pain.2010.06.019. [DOI] [PubMed] [Google Scholar]
- 8.Pfizer Inc. Lyrica® prescribing information. [cited Nov 14, 2014]. Available from: http://labeling.pfizer.com/showlabeling.aspx?id=561.
- 9.Brawek B, Loffler M, Weyerbrock A, Feuerstein TJ. Effects of gabapentin and pregabalin on K+-evoked 3H-GABA and 3H-glutamate release from human neocortical synaptosomes. Naunyn-Schmiedeberg’s archives of pharmacology. 2009;379(4):361–9. doi: 10.1007/s00210-008-0370-z. [DOI] [PubMed] [Google Scholar]
- 10.Freiman TM, Surges R, Kukolja J, Heinemeyer J, Klar M, van Velthoven V, et al. K(+)-evoked [(3)H]-norepinephrine release in human brain slices from epileptic and non-epileptic patients is differentially modulated by gabapentin and pinacidil. Neuroscience research. 2006;55(2):204–10. doi: 10.1016/j.neures.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 11.Fehrenbacher JC, Taylor CP, Vasko MR. Pregabalin and gabapentin reduce release of substance P and CGRP from rat spinal tissues only after inflammation or activation of protein kinase C. Pain. 2003;105(1–2):133–41. doi: 10.1016/s0304-3959(03)00173-8. [DOI] [PubMed] [Google Scholar]
- 12.Field MJ, Cox PJ, Stott E, Melrose H, Offord J, Su TZ, et al. Identification of the alpha2-delta-1 subunit of voltage-dependent calcium channels as a molecular target for pain mediating the analgesic actions of pregabalin. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(46):17537–42. doi: 10.1073/pnas.0409066103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harris RE, Napadow V, Huggins JP, Pauer L, Kim J, Hampson J, et al. Pregabalin rectifies aberrant brain chemistry, connectivity, and functional response in chronic pain patients. Anesthesiology. 2013;119(6):1453–64. doi: 10.1097/ALN.0000000000000017. [DOI] [PubMed] [Google Scholar]
- 14.Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: anatomy, function, and relevance to disease. Annals of the New York Academy of Sciences. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- 15.May A. Chronic pain may change the structure of the brain. Pain. 2008;137(1):7–15. doi: 10.1016/j.pain.2008.02.034. [DOI] [PubMed] [Google Scholar]
- 16.Kuchinad A, Schweinhardt P, Seminowicz DA, Wood PB, Chizh BA, Bushnell MC. Accelerated brain gray matter loss in fibromyalgia patients: premature aging of the brain? The Journal of neuroscience : the official journal of the Society for Neuroscience. 2007;27(15):4004–7. doi: 10.1523/JNEUROSCI.0098-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schmidt-Wilcke T, Luerding R, Weigand T, Jurgens T, Schuierer G, Leinisch E, et al. Striatal grey matter increase in patients suffering from fibromyalgia--a voxel-based morphometry study. Pain. 2007;132(Suppl 1):S109–16. doi: 10.1016/j.pain.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 18.Seifert F, Maihofner C. Functional and structural imaging of pain-induced neuroplasticity. Current opinion in anaesthesiology. 2011;24(5):515–23. doi: 10.1097/ACO.0b013e32834a1079. [DOI] [PubMed] [Google Scholar]
- 19.Cifre I, Sitges C, Fraiman D, Munoz MA, Balenzuela P, Gonzalez-Roldan A, et al. Disrupted functional connectivity of the pain network in fibromyalgia. Psychosom Med. 2012;74(1):55–62. doi: 10.1097/PSY.0b013e3182408f04. [DOI] [PubMed] [Google Scholar]
- 20.Eroglu C, Allen NJ, Susman MW, O’Rourke NA, Park CY, Ozkan E, et al. Gabapentin receptor alpha2delta-1 is a neuronal thrombospondin receptor responsible for excitatory CNS synaptogenesis. Cell. 2009;139(2):380–92. doi: 10.1016/j.cell.2009.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wolfe F. Fibromyalgia. Rheumatic diseases clinics of North America. 1990;16(3):681–98. [PubMed] [Google Scholar]
- 22.Hartrick CT, Kovan JP, Shapiro S. The numeric rating scale for clinical pain measurement: a ratio measure? Pain practice : the official journal of World Institute of Pain. 2003;3(4):310–6. doi: 10.1111/j.1530-7085.2003.03034.x. [DOI] [PubMed] [Google Scholar]
- 23.Melzack R. The short-form McGill Pain Questionnaire. Pain. 1987;30(2):191–7. doi: 10.1016/0304-3959(87)91074-8. [DOI] [PubMed] [Google Scholar]
- 24.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67(6):361–70. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 25.Harte SE, Clauw DJ, Napadow V, Harris RE. Pressure Pain Sensitivity and Insular Combined Glutamate and Glutamine (Glx) Are Associated with Subsequent Clinical Response to Sham But Not Traditional Acupuncture in Patients Who Have Chronic Pain. Medical acupuncture. 2013;25(2):154–60. doi: 10.1089/acu.2013.0965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gracely RH, Lota L, Walter DJ, Dubner R. A multiple random staircase method of psychophysical pain assessment. Pain. 1988;32(1):55–63. doi: 10.1016/0304-3959(88)90023-1. [DOI] [PubMed] [Google Scholar]
- 27.Gracely RH, Kwilosz DM. The Descriptor Differential Scale: applying psychophysical principles to clinical pain assessment. Pain. 1988;35(3):279–88. doi: 10.1016/0304-3959(88)90138-8. [DOI] [PubMed] [Google Scholar]
- 28.Ashburner J, Friston KJ. Unified segmentation. Neuroimage. 2005;26(3):839–51. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 29.Alemán-Gómez Y, Melie-García L, PV-H IBASPM: Toolbox for automatic parcellation of brain structures. Presented at the 12th Annual Meeting of the Organization for Human Brain Mapping; June 11–15, 2006; Florence, Italy. Available on CD-Rom in NeuroImage. [Google Scholar]
- 30.Matthew Brett J-LA, Valabregue Romain, Poline Jean-Baptiste. Region of interest analysis using an SPM toolbox HBM. Sendai, Japan: 2002. [Google Scholar]
- 31.Ichesco E, Schmidt-Wilcke T, Bhavsar R, Clauw DJ, Peltier SJ, Kim J, et al. Altered resting state connectivity of the insular cortex in individuals with fibromyalgia. J Pain. 2014 doi: 10.1016/j.jpain.2014.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Birn RM. The role of physiological noise in resting-state functional connectivity. NeuroImage. 2012;62(2):864–70. doi: 10.1016/j.neuroimage.2012.01.016. [DOI] [PubMed] [Google Scholar]
- 33.Glover GH, Li TQ, Ress D. Image-based method for retrospective correction of physiological motion effects in fMRI: RETROICOR. Magnetic resonance in medicine : official journal of the Society of Magnetic Resonance in Medicine/Society of Magnetic Resonance in Medicine. 2000;44(1):162–7. doi: 10.1002/1522-2594(200007)44:1<162::aid-mrm23>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 34.Whitfield-Gabrieli S, Nieto-Castanon A. Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain connectivity. 2012;2(3):125–41. doi: 10.1089/brain.2012.0073. [DOI] [PubMed] [Google Scholar]
- 35.Harris RE, Sundgren PC, Craig AD, Kirshenbaum E, Sen A, Napadow V, et al. Elevated insular glutamate in fibromyalgia is associated with experimental pain. Arthritis and rheumatism. 2009;60(10):3146–52. doi: 10.1002/art.24849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Laird AR, Eickhoff SB, Li K, Robin DA, Glahn DC, Fox PT. Investigating the functional heterogeneity of the default mode network using coordinate-based meta-analytic modeling. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29(46):14496–505. doi: 10.1523/JNEUROSCI.4004-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kucyi A, Moayedi M, Weissman-Fogel I, Goldberg MB, Freeman BV, Tenenbaum HC, et al. Enhanced medial prefrontal-default mode network functional connectivity in chronic pain and its association with pain rumination. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2014;34(11):3969–75. doi: 10.1523/JNEUROSCI.5055-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Napadow V, LaCount L, Park K, As-Sanie S, Clauw DJ, Harris RE. Intrinsic brain connectivity in fibromyalgia is associated with chronic pain intensity. Arthritis and rheumatism. 2010;62(8):2545–55. doi: 10.1002/art.27497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Napadow V, Kim J, Clauw DJ, Harris RE. Decreased intrinsic brain connectivity is associated with reduced clinical pain in fibromyalgia. Arthritis and rheumatism. 2012;64(7):2398–403. doi: 10.1002/art.34412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Seminowicz DA, Wideman TH, Naso L, Hatami-Khoroushahi Z, Fallatah S, Ware MA, et al. Effective treatment of chronic low back pain in humans reverses abnormal brain anatomy and function. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31(20):7540–50. doi: 10.1523/JNEUROSCI.5280-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim SH, Lee Y, Lee S, Mun CW. Evaluation of the effectiveness of pregabalin in alleviating pain associated with fibromyalgia: using functional magnetic resonance imaging study. PloS one. 2013;8(9):e74099. doi: 10.1371/journal.pone.0074099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ab Aziz CB, Ahmad AH. The role of the thalamus in modulating pain. The Malaysian journal of medical sciences : MJMS. 2006;13(2):11–8. [PMC free article] [PubMed] [Google Scholar]
- 43.Baliki MN, Geha PY, Apkarian AV. Parsing pain perception between nociceptive representation and magnitude estimation. Journal of neurophysiology. 2009;101(2):875–87. doi: 10.1152/jn.91100.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Apkarian AV, Hashmi JA, Baliki MN. Pain and the brain: specificity and plasticity of the brain in clinical chronic pain. Pain. 2011;152(3 Suppl):S49–64. doi: 10.1016/j.pain.2010.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gracely RH, Petzke F, Wolf JM, Clauw DJ. Functional magnetic resonance imaging evidence of augmented pain processing in fibromyalgia. Arthritis and rheumatism. 2002;46(5):1333–43. doi: 10.1002/art.10225. [DOI] [PubMed] [Google Scholar]
- 46.Jensen KB, Srinivasan P, Spaeth R, Tan Y, Kosek E, Petzke F, et al. Overlapping structural and functional brain changes in patients with long-term exposure to fibromyalgia pain. Arthritis and rheumatism. 2013;65(12):3293–303. doi: 10.1002/art.38170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gustin SM, McKay JG, Petersen ET, Peck CC, Murray GM, Henderson LA. Subtle alterations in brain anatomy may change an individual’s personality in chronic pain. PloS one. 2014;9(10):e109664. doi: 10.1371/journal.pone.0109664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kairys AE, Schmidt-Wilcke T, Puiu T, Ichesco E, Labus JS, Martucci K, et al. Increased brain gray matter in the primary somatosensory cortex is associated with increased pain and mood disturbance in patients with interstitial cystitis/painful bladder syndrome. The Journal of urology. 2015;193(1):131–7. doi: 10.1016/j.juro.2014.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Peyron R, Garcia-Larrea L, Gregoire MC, Costes N, Convers P, Lavenne F, et al. Haemodynamic brain responses to acute pain in humans: sensory and attentional networks. Brain : a journal of neurology. 1999;122(Pt 9):1765–80. doi: 10.1093/brain/122.9.1765. [DOI] [PubMed] [Google Scholar]
- 50.Borsook D, Becerra L, Hargreaves R. A role for fMRI in optimizing CNS drug development. Nature reviews Drug discovery. 2006;5(5):411–24. doi: 10.1038/nrd2027. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Overview of study design. Prior to imaging, subjects underwent a baseline visit for evaluation of inclusion/exclusion criteria. Subjects included in the study were consented and randomized to receive either PGB or PBO treatment first and underwent a baseline MRI session 7 days later, prior to starting treatment regimens. Following baseline imaging, the PGB group underwent dose escalation of Pregabalin to 450 mg/day over the course of 14 days, with a maintained fixed dose of 450 mg/day for the last 3 days. The PBO treatment arm took matching placebo pills over the course of 14 days. Following the first treatment arm, both treatment groups underwent post-treatment imaging, and subsequently underwent a 7-day taper and 8 days of placebo treatment for washout. After washout, participants crossed over to the other study drug during the second treatment arm (the PGB group underwent PBO treatment and vice versa). Imaging during the second treatment arm was identical to the first. Subjects were informed that they would receive placebo or pregabalin at various times, but were not told when they were transferred from one treatment to the other. Additionally, all investigators and members of the research team were blinded to the study. *MRI indicates both structural (VBM) and functional (evoked-pain) imaging. Abbreviations: txt= treatment; MRI: Magnetic Resonance Imaging; PBO= Placebo; PGB= Pregabalin; VBM= Voxel-Based Morphometry.
Evoked-pain paradigm. Diagram representing a portion of the pressure-pain paradigm; stimuli were applied to the thumbnail in fluctuating manner for 25 seconds; “light touch” consisted of a pressure of no more than 0.25 kg/cm2, “mild-moderate pain” consisted of a pressure that elicited between a 7 and 8 on the GBS, and “slightly intense pain” consisted of a pressure that elicited between a 13 and a 14 on the GBS. For each subject, either mild-moderate or slightly intense pain were adjusted to 2 kg/cm2, based on the subject’s pressure pain sensitivity. Abbreviations: GBS= Gracely Box Scale.
No significant changes in clinical pain as a result of PGB or PBO treatment for VBM subjects (n=16). Horizontal bars indicate mean, box indicates interquartile range, and whiskers indicate upper and lower quartiles. Abbreviations: PGB= Pregabalin; PBO= Placebo; VAS= Visual Analog Scale; VBM= Voxel-Based Morphometry.
Changes in right posterior insula GMV are largely reversible following PGB treatment for subjects receiving PGB first (n=7). Black lines indicate subject-specific changes in GMV; Red line indicates mean; Abbreviations: GMV= Gray Matter Volume; PBO= Placebo; PGB= Pregabalin.
Demographics
Sources and coordinates for small volume corrections (SVCs)*