Significance
Much research has focused on the influence of animal migrations on terrestrial ecosystems. Mass drownings are an understudied phenomenon associated with migrations that may have substantial impacts on aquatic ecosystems. Here, we show that mass drownings of wildebeest occur nearly annually during the Serengeti wildebeest migration, and these mass drownings contribute the equivalent biomass of 10 blue whale carcasses per year to this moderately sized river. Soft tissues of the carcass decompose within several weeks and are assimilated by both in-stream and terrestrial consumers. Bones decompose over years, which may influence nutrient cycling and food webs in the river on decadal time scales. The loss of migrations and associated mass drownings may fundamentally alter river ecosystems in ways previously unrecognized.
Keywords: animal migration, mass drowning, nutrient cycling, Serengeti wildebeest, stable isotope
Abstract
The annual migration of ∼1.2 million wildebeest (Connochaetes taurinus) through the Serengeti Mara Ecosystem is the largest remaining overland migration in the world. One of the most iconic portions of their migration is crossing of the Mara River, during which thousands drown annually. These mass drownings have been noted, but their frequency, size, and impact on aquatic ecosystems have not been quantified. Here, we estimate the frequency and size of mass drownings in the Mara River and model the fate of carcass nutrients through the river ecosystem. Mass drownings (>100 individuals) occurred in at least 13 of the past 15 y; on average, 6,250 carcasses and 1,100 tons of biomass enter the river each year. Half of a wildebeest carcass dry mass is bone, which takes 7 y to decompose, thus acting as a long-term source of nutrients to the Mara River. Carcass soft tissue decomposes in 2–10 wk, and these nutrients are mineralized by consumers, assimilated by biofilms, transported downstream, or moved back into the terrestrial ecosystem by scavengers. These inputs comprise 34–50% of the assimilated diet of fish when carcasses are present and 7–24% via biofilm on bones after soft tissue decomposition. Our results show a terrestrial animal migration can have large impacts on a river ecosystem, which may influence nutrient cycling and river food webs at decadal time scales. Similar mass drownings may have played an important role in rivers throughout the world when large migratory herds were more common features of the landscape.
Large animal migrations can have important impacts on ecosystem structure and function (1), but, globally, most large animal migrations have declined or been lost all together (2). The Serengeti wildebeest (Connochaetes taurinus) migration is the largest remaining terrestrial migration (3), and a great deal of research has been done on the migration’s role in facilitating terrestrial nutrient cycling and promoting vegetation, prey, and predator biomass in the savanna grasslands of the Serengeti Mara Ecosystem (4–6). This migration also may impact the ecology of aquatic ecosystems in the region, particularly through annual mass drownings that occur during river crossings when the migration is in the northern portion of its route (7) (Fig. 1A). Although wildebeest mass drownings during the migration have been documented in the region (8–10), previous studies have not investigated the size and frequency of these mass drownings or the fate of the carcasses and associated nutrients.
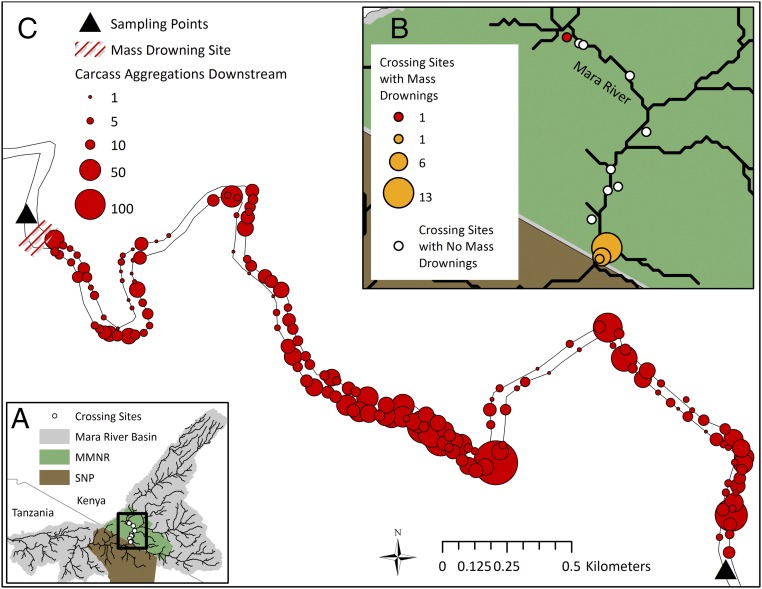
Fig. 1.
Map showing the Mara River Basin, Kenya, and its relationship to the Maasai Mara National Reserve (MMNR) and Serengeti National Park (SNP) (A), the distribution of wildebeest crossing sites along the Mara River coded by the number of mass drownings confirmed to have occurred at each site (for 21 of the 23 drownings) (B), and the distribution of 3,380 wildebeest carcasses over 5 km of river after a mass drowning in July 2011 at the crossing site coded red in B (C).
Catastrophic mortality by drowning has been documented as animal herds cross large bodies of water in a range of ecosystems (9, 11), and has been implicated in the occurrence of large dinosaur bone beds (12). Mass drownings move large amounts of resources from terrestrial to aquatic systems in the form of carcasses, which are complex, high-quality resources that can alter nutrient cycling, productivity, species diversity, and succession patterns on time scales of years to decades (13–15). Mass drownings likely played a large role historically when large migrations and unregulated rivers were more common features of the landscape. For example, there are numerous accounts of hundreds to thousands of bison drowning in rivers of the western United States in the late 18th and early 19th centuries when large bison herds were still intact (16, 17). River basins that no longer contain terrestrial migrations may have lost the annual input of resources from mass drownings, which may fundamentally alter how those river ecosystems function now compared with the past. The more well-studied annual migrations of anadromous fishes and subsequent carcass inputs have been shown to be important in rivers (18, 19), but equivalent examination of the influence of terrestrial migrations is lacking.
Here, we present an estimation of annual mass drownings of one of the world’s largest remaining intact mammal migrations and investigate the input rate and fates of these carcasses in a river ecosystem. We used historical reports from 2001 to 2010 and field surveys from 2011 to 2015 to quantify the frequency and size of wildebeest mass drownings in the Kenyan portion of the Mara River. We used field measurements, modeling approaches, and stable isotope analysis to estimate the total loading of carbon (C) and nutrients from carcasses and to provide an approximation of the fate of carcass material in the ecosystem.
Results and Discussion
Historical reports from a tourist lodge and the Mara Conservancy and field surveys demonstrate that mass drownings occurred in the Kenyan portion of the Mara River in at least 13 of 15 y from 2001 to 2015. During those years, the wildebeest migration arrived in the Mara River Region (Fig. 1A) between early June and late July and stayed until November or December, for 172 ± 45 (mean ± 1 SD) d. River crossings occurred on 52 ± 16 d per year. From 2001 to 2010, mass drownings were noted in eight of 10 years, although reports did not contain sufficient detail to quantify the size of mass drownings. For the 5-y period from 2011 to 2015, when we conducted detailed surveys of mass drownings, we observed 23 mass drownings, with 4.6 ± 1.8 mass drownings and 6,250 ± 3,000 wildebeest carcasses per year. The frequency and size of mass drownings have likely increased as the herd size has grown from ∼200,000 in the 1950s to the fairly stable size of 1.2 million in the 1970s to the present (3, 20). Although an estimated 175,000 zebra also migrate with the wildebeest (3), and often fall prey to crocodile attacks during crossings of the Mara River, we have counted fewer than five zebra carcasses throughout all of our surveys.
There are 12 major river-crossing sites in the Kenyan portion of the Mara River, and all of the mass drownings we documented occurred at four of those sites (Fig. 1B). These data suggest crossing site geomorphology may be a factor in determining drowning occurrence. Discharge also plays a role in the occurrence of mass drownings, because mass drownings do not typically occur at very low discharge. At higher discharge, mass drownings appear to result from a complex interaction between river bank geomorphology, discharge, herd size, and tourist behavior, and we are conducting ongoing research to quantify these relationships.
Using the mean number of individuals drowning in each year from 2011 to 2015 and the mean body mass of an individual wildebeest (175 kg) (21) (SI Materials and Methods), we estimate mass drownings contribute 1,096 ± 526 tons of biomass to the Mara River each year (301 ± 145 tons of dry mass). The Mara River is incised several meters beneath the upper river bank; therefore, carcasses from mass drownings are contained within the wetted width of the river channel. We measured the elemental composition of wildebeest carcasses [36% C, 8% nitrogen (N), and 4% phosphorus (P)] (Fig. 2A) and estimate that these mass drownings contribute 107 ± 51 tons of C, 25 ± 12 tons of N, and 13 ± 6 tons of P to the Mara River each year (Table S1). These nutrients are distributed along several kilometers of the Mara River in small carcass aggregations at bends and rock outcroppings that form potential hot spots of biogeochemical cycling (22–24) (Fig. 1C). Carcasses from each mass drowning persist in the Mara River for ∼28 d before carcasses are no longer visible and background nutrient levels return to normal. During this 28-d period, inputs from carcasses contributed 18–191% of the dissolved organic carbon (DOC), 6–78% of the total nitrogen (TN), and 31–451% of the total phosphorus (TP) being transported from upstream reaches of the Mara River (n = 3 drowning events). These carcass inputs are remarkably high, even compared with other ecosystems in which animal carcass inputs have been documented (18, 19). Total carcass inputs into this medium-sized river system (mean discharge = 12.5 m3⋅s−1) are equivalent to the input of 10 blue whale carcasses per year (assuming a mean mass of 105 tons) (25), and mean carcass biomass (56 kg⋅m−1) is nearly fourfold higher than the mean spawning biomass of Pacific Salmon runs in British Columbia (in which 10 kg⋅m−1 was identified as a conservation goal) (26).
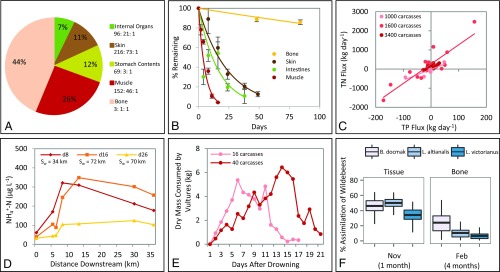
Fig. 2.
Input and fate of wildebeest carcasses from mass drownings in the Mara River. (A) Percentage of dry mass composition of an average wildebeest carcass, with stoichiometric ratios by mass shown as C/N/P. (B) Microbial decomposition rates for wildebeest bone, skin, intestines, and muscle in the Mara River (mean ± SEM). (C) Daily estimations of total nitrogen (TN) vs. total phosphorus (TP) flux downstream of three carcass aggregations ranging from 1,000–3,400 carcasses. (D) Declines in NH4+-N concentration downstream of wildebeest carcasses and estimations of nutrient uptake length (Sw) on days 8, 16, and 26 after the drowning. (E) Total dry mass of wildebeest carcasses consumed by avian scavengers on aggregations of 16 and 40 carcasses. (F) Assimilation of wildebeest tissue (in November 2013, 1 mo after carcasses were present) and bone biofilm (in February 2014, 4 mo after carcasses were present) by three common fish taxa (mean ± 95% credibility intervals).
Table S1.
Number and elemental composition of wildebeest carcasses entering the Mara River from 2011 to 2015
| Year | No. of mass drownings | No. of carcasses | Carcass dry mass,* 103 kg | Average aerial loading,† g of DM per m−2 | C, 103 kg | N, 103 kg | P, 103 kg |
| 2011 | 3 | 8,000 | 385 | 590 | 137 | 32 | 16 |
| 2012 | 6 | 9,400 | 453 | 250 | 161 | 37 | 19 |
| 2013 | 7 | 7,750 | 373 | 360 | 133 | 31 | 16 |
| 2014 | 4 | 2,700 | 130 | 640 | 46 | 11 | 5 |
| 2015 | 3 | 3,400 | 164 | 140 | 58 | 13 | 7 |
| Mean (SD) | 4.6 (1.8) | 6,250 (3,000) | 301 (144) | 400 (210) | 107 (51) | 25(12) | 13(6) |
DM, dry matter.
Total carcass biomass assuming a mean biomass of 175 kg of wet mass and 48 kg of dry mass per carcass.
Average aerial loading for mass drownings, where detailed carcass counts could be conducted over a spatially explicit area (12 of 23 mass drownings).
Carcasses are complex and heterogeneous resources, and different portions of the carcass have different fates in the ecosystem. Carcasses are composed of 44 ± 4% bone and 56 ± 4% soft tissue (muscle, organs, and skin). Bone contributes, on average, 132 ± 63 tons of dry mass annually to the Mara River. Bones are very high in P (24% C, 5% N, and 9% P) and take a mean of 7.4 y to 95% decay (Fig. 2B and Table S2). Relative to other ecological and biogeochemical rates in the Mara River, bones act as long-term storage of 32 ± 15 tons of C (29% of total inputs), 6 ± 3 tons of N (25%), and 12 ± 6 tons of P (95%) each year. The slow leaching of nutrients from bones likely provides a constant source of nutrients, particularly P, to the river ecosystem until bones are buried, washed downstream, or completely decomposed. The soft tissue contributes, on average, 169 ± 81 tons of dry mass to the Mara River that is composed of mostly C and N (45% C, 11% N, and 0.4% P). Soft tissue contributes 75 ± 36 tons of C, 18 ± 9 tons of N, and 0.6 ± 0.3 tons of P annually that decays rapidly, with 95% of mass lost in 16–70 d (Fig. 2B and Table S2).
Table S2.
Elemental composition and decomposition rates of wildebeest carcass components
| Carcass element | Percentage of carcass,* mean (SD) | C/N/P† | Decay rate, k⋅d−1 (95% CI) | Days to 95% biomass loss, mean days (95% CI) |
| Stomach contents | 12.4 (2.9) | 69.2:3.3:1.0 | ||
| Muscle | 25.7 (0.7) | 152.0:45.7:1.0 | −0.188 (−0.168 to −0.208) | 16 (14–18) |
| Internal organs‡ | 7.2 (1.7) | 96.4:21.2:1.0 | −0.068 (−0.047 to −0.089) | 44 (38–63) |
| Skin | 10.9 (2.0) | 215.5:72.5:1.0 | −0.043 (−0.038 to −0.048) | 70 (63–80) |
| Bone | 43.7 (3.7) | 2.6:0.5:1.0 | −0.001 (−0.0013 to −0.0009) | 2,709 (2,285–3,327) |
| Total carcass | 100.0 | 8.5:2.0:1.0 |
Based on dry mass.
C/N/P ratio is shown by mass.
Percentage of carcass and stoichiometry averaged across all internal organs; decay rate based on intestine.
Using multiple approaches, we traced the fate of soft tissue nutrients (because bone nutrients are largely unavailable over the short term) to estimate how the river ecosystem responds to large pulses of carcasses. The decomposing carcasses in the river represent a large nutrient source, and we used the declines in ammonium (NH4+) and soluble reactive phosphorus (SRP) concentration downstream to estimate nutrient uptake length (the mean distance a solute travels before being removed by biological assimilation or uptake), uptake velocity (the speed at which a nutrient molecule moves from the water column to an uptake compartment), and total aerial uptake (total flux of nutrients from the water column to the stream bottom) at three time points from 1 to 4 wk after the drowning occurred (27, 28). Total aerial uptake over this time accounted for between 149 and 879 kg of N (based on minimum and maximum estimations, as described in Materials and Methods), or 1–6% of soft tissue N loading, and between 109 and 136 kg of P, or 24–30% of soft tissue P loading. Nutrient uptake length increased from 34 to 70 km for NH4+ and from 36 to 103 km for SRP between 8 and 26 d after the mass drowning (Fig. 2D and Table S3). Increasing uptake lengths after the drowning suggest the river reached saturation with respect to these nutrients (i.e., these nutrients were no longer limiting to production). We were only able to estimate nutrient uptake for one drowning, because carcasses needed to be located sufficiently upstream of the Kenya-Tanzania border to enable the necessary measurements. However, these uptake lengths are on the order of the uptake lengths documented for other rivers of similar size with high background nutrient concentrations (27–29).
Table S3.
Nutrient cycling in the Mara River after a drowning of 5,000 wildebeest
| Days after drowning | NH4+ | SRP | ||||||
| Sw,* km | Vf,† m⋅d−1 | Umin,‡ mg⋅m−2⋅d−1 | Umax,§ mg⋅m−2⋅d−1 | Sw, km | Vf, m⋅d−1 | Umin, mg⋅m−2⋅d−1 | Umax, mg⋅m−2⋅d−1 | |
| 8 | 34.4 | 0.5 | 31.2 | 164.6 | 35.6 | 0.5 | 18.2 | 25.9 |
| 16 | 72.5 | 0.3 | 13.0 | 115.8 | 51.6 | 0.5 | 16.6 | 16.6 |
| 26 | 69.9 | 0.3 | 10.9 | 42.7 | 103.1 | 0.2 | 6.9 | 8.4 |
Nutrient uptake length.
Nutrient uptake velocity.
Minimum aerial uptake, based on upstream nutrient concentration.
Maximum aerial uptake, based on peak nutrient concentrations measured within the sampling reach.
This saturation of uptake by the river ecosystem suggests that a large proportion of dissolved nutrients from carcasses were transported downstream in the water column. We measured net nutrient flux downstream of carcasses during three mass drownings (the only ones for which the carcasses were present for at least 25 d). We collected water samples upstream and downstream of carcasses every 0.5–7 d for a period of 25–29 d after each drowning, and found carcass aggregations acted as both a net sink and source of nutrients throughout the 4 wk after the drowning (Fig. 2C). There was a large amount of variability in fluxes across the drowning events, and further research is needed to understand the drivers of this variability. Downstream transport accounted for −3 to 43% of the C and −6 to 47% of the N from soft tissue, mostly in the form of dissolved inorganic N (Table S4). We measured less TP downstream of carcasses than upstream (−134 to 17% of soft tissue loading), but a −2 to 80% increase in SRP, suggesting carcass aggregations may be a source for soluble inorganic P but a sink for organic and sediment-bound P via the physical accumulation of suspended sediments through deposition within carcass aggregations.
Table S4.
C and nutrient flux upstream and downstream of wildebeest carcass aggregations
| Date | No. of carcasses | Average Q, m3⋅s−1 | Upstream DOC flux, kg | Total carcass C loading, kg | Soft tissue C loading, kg | Net DOC flux, kg | Upstream TN flux, kg | Total carcass N loading, kg | Soft tissue N loading, kg | Net TN flux, kg | Upstream TP flux, kg | Total carcass P loading, kg | Soft tissue P loading, kg | Net TP flux, kg |
| 7/4/2011 | 3,380 | 9.7 | 30,310 | 57,900 | 40,800 | −1,290 | 17,080 | 13,400 | 10,000 | 1,270 | 1,510 | 6,800 | 320 | 50 |
| 11/5/2012 | 990 | 15.6 | 96,270 | 17,000 | 12,000 | 3,480 | 64,290 | 3,900 | 2,900 | −180 | 6,450 | 2,000 | 90 | −40 |
| 9/25/2013 | 1,610 | 16.6 | 10,8490 | 27,600 | 19,400 | 8,350 | 60,720 | 6,400 | 4,700 | 2,240 | 6,250 | 3,300 | 150 | −210 |
| Mean (SD) | 1,990 (1,240) | 13.9 (3.7) | 78,360 (42,060) | 34,200 (21,300) | 24,100 (15,000) | 3,510 (4,820) | 47,370 (26,290) | 7,900 (4,900) | 5,900 (3,700) | 1,110 (1,220) | 4,740 (2,790) | 4,000 (2,500) | 190 (120) | −65 |
All fluxes were calculated over a period of 25–29 d. Q, discharge; TN, total nitrogen.
Carcass aggregations also provide important resource pulses for scavengers, which can facilitate carcass decomposition and transport (30, 31). Many of the scavengers in this ecosystem are birds, which feed on partially submerged carcasses within the river and can transport C and nutrients out of the river into the nearby terrestrial ecosystem. We used two photographic time series of scavengers on carcass aggregations and a metabolic model to estimate the proportion of carcasses consumed by scavengers (Table S5). Marabou storks (Leptoptilos crumenifer), white-backed vultures (Gyps africanus), Rüppell’s vultures (Gyps rueppellii), and hooded vultures (Necrosyrtes monachus) were the most common avian species. We conservatively estimated that avian scavengers consumed 6–9% of C, N, and P from soft tissue loading (Fig. 2E). Some proportion of nutrients consumed is likely released back into or near the river; however, digestion time can exceed 24 h and vultures may hold food in their crop to transport back to fledglings, suggesting most nutrients consumed are transported out of the river channel (32, 33). These estimations are based on avian scavenger use of two carcass aggregations, so there remains much uncertainty around this estimation. Further research is needed on this important pathway.
Table S5.
Metabolic model for avian scavenger consumption of wildebeest carcasses
| Species | Average mass, kg | Daily energy expenditure, kJ⋅d−1 | Daily energy consumed, kJ⋅d−1 | Daily dry mass consumed, g⋅d−1 | Daily C consumed, g⋅d−1 | Daily N consumed, g⋅d−1 | Daily P consumed, g⋅d−1 |
| Maribou stork | 6.45 | 2,577 | 2,990 | 115 | 51 | 13 | 0.4 |
| White-backed vulture | 5.43 | 2,321 | 2,692 | 104 | 46 | 11 | 0.4 |
| Rüppell's vulture | 7.40 | 2,803 | 3,251 | 125 | 56 | 14 | 0.4 |
| Hooded vulture | 2.04 | 1,277 | 14,812 | 57 | 25 | 6 | 0.2 |
Aquatic consumers also are likely important consumers of wildebeest carcasses. We analyzed C and N stable isotope ratios of three common fish taxa from the Mara River. All three species have omnivorous diets, although Bagrus docmak is largely predacious, Labeobarbus altianalis is a generalist consumer, and Labeo victorianus feeds primarily on algae, suggesting they may assimilate carcass inputs via different pathways (34, 35) (more details are provided in SI Materials and Methods). B. docmak and L. altianalis may consume carcasses directly or indirectly via aquatic and terrestrial insects feeding on the carcasses. L. victorianus is more likely to consume biofilms growing on rocks and bones in response to carcass inputs. When wildebeest carcasses were present, wildebeest tissue comprised 34(±10)–50(±8)% [mean (± SD)] of the assimilated diet of fish, depending on taxa (Fig. 2F). Months after carcasses decomposed, wildebeest bones remaining in the river were covered in biofilm (a mixture of bacteria, fungi, and algae) that continued to be an important resource (36). Biofilm growing on bone had a distinct isotopic signature compared with biofilm growing on rocks, and it comprised 7(±5)–24(±14)% of the assimilated diet of the three common fish species 4 mo after the last drowning (Fig. 2F). Our data suggest wildebeest carcasses have a substantial and persistent influence on the river food web, although more data for additional individuals and species are needed to quantify further the extent to which carcasses support the river food web.
Nile crocodiles (Crocodylus niloticus) are another potential consumer of wildebeest, and high densities of adult crocodiles at river-crossing sites are a major cause of individual mortality, which augments carcass inputs to the river from drowning. However, crocodiles have a relatively low metabolic rate, and they become easily satiated, leading to intermittent feeding. We used a literature estimation of crocodile consumption rate (37) and limited survey data we collected to estimate that crocodiles in the river reach where the majority of mass drownings occur would consume 150 wildebeest throughout the entire period the migration is in the Mara region, or 2% of total carcass input to the river. This consumption includes both animals killed during crossings and carcasses available after mass drownings. More detailed crocodile surveys are needed to estimate the total number of wildebeest that may be consumed by crocodiles in the Mara River during the migration period. These data suggest that consumption by fish and other aquatic animals may be an important pathway for wildebeest carcasses, although further analysis of secondary production is needed to quantify this pathway (38). Moreover, pulsed inputs of wildebeest carcasses may influence the river food web at decadal time scales through the contribution of bone to basal resources.
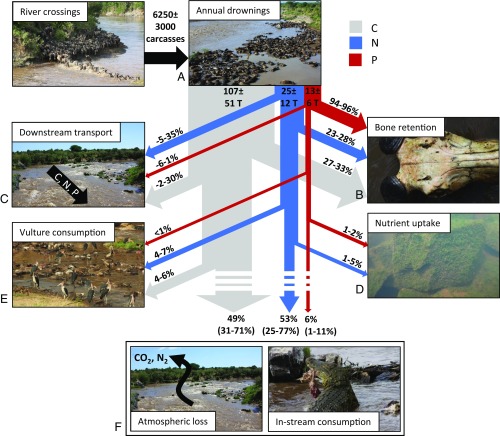
Using these approaches, we accounted for the fate of a mean of 51% of C, 47% of N, and 94% of P from wildebeest carcasses after mass drownings (Fig. 3). For C, a mean of 29% is held in bones, 16% is transported downstream in the water column, and 5% is consumed by avian scavengers. A large portion of C that is unaccounted for is likely either consumed by aquatic insects and fish or lost to the atmosphere through respiration during microbial breakdown of the carcasses (Fig. 3F). The largest proportion of N for which we could account is stored in bones (mean = 25%). A mean of 3% of total loading is taken up by the river near the carcass aggregations, 13% is transported downstream in the water column, and 5% is consumed by avian scavengers. Much of the N that is unaccounted for may be consumed by aquatic animals or lost to the atmosphere via denitrification by microbial activity near the carcasses (24) (Fig. 3F). We have measured zones of hypoxia near carcass aggregations, which can increase rates of denitrification. In addition, a large amount of N might be lost via volatilization of ammonia gas from the decaying carcasses (39). Almost all of the P from carcasses (mean = 95%) is held in bones that slowly decompose in the ecosystem over multiple years. Some inorganic P is quickly mineralized and transported downstream, but carcass aggregations are a short-term sink for total P and likely a long-term source of P due to the slow decomposition of bones. Indeed, we have observed large numbers of bones in the river below the main wildebeest crossing sites. Our estimations of carcass fate are based on opportunistic sampling of stochastic events, resulting in limited data for several pathways. These estimations present a first step at quantifying the fate of carcass inputs, but the range of variability inherent in these processes will necessitate more research on each pathway.
Fig. 3.
Illustrative diagram showing estimated annual loading and transport pathways of C, N, and P from mass drownings of wildebeest in the Mara River, Kenya. Mean ± SD total loading estimations are at the top of the diagram in terms of carcasses and metric tons of C, N, and P, and the range of estimations (minimum–maximum) for the amount transported in each pathway are presented as the percentage of total loading. A through F align with data presented in Fig. 2. Fluxes through the two pathways illustrated in F are currently unknown.
SI Materials and Methods
Drowning Occurrences and Carcass Numbers.
We documented wildebeest crossings and mass drownings in the Kenyan portion of the Mara River from 2001 to 2015 from two sets of historical reports and from field surveys. We collected information on the occurrence of mass drownings from Mara Conservancy monthly newsletters from September 2001 through December 2014. We also collected information from 2003 to 2014 from Governor’s Camp monthly newsletters from June through December (the period overlapping the wildebeest migration).
From 2011 to 2015, we worked with Mara Conservancy rangers to collect historical and current locations for wildebeest crossing sites (Fig. 1), and we actively monitored mass drownings during field seasons (2011, June–August; 2012, July–December; 2013–2015, August–December) through direct observation, communication from reserve rangers, and observation of carcass aggregations (Table S1). Field seasons did not always encompass the full period when the migration was present in the Mara region, but they did cover most of the time when river crossings were occurring in every year except 2011. In 2011, discussions with rangers and management staff suggest we did not miss any mass drowning events, but our counts may be an underestimation for that year. We counted the number of carcasses floating downstream from a stationary location if we were present when the drowning occurred, or we counted carcasses as soon as possible after the drowning by walking the riverbank and counting all carcasses until the carcasses became sufficiently scarce that spot checks could be conducted by vehicle at river bends. Final carcass counts were rounded to 10s. When our counts were artificially abbreviated by the Tanzanian border, we estimated the total number of wildebeest drowned by working with reserve rangers who had observed either the drownings or carcass aggregations downstream of the border. These estimations were rounded to 100s. On July 21, 2011, 7 d after a mass drowning at the uppermost crossing site, we mapped carcass locations in the river using handheld maps and a global positioning system unit (Oregon 300; Garmin International, Inc.) and imported data into ArcGIS (Fig. 1C).
Carcass Composition.
Carcass composition was measured by collecting freshly drowned wildebeest carcasses from the river (1 adult male and 1 subadult male in 2012, and 1 adult female in 2013), dissecting them into their primary components, and weighing each component (Table S2). The percentage of dry mass was measured on triplicate subsamples of each component for the adult female and used to estimate dry mass for components of all carcasses. Triplicate subsamples of each carcass component from each individual were analyzed for C, N, and P composition. Samples were dried at 72 °C (to meet US Department of Agriculture permit import regulations) and finely ground using a cryogenic ball mill. C and N composition was measured using a Costech Elemental Analyzer (Costech Analytical Technologies, Inc.). P composition was measured by digesting preweighed combusted material using 1 M HCl at 80 °C for 2 h, treating with an ammonium molybdate color reagent, and analyzing on a spectrophotometer at 885 nm. Average wildebeest mass values were taken from the literature (21). We estimated a herd to be composed of one-third juveniles (145-kg female, 159-kg male) and two-thirds adults (165-kg female, 210-kg male), with equal sex ratios, giving us an average mass of 175 kg per individual (175 kg⋅individual−1).
Microbial Decomposition.
We measured the decay rate of carcass components due to microbial decomposition by placing subsamples of muscle, intestine, skin, and bone in fine-mesh bags, which were secured inside a metal cage in the river (Table S2). Triplicate samples of muscle, intestine, and skin were collected at five different time points that were predetermined using data from a preliminary decomposition experiment (muscle, days 2–16; intestine, days 4–38; skin, days 8–49). After collection, samples were weighed, dried, and reweighed to measure the percentage of dry mass. Due to the large variability across bone types, triplicate subsamples of leg, rib, scapula, and vertebrae were placed in fine-mesh bags inside the metal cage in the river. Wet weight was measured at five time intervals (days 48–216), and samples were replaced in the bags after weighing. The percentage of dry mass of bone was measured for the final time interval. The decay rate was calculated in R using a single exponential decay function, with a fixed intercept of 100% remaining at time step 0 (44). Days to 95% biomass loss were calculated by dividing ln(0.05) by k (decay rate) (45). The decay rate was calculated using dry mass for muscle, intestine, and skin, and using average wet mass across the different types of bone.
Discharge Data.
We collected discharge data for the Mara River from 2011 to 2014, which were used in estimations of nutrient flux and uptake. Discharge was measured at Purungat Bridge, at the lower reach of our study region on the border between Kenya and Tanzania. Stage height data were measured every 15 min from June 2011 through November 2012 using a Rugged TROLL 100 depth transducer corrected with a BaroTROLL barometric pressure logger (In-Situ, Inc.). From December 2012 through December 2014, stage height data were measured every 15 min using a depth transducer probe connected to a Manta2 sonde (Eureka Water Probes).
Stage heights were converted to discharge using a rating curve we developed by measuring discharge on multiple days using the area-velocity method. We measured depth using a handheld staff gauge or weighted measuring tape and velocity using a velocimeter, or we measured depth and velocity with a HydroSurveyor (SonTek/Xylem, Inc.) (46). Discharge was measured 10 times in 2011 and one time in 2014. Due to the bedrock substrate at the channel reach, the channel geomorphology appeared to be consistent over this time period. Three outliers were removed from the rating curve because they fell outside the 95% CI and were collected during the rising or falling limb of a flood, when it is not appropriate to collect data for rating curves. The final rating curve had an adjusted R2 = 0.95.
Nutrient Uptake.
After a mass drowning of 5,000 wildebeest on July 14, 2011, we measured nutrient uptake length (Sw), uptake velocity (vf), and aerial uptake (U) using the carcasses as a high-input nutrient source and declines in concentration downstream as an indication of nutrient uptake in the river (27, 28, 47) (Table S3). Although nutrient uptake is often measured using additions of inorganic nutrients accompanied by a conservative tracer (48), a high-input source and downstream declines have been used to measure nutrient uptake downstream of wastewater treatment plants (27, 28). Measurement of a conservative tracer (Cl− or Fl−) is often still used in this approach to detect any influence of dilution. However, discharge data showed this portion of the river received minimal inputs of water from other sources during this time, so we assumed there was no dilution over the study reach.
Water samples were collected directly upstream of the carcasses and at five points downstream of the carcasses, ranging from 5.0–36.9 km, on days 8, 16, and 26 after the drowning occurred. Although this distance is longer than commonly used in studies of nutrient uptake length, we anticipated that uptake lengths would be long in a river of this size with this level of nutrient loading. NH4+ and SRP concentrations were analyzed as described in the section below. For NH4+, we accounted for changes in background NH4+ concentrations (31.6–60.9 μg NH4+-N⋅L−1) throughout the duration of the sampling period (26 d) by subtracting upstream values from downstream values. We then measured the decline of the corrected values. For SRP, upstream concentrations did not change significantly over the sampling period (29.4–36.8 µg⋅L−1). Furthermore, upstream values were higher than the furthest downstream values in some cases, possibly due to elevated rates of nutrient uptake. We used non–background-corrected SRP values and simply investigated declines in concentration. DOC concentrations did not decrease steadily downstream of carcasses, so we were not able to calculate DOC uptake.
We used the following equation to estimate nutrient uptake length for each of these three time periods:
where Cx is the background-corrected nutrient concentration x kilometers from the top of the sampling reach (just downstream of the majority of the carcasses), C0 is the background-corrected nutrient concentration at the top of the sampling reach, and kc is the per meter nutrient uptake rate (27, 47). Nutrient uptake length, which is the average distance downstream a nutrient molecule travels before being removed from the water column, is calculated as kc−1 (47).
We calculated nutrient vf, which is a measure of the speed at which a nutrient molecule moves from the water column to an uptake compartment, using the following equation:
where Q is discharge [cubic meters per day (m3⋅d−1)], w is wetted width (measured in meters), and Sw is measured in meters (27, 55). We measured w every ∼0.15 km along the river length using Google Earth version 7 (Google) and satellite imagery (DigitalGlobe).
We calculated total U, which is the total flux of nutrient from the water column to the stream bottom, expressed on the basis of stream bottom area, as
where vf is measured as meters per day (m⋅d−1) and Cb is the background concentration of the nutrient [milligrams per cubic meter (mg⋅m−3)] (55). In a nutrient addition experiment, Cb would be the nutrient concentration in the river reach before the experiment. For this study, Cb could be approximated as the nutrient concentration upstream of the carcasses. However, vf is calculated for an extended period of elevated nutrient concentrations rather than the upstream level. Therefore, we calculated a range of U values from minimum (Umin; based on upstream nutrient concentrations) to maximum (Umax; based on the highest measured nutrient concentrations during the sampling period). Actual aerial nutrient flux values will fall somewhere in this range. We calculated both Umin and Umax values for NH4+ and SRP on days 8, 16, and 26 after the drowning. We calculated total aerial uptake over this time period (assuming equal aerial uptake from the beginning of the drowning to day 8) for the 6.5-km reach in which the majority of carcasses were located by measuring the area under the curve in Sigmaplot 12.0 (Systat Software, Inc.). We compared these amounts with the total amount of N and P loaded by the 4,820 carcasses within this reach.
Nutrient Flux.
We measured the flux of total and dissolved inorganic nutrient levels and DOC upstream and downstream of carcasses for six different drowning events in 2011, 2012, and 2013. For three of these events, we were able to measure flux for at least 25 d after the drowning, after which carcasses were no longer visibly present in the river and downstream nutrient values had generally returned to baseline levels. We used flux estimations from these three drownings to estimate total downstream transport of carcass nutrients (Table S4). Measurements were taken every 0.5–7 d for a period of 25–29 d per drowning, for a total of 34 sampling days across the three drownings. Water samples were collected for nutrient analysis upstream and downstream of the majority of carcasses from a drowning. The distance between upstream and downstream samples ranged from 3.1 to 5.0 km.
Water samples were kept refrigerated after collection and processed as soon as possible. For DOC analysis, samples were filtered through a glass fiber filter (Whatman GF/F; GE Healthcare Bio-Sciences), acidified with sulfuric acid to pH < 2 for preservation, and analyzed on a total organic carbon analyzer (Shimadzu Scientific Instruments). For TN and TP analysis, unfiltered samples were acidified for preservation, diluted to acceptable sediment levels (<150 mg⋅L−1) to avoid interference with colorimetric measurements, digested using an alkaline potassium persulfate digestion reagent, and analyzed on an Astoria Analyzer (Astoria-Pacific).
Inorganic nutrient samples were filtered after collection and either analyzed in the field or preserved and analyzed in the laboratory. NH4+-N was analyzed in the field using fluorometric methods in 2011–2012 (56, 57) and using the gas exchange method on a portable flow injection analyzer in 2013 (58). Nitrate (NO3−-N) was analyzed in the laboratory using cadmium reduction on an Astoria Analyzer in 2011 and in the field using zinc reduction on a portable flow injection analyzer in 2012–2013 (59, 60). SRP was analyzed using the molybdate blue method in the laboratory in 2011 and on a portable flow injection analyzer in 2012–2013 (61).
Nutrient concentrations were multiplied by average hourly discharge for the time the sample was collected, and were scaled to estimate nutrient flux in kilograms per day (kg⋅d−1). Upstream flux was calculated over this time period to compare with carcass loading. Net nutrient flux from the carcasses was calculated by subtracting upstream values from downstream values. Total flux was estimated by measuring the area under the curve in Sigmaplot 12.0 (Systat Software, Inc.). Flux values were compared with total carcass loading levels for C, N, and P, which were quantified by multiplying the number of carcasses between water sampling points by the percentage of C, N, and P composition of a carcass (described above) (Table S2).
Terrestrial Transport via Scavengers.
We used game cameras and metabolic models to estimate the use of wildebeest carcasses and fate of nutrients and C consumed by scavengers. We estimated scavenger abundance using a game camera (Trophy Cam HD Max Black LED; Bushnell) placed on two different aggregations of wildebeest carcasses after drownings: one aggregation of 16 carcasses in November 2012 and one aggregation of 40 carcasses in October 2013. The camera was programmed to take a picture every 15 min, and species identification and abundance were recorded every hour from days 5–17 after the drowning occurred in 2012 and days 4–21 in 2013. The majority of scavengers were avian, and the most common species observed were Marabou storks (Leptoptilos crumenifer), white-backed vultures (Gyps africanus), Rüppell’s vultures (Gyps rueppellii), and hooded vultures (Necrosyrtes monachus) (all referred to below as vultures for ease of description). Individuals of these four species accounted for 80% of all scavengers observed in 2012 and 86% in 2013.
We developed a metabolic model for vulture consumption of carcasses (Table S5) based on estimations of daily energy expenditure (kilojoules per day) of free-living Cape vultures (Gyps coprotheres) of 826.7 kJ⋅d−1 * kg0.61 (49). We used this equation and average mass estimations (50) for our four most common species to estimate total energy required daily. We divided this estimation by vulture mean energy assimilation efficiency (86%) to estimate the total daily energy intake required, and we divided that amount by carcass energy content (based on literature estimations of 26 kJ⋅g−1 of ash-free dry mass) to estimate the total dry mass of carcass consumed daily (49). We multiplied this mass by the mean percentage of C, N, and P of wildebeest soft tissue (excluding bone) to estimate total C, N, and P consumed.
We multiplied individual consumption rates by the maximum number of vultures documented daily at each carcass aggregation. We assumed that avian scavengers were obtaining 100% of their energy during this time period from the carcasses, so we used the maximum number of individuals recorded at any point in time each day for each species as our estimation of the number of vultures. Our photographs began on days 4 and 5 after the drowning. We assumed there were no vultures feeding on the first day and a linear increase in vultures to the number present on the first day of photographs. The maximum number of vultures counted on any given day ranged from two to 46 for the 2012 photograph series and from four to 57 for the 2013 photograph series. This number could be a very conservative estimation if vultures only spend several hours feeding at the river and then leave to be replaced by different individuals; thus, we could be underestimating total vulture consumption. We summed the daily consumption of C, N, and P by vultures and compared it with the total amount of C, N, and P loaded by the number of carcasses present in the photographs.
Assimilation by Aquatic Consumers.
We used analysis of δ13C and δ15N stable isotopes to quantify assimilation of wildebeest carcass by fish. We collected basal resources and fish samples in November 2013 (1 mo after a series of large wildebeest mass drownings) and February 2014 (4 mo after the last drowning). Basal resources included biofilm, coarse particulate organic matter (CPOM), and wildebeest tissue. We collected biofilm from rocks and wildebeest bones, which were the dominant substrates in the river, by scraping the surface of the substrate and filtering the slurry onto a precombusted glass fiber filter (Whatman GF/C). We collected a composite CPOM sample by holding a 1-mm mesh net in a well-mixed portion of the river to collect suspended material, which we subsampled for analysis. There is no emergent vegetation in this region of the river and minimal riparian vegetation, and CPOM reflects the majority of terrestrial vegetation that has entered the river, which is predominantly grass loaded by hippos (62). We collected muscle tissue from three recently drowned wildebeest carcasses (one adult male and one subadult male in 2012, and one adult female in 2013). Consumers included three common taxa of fish. Bagrus docmak [n = 3 in November (125- to 156-mm standard total length) and n = 3 in February (147–265 mm)] is largely predacious, consuming insects, crustaceans, and fishes, although debris and vegetative matter are also consumed (34, 35). Labeobarbus altianalis [n = 10 in November (69–318 mm) and n = 5 in February (132–311 mm)] is a generalist consumer, with a diet including molluscs, insects, plants, and crustaceans as well as, to a lesser extent, fishes (34). Labeo victorianus [n = 9 in November (71–333 mm) and n = 8 in February (81–233 mm)] largely feeds on epilithic and epiphytic algae (34). We captured fish using gillnets and an electroshocker, and collected tissue plugs from the lateral muscle near the dorsal fin. Fish muscle tissue typically has a turnover rate of several weeks to months, so it provides an integrated signal of diet at a time scale relevant to the occurrence of carcasses in the river and the timing of our sample collection (63). All samples were kept frozen until they could be dried at 60 °C, ground into a fine powder, and analyzed for δ13C and δ15N on a Costech Elemental Analyzer.
We estimated the contribution of basal resources to the fish tissue assimilation using Bayesian mixing models in MixSIAR (51, 52). For trophic enrichment factors, we used 0.4 ± 1.3 for δ13C (53) and 4.3 ± 1.5 for δ15N (54), which incorporate variability in trophic structure for these omnivorous fish. In November 2013, when carcasses were present in the river, the isotopic signature of bone biofilm was very similar to wildebeest tissue, so we only included wildebeest tissue as a potential source. For basal resource end members, we used CPOM (δ13C = −14.3 ± 0.3, δ15N = 3.1 ± 0.5; n = 3), wildebeest tissue (δ13C = −11.0 ± 0.5, δ15N = 7.3 ± 0.4; n = 3), and rock biofilm (δ13C = −23.4 ± 2.0, δ15N = 6.8 ± 2.2; n = 3). In February 2014, wildebeest tissue had not been present in the river for 4 mo, so we replaced wildebeest tissue with bone biofilm as a potential source. Our basal resource end members were CPOM (δ13C = −14.1 ± 0.5, δ15N = 4.7 ± 0.3; n = 3), bone biofilm (δ13C = −15.2 ± 2.3, δ15N = 11.2 ± 1.8; n = 3), and rock biofilm (δ13C = −19.2 ± 0.6, δ15N = 13.0 ± 1.2; n = 3). Fish may have retained some of the isotopic signal from wildebeest tissue even after carcasses were gone; however, we estimated the average isotopic half-life for muscle tissue from fish in our study was 71 d (63), suggesting that signal would be largely gone by February. Furthermore, the bone biofilm signature in February 2014 was distinct from the wildebeest tissue signature. Visual analysis of isospace plots confirmed that consumer data were within the minimum convex polygon of source data, suggesting we were not missing any major diet sources (64).
We estimated consumption of carcasses by Nile crocodiles (Crocodylus niloticus) using literature and field estimations. Captive adult Nile crocodiles (2–4 m long) fed to satiation consumed, on average, 1–3 kg of fish per day (37). During two carcass surveys, we counted 33–41 Nile crocodiles in the 3.5 km of river in which the majority of mass drownings occurred. If all individuals ate to satiation throughout the average 172-d period when the wildebeest migration is in the Mara region, they would consume 21,160 kg of carcass (wet mass), which is equivalent to the total mass in 120 carcasses and the soft tissue mass in 150 carcasses.
All data used in these analyses will be made available on the Dryad Digital Repository.
Conclusion
We have presented a systematic quantification of resource movement from a terrestrial to aquatic ecosystem through mass drownings within a large, intact ungulate migration. These mass drownings have little impact on the wildebeest herd, comprising only 0.5% of the total herd size, but they provide huge short-term and long-term sources of nutrients to the Mara River that form hot spots of biogeochemical cycling, stimulate nutrient uptake, and transport nutrients downstream and back to the terrestrial ecosystem. Large migrations of terrestrial vertebrates have declined or been lost from many natural ecosystems (2, 40), with an associated loss in ecosystem functions they maintained (1, 41). Many river ecosystems likely experienced mass drownings in the past, although their occurrence has likely declined in concert with loss of migrations and increased human presence along rivers. The widespread loss of mass drownings may have fundamentally altered river ecosystem structure and function in ways hitherto unrecognized. For example, decreasing P loading through bone deposits may contribute to P limitation in many freshwater ecosystems (42), although this effect may be offset in some ecosystems by anthropogenic nutrient inputs (43). This study provides an examination of the potential importance that large migrations may have had on rivers throughout much of the world.
Materials and Methods
We documented wildebeest crossings and mass drownings in the Kenyan portion of the Mara River from 2001 to 2014 from two sets of historical reports and from 2011 to 2015 from field surveys (Table S1). Carcass composition was measured by collecting freshly drowned wildebeest carcasses from the river (n = 3), dissecting them into their primary components, and measuring wet and dry mass and C, N, and P composition for each component (Table S2). We measured the decomposition of carcass components using fine-mesh litterbags (Table S2), and calculated the decay rate (k) using a single exponential decay function, with a fixed intercept of 100% at time step 0 (44, 45).
We measured river discharge from 2011 to 2014 at the lower reach of our study region using a depth transducer at a rated cross-section of the river (46). We measured nutrient uptake length (Sw), uptake velocity (vf), and aerial uptake (U) for NH4+ and SRP after a mass drowning of 5,000 wildebeest in 2011, using the carcasses as a high-input nutrient source and declines in concentration downstream as an indication of nutrient uptake in the river (27, 28, 47, 48) (Table S3). We calculated minimum U (Umin; based on upstream nutrient concentrations) and maximum U (Umax; based on the highest nutrient concentrations during the sampling period) for NH4+ and SRP on days 8, 16, and 26 after the drowning. We calculated total aerial uptake over this time period for the 6.5-km reach in which the majority of carcasses were located and compared these amounts with the total loading of N and P by the carcasses within this reach. We measured net flux of total and dissolved inorganic nutrient levels and DOC from carcasses for three drownings for which we could collect data for at least 25 d (Table S4). Upstream flux was calculated over this time period to compare with carcass loading. Flux values were compared with total loading of C, N, and P by the carcasses between upstream and downstream sampling points (Table S2).
We used game cameras and metabolic models to estimate the use of carcasses by avian scavengers for two different carcass aggregations. We developed a metabolic model for vulture consumption of carcasses (Table S5) based on estimations of daily energy expenditure [kilojoules per day (kJ⋅d−1)] (49), average mass estimations (50), vulture mean energy assimilation efficiency, and carcass energy content (49) to estimate the total dry mass of carcass consumed daily. We multiplied this mass by the mean percentage of C, N, and P of wildebeest soft tissue (excluding bone) to estimate total C, N, and P consumed. We multiplied individual consumption rates by the maximum number of vultures documented daily at each carcass aggregation. We summed the daily consumption of C, N, and P by vultures and compared it with the total amount of C, N, and P loaded by the number of carcasses present in the photographs.
We used analysis of δ13C and δ15N stable isotopes to quantify assimilation of wildebeest carcass by fish. We collected basal resources [biofilm from rocks and bones, coarse particulate organic matter, and wildebeest tissue] and fish samples in November 2013 (1 mo after a series of large wildebeest mass drownings) and February 2014 (4 mo after the last drowning). In November 2013, when carcasses were present in the river, the isotopic signature of bone biofilm was very similar to wildebeest tissue, so we only included wildebeest tissue as a potential source. In February 2014, wildebeest tissue had not been present in the river for 4 mo, so we replaced wildebeest tissue with bone biofilm as a potential source. We analyzed muscle tissue (which typically has a turnover rate of weeks to months) for three common taxa of fish: B. docmak (n = 3 in November, n = 3 in February), L. altianalis (n = 10 in November, n = 5 in February), and L. victorianus (n = 9 in November, n = 8 in February). We estimated the contribution of basal resources to fish tissue assimilation using Bayesian mixing models in MixSIAR (51, 52). For trophic enrichment factors, we used 0.4 ± 1.3 for δ13C (53) and 4.3 ± 1.5 for δ15N (54), which incorporate variability in trophic structure for these omnivorous fish. We estimated total consumption of carcasses by Nile crocodiles (C. niloticus) throughout the entire 172-d period when the migration is the Mara region using literature values on consumption to satiation (37) and field estimations of crocodile abundance in the 3.5-km of river in which most mass drownings occurred.
All data used in these analyses are available on the Dryad Digital Repository. Further details regarding methods are available in SI Materials and Methods.
Acknowledgments
We thank Brian Heath, the Mara Conservancy, Patrick Reynolds, Paul Geemi, Laban Njoroge, Edward Njagi, Joseph Gathua, and Katherine Handler for their assistance with this research. Our research was conducted in affiliation with the National Museums of Kenya and permit NCST/RCD/12B/012/36 from the Kenya National Commission for Science, Technology, and Innovation. Fish were sampled in accordance with Institutional Animal Care and Use Committee Animal Use Protocol (AUP) 2012-10734 and AUP 2015-10734. Funding for this research was provided by the Yale Institute for Biospheric Studies, the Robert and Patricia Switzer Foundation, the National Geographic Society Committee for Research and Exploration, and National Science Foundation Grants DEB 1354053 and DEB 1354062.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
See Commentary on page 7489.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1614778114/-/DCSupplemental.
References
- 1.Doughty CE, et al. Global nutrient transport in a world of giants. Proc Natl Acad Sci USA. 2016;113:868–873. doi: 10.1073/pnas.1502549112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Harris G, Thirgood S, Hopcraft JGC, Cromsigt JPGM, Berger J. Global decline in aggregated migrations of large terrestrial mammals. Endanger Species Res. 2009;7:55–76. [Google Scholar]
- 3.Hopcraft JGC, et al. Serengeti IV: Sustaining Biodiversity in a Coupled Human–Natural System. Univ of Chicago Press; Chicago: 2013. Why are wildebeest the most abundant herbivore in the Serengeti ecosystem? [Google Scholar]
- 4.Sinclair ARE. Mammal population regulation, keystone processes and ecosystem dynamics. Philos Trans R Soc Lond B Biol Sci. 2003;358:1729–1740. doi: 10.1098/rstb.2003.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McNaughton SJ. Serengeti migratory wildebeest: Facilitation of energy flow by grazing. Science. 1976;191:92–94. doi: 10.1126/science.191.4222.92. [DOI] [PubMed] [Google Scholar]
- 6.Holdo RM, Holt RD, Coughenour MB, Ritchie ME. Plant productivity and soil nitrogen as a function of grazing, migration and fire in an African savanna. J Ecol. 2007;95:115–128. [Google Scholar]
- 7.Pennycuick L. Movements of the migratory wildebeest population in the Serengeti area between 1960 and 1973. Afr J Ecol. 1975;13:65–87. [Google Scholar]
- 8.Capaldo SD, Peters CR. Observations of wildebeest, Connochaetes taurinus (Artiodactyla, Bovidae), crossing Lake Masek (Serengeti ecosystem, Tanzania), including one small drowning. Mammalia. 1996;60:303–306. [Google Scholar]
- 9.Capaldo SD, Peters CR. Skeletal inventories from wildebeest drownings at Lakes Masek and Ndutu in the Serengeti Ecosystem of Tanzania. J Archaeol Sci. 1995;22:385–408. [Google Scholar]
- 10.Dechant-Boaz D. 1982. Modern riverine taphonomy: Its relevance to the interpretation of Plio-Pleistocene hominid paleoecology in the Omo Basin, Ethiopia. PhD dissertation (University of California, Berkeley)
- 11.Hummel M, Ray JC. Caribou and the North: A Shared Future. Dundurn; Toronto: 2008. [Google Scholar]
- 12.Eberth DA. Origins of dinosaur bonebeds in the Cretaceous of Alberta, Canada. Can J Earth Sci. 2015;52:655–681. [Google Scholar]
- 13.Smith CR, Baco AR. Ecology of whale falls at the deep-sea floor. Oceanogr Mar Biol. 2003;41:311–354. [Google Scholar]
- 14.Bump JK, et al. Ungulate carcasses perforate ecological filters and create biogeochemical hotspots in forest herbaceous layers allowing trees a competitive advantage. Ecosystems. 2009;12:996–1007. [Google Scholar]
- 15.Janetski DJ, Chaloner DT, Tiegs SD, Lamberti GA. Pacific salmon effects on stream ecosystems: A quantitative synthesis. Oecologia. 2009;159:583–595. doi: 10.1007/s00442-008-1249-x. [DOI] [PubMed] [Google Scholar]
- 16.Saindon RA. Explorations into the World of Lewis and Clark. Digital Scanning, Inc.; Scituate, MA: 2003. [Google Scholar]
- 17.Isenberg AC. The Destruction of the Bison. Cambridge Univ Press; Cambridge, MA: 2000. [Google Scholar]
- 18.Naiman RJ, Helfield JM, Bartz KK, Drake DC, Honea JM. 2009. Pacific salmon, marine-derived nutrients, and the characteristics of aquatic and riparian ecosystems. Challenges for Diadromous Fishes in a Dynamic Global Environment, American Fisheries Society Symposium, eds Haro A, et al. (American Fisheries Society, Bethesda), Vol 69, pp 395–425.
- 19.West DC, Walters AW, Gephard S, Post DM. Nutrient loading by anadromous alewife (Alosa pseudoharengus): Contemporary patterns and predictions for restoration efforts. Can J Fish Aquat Sci. 2010;67:1211–1220. [Google Scholar]
- 20.Mduma SAR, Sinclair ARE, Hilborn R. Food regulates the Serengeti wildebeest: A 40-year record. J Anim Ecol. 1999;68:1101–1122. [Google Scholar]
- 21.Talbot LM, Talbot MH. The wildebeest in western Masailand, East Africa. Wildl Monogr. 1963;12:3–88. [Google Scholar]
- 22.McIntyre PB, et al. Fish distributions and nutrient cycling in streams: Can fish create biogeochemical hotspots? Ecology. 2008;89:2335–2346. doi: 10.1890/07-1552.1. [DOI] [PubMed] [Google Scholar]
- 23.McClain ME, et al. Biogeochemical hot spots and hot moments at the interface of terrestrial and aquatic ecosystems. Ecosystems. 2003;6:301–312. [Google Scholar]
- 24.Levi PS, Tank JL, Tiegs SD, Chaloner DT, Lamberti GA. Biogeochemical transformation of a nutrient subsidy: Salmon, streams, and nitrification. Biogeochemistry. 2013;113:643–655. [Google Scholar]
- 25.Pershing AJ, Christensen LB, Record NR, Sherwood GD, Stetson PB. The impact of whaling on the ocean carbon cycle: why bigger was better. PLoS One. 2010;5:e12444. doi: 10.1371/journal.pone.0012444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hocking MD, Reimchen TE. Salmon species, density and watershed size predict magnitude of marine enrichment in riparian food webs. Oikos. 2009;118:1307–1318. [Google Scholar]
- 27.Gibson CA, Meyer JL. Nutrient uptake in a large urban river. J Am Water Resour Assoc. 2007;43:576–587. [Google Scholar]
- 28.Marti E, Aumatell J, Godé L, Poch M, Sabater F. Nutrient retention efficiency in streams receiving inputs from wastewater treatment plants. J Environ Qual. 2004;33:285–293. doi: 10.2134/jeq2004.2850. [DOI] [PubMed] [Google Scholar]
- 29.Tank JL, Rosi-Marshall EJ, Baker MA, Hall RO., Jr Are rivers just big streams? A pulse method to quantify nitrogen demand in a large river. Ecology. 2008;89:2935–2945. doi: 10.1890/07-1315.1. [DOI] [PubMed] [Google Scholar]
- 30.Hewson R. Use of salmonid carcasses by vertebrate scavengers. J Zool (Lond) 1995;235:53–65. [Google Scholar]
- 31.Muhametsafina A, Midwood JD, Bliss SM, Stamplecoskie KM, Cooke SJ. The fate of dead fish tagged with biotelemetry transmitters in an urban stream. Aquat Ecol. 2014;48:23–33. [Google Scholar]
- 32.Houston DC, Cooper JE. The digestive tract of the whiteback griffon vulture and its role in disease transmission among wild ungulates. J Wildl Dis. 1975;11:306–313. doi: 10.7589/0090-3558-11.3.306. [DOI] [PubMed] [Google Scholar]
- 33.Houston DC. Breeding of the white-backed and Ruppell’s Griffon vultures, Gyps africanus and G. rueppellii. Ibis. 1976;118:14–40. [Google Scholar]
- 34.Corbet PS. The food of non-cichlid fishes in the Lake Victoria Basin, with remarks on their evolution and adaptation to lacustrine conditions. Proc Zool Soc Lond. 1961;136:1–101. [Google Scholar]
- 35.Bailey RG. Guide to the fishes of the River Nile in the Republic of the Sudan. J Nat Hist. 1994;28:937–970. [Google Scholar]
- 36.Battin TJ, Besemer K, Bengtsson MM, Romani AM, Packmann AI. The ecology and biogeochemistry of stream biofilms. Nat Rev Microbiol. 2016;14:251–263. doi: 10.1038/nrmicro.2016.15. [DOI] [PubMed] [Google Scholar]
- 37.Whitfield AK, Stephen JMB. Predation on striped mullet (Mugil cephalus) by Crocodylus niloticus at St. Lucia, South Africa. Copeia. 1979;(2):266–269. [Google Scholar]
- 38.Cross WF, et al. Food-web dynamics in a large river discontinuum. Ecol Monogr. 2013;83:311–337. [Google Scholar]
- 39.Wheeler TA, Kavanagh KL, Daanen SA. Terrestrial salmon carcass decomposition: Nutrient and isotopic dynamics in central Idaho. Northwest Sci. 2014;88:106–119. [Google Scholar]
- 40.Bolger DT, Newmark WD, Morrison TA, Doak DF. The need for integrative approaches to understand and conserve migratory ungulates. Ecol Lett. 2008;11:63–77. doi: 10.1111/j.1461-0248.2007.01109.x. [DOI] [PubMed] [Google Scholar]
- 41.Doughty CE, Wolf A, Malhi Y. The legacy of the Pleistocene megafauna extinctions on nutrient availability in Amazonia. Nat Geosci. 2013;6:761–764. [Google Scholar]
- 42.Hecky RE, Kilham P. Nutrient limitation of phytoplankton in freshwater and marine environments - a review of recent evidence on the effects of enrichment. Limnol Oceanogr. 1988;33:796–822. [Google Scholar]
- 43.Twining CW, West DC, Post DM. Historical changes in nutrient inputs from humans and anadromous fishes in New England’s coastal watersheds. Limnol Oceanogr. 2013;58:1286–1300. [Google Scholar]
- 44.R Core Team . R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; Vienna: 2016. [Google Scholar]
- 45.Wider RK, Lang GE. A critique of the analytical methods used in examining decomposition data obtained from litter bags. Ecology. 1982;63:1636–1642. [Google Scholar]
- 46.Dutton C, Anisfeld S, Ernstberger H. A novel sediment fingerprinting method using filtration: Application to the Mara River, East Africa. J Soils Sediments. 2013;13:1708–1723. [Google Scholar]
- 47.Newbold JD, Elwood JW, O’Neill RV, Winkle WV. Measuring nutrient spiralling in streams. Can J Fish Aquat Sci. 1981;38:860–863. [Google Scholar]
- 48.Mulholland PJ, et al. Can uptake length in streams be determined by nutrient addition experiments? Results from an interbiome comparison study. J N Am Benthol Soc. 2002;21:544–560. [Google Scholar]
- 49.Komen J. Energy requirements of adult cape vulture (Gyps coprotheres) J Raptor Res. 1992;26:213–218. [Google Scholar]
- 50.Dunning JJB. CRC Handbook of Avian Body Masses. 2nd Ed CRC; Boca Raton, FL: 2008. [Google Scholar]
- 51.Moore JW, Semmens BX. Incorporating uncertainty and prior information into stable isotope mixing models. Ecol Lett. 2008;11:470–480. doi: 10.1111/j.1461-0248.2008.01163.x. [DOI] [PubMed] [Google Scholar]
- 52.Stock BC, Semmens BX. 2016 doi: 10.1002/ecy.1517. MixSIAR GUI User Manual. Version 3.1. Available at https://github.com/brianstock/MixSIAR. Accessed June 29, 2016. [DOI] [PubMed]
- 53.Post DM. Using stable isotopes to estimate trophic position: models, methods, and assumptions. Ecology. 2002;83:703–718. [Google Scholar]
- 54.Bunn SE, Leigh C, Jardine TD. Diet-tissue fractionation of δ15N by consumers from streams and rivers. Limnol Oceanogr. 2013;58:765–773. [Google Scholar]
- 55.Workshop SS. Concepts and methods for assessing solute dynamics in stream ecosystems. J N Am Benthol Soc. 1990;9:95–119. [Google Scholar]
- 56.Holmes RM, Aminot A, Kérouel R, Hooker BA, Peterson BJ. A simple and precise method for measuring ammonium in marine and freshwater ecosystems. Can J Fish Aquat Sci. 1999;56:1801–1808. [Google Scholar]
- 57.Taylor BW, et al. Improving the fluorometric ammonium method: Matrix effects, background fluorescence, and standard additions. J N Am Benthol Soc. 2007;26:167–177. [Google Scholar]
- 58.Gray SM, Ellis PS, Grace MR, McKelvie ID. Spectrophotometric determination of ammonia in estuarine waters by hybrid reagent-injection gas‐diffusion flow analysis. Spectrosc Lett. 2006;39:737–753. [Google Scholar]
- 59.Ellis PS, Shabani AMH, Gentle BS, McKelvie ID. Field measurement of nitrate in marine and estuarine waters with a flow analysis system utilizing on-line zinc reduction. Talanta. 2011;84:98–103. doi: 10.1016/j.talanta.2010.12.028. [DOI] [PubMed] [Google Scholar]
- 60.APHA . Standard Methods for the Examination of Water and Wastewater. American Public Health Association, American Water Works Association, Water Environment Federation; Washington, DC: 2006. [Google Scholar]
- 61.Lyddy-Meaney AJ, Ellis PS, Worsfold PJ, Butler ECV, McKelvie ID. A compact flow injection analysis system for surface mapping of phosphate in marine waters. Talanta. 2002;58:1043–1053. doi: 10.1016/s0039-9140(02)00428-9. [DOI] [PubMed] [Google Scholar]
- 62.Subalusky AL, Dutton CL, Rosi-Marshall EJ, Post DM. The hippopotamus conveyor belt: Vectors of carbon and nutrients from terrestrial grasslands to aquatic systems in sub-Saharan Africa. Freshw Biol. 2014;60:512–525. [Google Scholar]
- 63.Vander Zanden MJ, Clayton MK, Moody EK, Solomon CT, Weidel BC. Stable isotope turnover and half-life in animal tissues: A literature synthesis. PLoS One. 2015;10:e0116182. doi: 10.1371/journal.pone.0116182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Phillips DL, et al. Best practices for use of stable isotope mixing models in food-web studies. Can J Zool. 2014;92:823–835. [Google Scholar]