Significance
Since birth, infants begin to learn a myriad of sounds. Yet our knowledge of their abilities to remember specific sounds is primarily based on habituation studies that presented them with massive repetitions of single sounds. The present work opens vistas on the study of language acquisition and memory development by suggesting that the presence of various sounds during encoding does not impair recognition in newborns. Results also indicate that a coordinated interplay between temporal and frontal regions of the brain supports newborns’ ability to memorize specific words in this—previously thought—disruptive context.
Keywords: newborns, language, habituation, memory, fNIRS effective connectivity
Abstract
Perception and cognition in infants have been traditionally investigated using habituation paradigms, assuming that babies’ memories in laboratory contexts are best constructed after numerous repetitions of the very same stimulus in the absence of interference. A crucial, yet open, question regards how babies deal with stimuli experienced in a fashion similar to everyday learning situations—namely, in the presence of interfering stimuli. To address this question, we used functional near-infrared spectroscopy to test 40 healthy newborns on their ability to encode words presented in concomitance with other words. The results evidenced a habituation-like hemodynamic response during encoding in the left-frontal region, which was associated with a progressive decrement of the functional connections between this region and the left-temporal, right-temporal, and right-parietal regions. In a recognition test phase, a characteristic neural signature of recognition recruited first the right-frontal region and subsequently the right-parietal ones. Connections originating from the right-temporal regions to these areas emerged when newborns listened to the familiar word in the test phase. These findings suggest a neural specialization at birth characterized by the lateralization of memory functions: the interplay between temporal and left-frontal regions during encoding and between temporo-parietal and right-frontal regions during recognition of speech sounds. Most critically, the results show that newborns are capable of retaining the sound of specific words despite hearing other stimuli during encoding. Thus, habituation designs that include various items may be as effective for studying early memory as repeated presentation of a single word.
Habituation, namely the decreased response to repeated presentations of stimuli, has been widely used to investigate infant cognition. Both behavioral (1) and neuroimaging (2, 3) habituation paradigms are regularly implemented for assessing a wide range of mental processes including categorization, object representation, and memory in very young babies. Despite this long tradition, it remains elusive the extent to which the results of habituation studies (which often consist of massive repetition of a single stimulus) can inform our understanding of memory functions in everyday-like situations—that is, in the presence of various stimuli.
Developmental studies in the language domain have uncovered basic perceptual (4–6), discrimination (7, 8), and mnemonic capacities (9–14) in newborns and fetuses, supporting the hypothesis that memories for sounds begin to be established very early in life. However, infants’ memory for specific words seems to be highly vulnerable to interference. Newborns are capable of remembering a single word that they have heard in a classic habituation paradigm (i.e., consecutive/massed repetitions) and after a silent pause but readily forget the word if interfering words are introduced in the interval before testing (10). Whether these findings reflect memory constraints that allow newborns to remember specific words only if presented in isolation is an open yet crucial question whose answer will contribute to better characterizing the initial state of human memory and will advance our understanding of the foundations of learning and language acquisition in infancy.
Here, we used a modified habituation paradigm that incorporated various words to examine a counterintuitive hypothesis: Newborns may remember specific words even in the presence of other—potentially distracting—words but may be better able to do so when the different words are presented in an interleaved fashion during habituation than when a word is presented alone during habituation and the “distracting” word appears afterward. In other words, additional words may not cancel or weaken infants’ recognition of the most frequently presented word in habituation paradigms, as long as the former are distributed during the encoding stage.
This hypothesis finds support on at least two pieces of evidence. First, there is a phenomenon robustly and consistently observed in adults known as the spacing effect. This refers to the observation that repeated presentations of a stimulus induce better recollection if intervening stimuli occur between the various presentations (i.e., spaced condition) compared with a situation in which no intervening stimuli occur between the repetitions (i.e., massed condition) (15–20). Because the spacing effect has been observed in adults even during incidental learning—when participants are not intentionally memorizing or paying attention to the stimuli (21, 22)—it is likely that this effect influences the functioning of infants’ memory as well.
The second source of information that supports our hypothesis derives from studies showing that specific word-sound representations in infants and toddlers become more robust, generalizable, and phonologically precise with increased input variability and conversely that low variability during encoding appears to degrade later word recognition (23–25). In a related line of research, studies on statistical learning further show that 8-mo-old infants successfully use statistical cues to segment words in conditions of high acoustic variation, whereas low acoustic variation hinders infants’ efficiency in segmenting words from continuous speech (26). A similar advantage provided by input variability has been reported in an artificial language study, where increasing variability between adjacent elements led to better detection of nonadjacent dependencies, both in 14-mo-old infants and adults (27). Altogether, these studies endorse our initial hypothesis that input variability during encoding should at the very least not interfere with the recognition of the words and, if anything, might rather favor a more stable representation of these words.
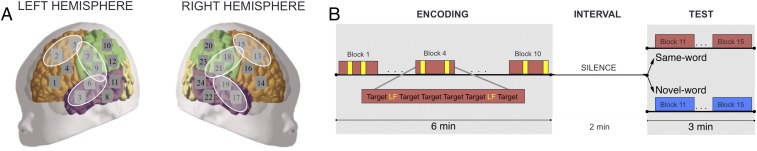
The structure of the present experiment consisted of an encoding phase that incorporated various items followed by a silent retention interval of 2 min and a test phase. During the encoding phase, neonates were presented with 10 blocks containing 8 sounds each. These sounds were either a high-frequency word (hereafter the target) presented six times or a low-frequency word presented two times (Fig. 1B). The low-frequency word plays an analog role to that of interfering stimuli in previous studies. The total number of instances of the target and low-frequency words was the same as in previous studies (experiment 3 in ref. 10). Unlike previous work, however, the low-frequency word was randomly distributed in the blocks during the encoding phase rather than being presented after mass repetition of the target word and before testing. We expected newborns’ brain activity to exhibit a recognition response to the target despite the presence of the potentially distracting low-frequency word during the encoding phase. During the test phase, brain hemodynamic responses to the target word (same-word group) and a completely novel word sound (novel-word group) were assessed in six regions of interest (ROIs).
Fig. 1.
(A) Location of channels (gray squares) and ROIs (white ellipses) on a schematic neonate brain; see also Fig. S1 depicting a projection of the probes to a label map of a MRI neonate template and the SI Materials and Methods for a detailed description of the cortical regions underlying the channels. (B) Experimental design. Each rectangle represents a block consisting of a series words separated by short pauses of 0.5 or 1.5 s. During the encoding phase, all neonates heard 10 blocks composed of 6 repetitions of the target word and 2 repetitions of a potentially distracting word (low-frequency word, LF). In the test, half of the neonates heard the target word and the other half heard a completely novel word. A 2-min silent interval separated the encoding and test phases.
We examined brain local hemodynamic activity in frontal, parietal, and temporal cortical regions as well as functional interactions among these regions during the encoding and recognition phases. Each of these areas appears to support relatively specialized functions in newborns. Temporal areas are mainly involved in perception of the speech signal, with those in the left hemisphere dedicated to the processing of fast phonemic transitions and those in the right one to the processing of the slow acoustic modulations associated with prosody (28). Frontal and parietal regions are believed to sustain important domain-general functions from early infancy (29). Frontal regions are functionally involved in memory tasks (11, 30, 31), novelty and invariance detection (32, 33), and attentional orienting (33–35). Parietal regions have connections with the frontal regions and have also been associated with mnemonic (36, 37), attentional (38), and verbal working memory processes in adults (39, 40).
We evaluated the coordinated interactions among these cortical regions during the elaboration of verbal memories in newborns using Structural Equations Modeling (SEM). Previous studies have shown that frontal and temporal regions of the language network appear anatomically connected already in the first postnatal weeks (41–43). Moreover, these regions show time-lagged cross-correlations in response to passive listening to speech stimuli (44) and exhibit phase synchronization particularly in the left hemisphere (45). Our study aimed to provide a characterization of the spatiotemporal dynamics within this network during memory assessments by exploring the different configurations as a function of the familiarity of a given speech stimulus—namely, when the system processes a word that is familiar to the newborn versus a totally novel word.
Results
Encoding Phase.
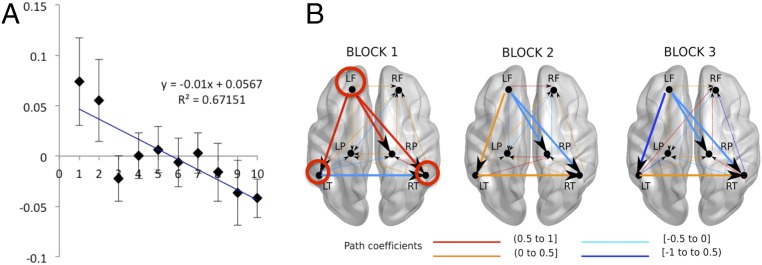
As expected, there were no significant differences in this phase between the hemodynamic activity associated with the same-word and novel-word groups (permutation test, P = 0.49). Hence, data from all participants were pooled together for the analysis. A robust linear regression computed over the 10 encoding blocks showed a significant reduction of oxyhemoglobin (OxyHb) in the left-frontal (LF) ROI [R2 = 0.671; y = –0.01x (P = 0.002) + 0.06 (P = 0.018, Bonferroni-corrected)]. This reduction was most evident in the first three blocks of the encoding phase (Fig. 2A). Computing regressions separately for each participant in this ROI yielded results consistent with the group analysis: The slope values across participants were altogether significantly lower than zero [mean slope = –0.01; t(39) = –2.22, P = 0.03]. No other ROI showed significant changes in OxyHb responses during encoding.
Fig. 2.
(A) Time course of the relative OxyHb changes in the LF area averaged across subjects. The plot depicts mean concentration changes in the encoding phase. The x axis shows block numbers. The y axis shows the changes in concentration of OxyHb in mmol∙mm. Error bars indicate standard error of the mean. (B) Effective connections observed among the bilateral frontal (LF, RF), temporal (LT, RT), and parietal (LP, RP) areas during the habituation response in the first three blocks of the encoding phase. The evaluation of different models with AMOS showed good data fit in all of the blocks. Block 1, χ2 (df = 2) =1.377, P = 0.502; block 2, χ2 (df = 3) = 4.03, P = 0.258; block 3, χ2 (df = 3) = 1.195, P = 0.754. Wider lines highlight the progressive weakening of the functional connections of the LF region and the strengthening of the connections between the temporal regions. Red circles highlight the regions with noteworthy connections. Fig. S2 depicts the hypothetical starting model.
A comparative analysis of effective connectivity across blocks in the encoding phase (where each combination of brain regions and connections among them represents the best fit model for each block) evidenced two systematic changes associated with the habituation effect observed in the first three blocks of the encoding phase (Fig. 2B): (i) weakening of the functional connections from the LF to the left-temporal (LT) (path coefficients block 1, 0.880; block 2, 0.433; block 3, –0.683), right-temporal (RT) (path coefficients block 1, 0.908; block 2, 0; block 3, –0.475), and right-parietal (RP) ROIs (path coefficients block 1, 0.562; block 2, –0.062; block 3, 0) and (ii) strengthening of connections between both temporal ROIs (path coefficients block 1, 0; block 2, 0.238; block 3, 0.476). These differences were confirmed statistically: Whereas connections from LF were weaker in block 1 compared with block 3 (LF to LT ∆ = 1.756, P = 0.001; LF to RT ∆ = 1.717, P = 0.001; LF to RP ∆ = 0.391, P = 0.001) and block 2 compared with block 3 (LF to LT ∆ = 0.271, P = 0.001; LF to RT ∆ = 1.467, P = 0.001; LF to RP ∆ = –0.007, P = 0.884), the interhemispheric connections between temporal regions were stronger (block 1 vs. block 3 ∆ = –0.424, P = 0.001; block 2 vs. block 3 ∆ = 0.17, P = 0.002).
Test Phase.
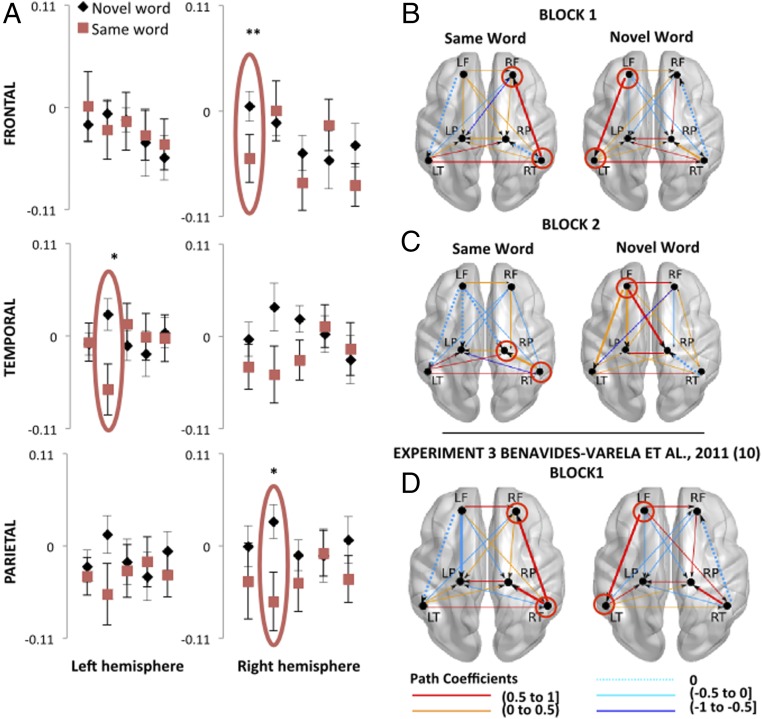
A robust linear regression, comparable to the one carried out in the encoding phase, consistently showed a significant reduction of OxyHb in the LF ROI in the test phase [R2 = 0.876; y = –0.01x (P = 0.02) + 0.004 (P = 0.58)] (Fig. 3A, Top Left). Permutation tests performed over each ROI showed significant differences between groups (same word vs. novel word) in the right-frontal (RF) region in the first block of the test phase (P = 0.006, Bonferroni-corrected) (Fig. 3A, Top Right). In agreement with previous studies reporting a differential response with similar functional near-infrared spectroscopy (fNIRS) paradigms (10, 11), we found that neonates in the novel-word group displayed greater hemodynamic responses than neonates in the same-word group. Furthermore, model comparisons within the connectivity analysis evidenced distinct functional configurations in the same-word versus the novel-word groups (χ2 = 1,365.39, df = 14, P < 0.0001); all but two pathways [LF to RP (∆ = 0.051, P = 0.390); LT to left-parietal (LP) (∆ = –0.123, P = 0.354)] differed between groups (all Ps < 0.005). Moreover, connections from the RT to the RF (the region showing hemodynamic differences between groups) were present only in the same-word (path coefficient, 0.53) but not in the novel-word group. The response in the novel-word group was characterized, like the encoding phase, by strong connections from the LF to the LT areas (path coefficient, 0.50) and from the RT to the RP areas (path coefficient, 0.38). These connections were absent in the same-word group (Fig. 3B).
Fig. 3.
(A) Relative OxyHb changes measured in six ROIs during the 5 blocks comprising the test phase. The y axes show concentrations of OxyHb in mmol·mm. Red ellipses indicate the blocks in which significant differences between groups were found. Fig. S3 shows the hemodynamic curves of the same-word and novel-word groups in the RF area during the first block of the test phase. In all graphs, error bars indicate standard error of the mean (permutation test, *P < 0.01; **P < 0.001). (B–D) Functional connections observed during the first (B) and second block (C) of the recognition test in the current study and the first block (D) of the recognition test in Benavides-Varela et al., 2011 (10), experiment 3. The models appropriately fit the data: block 1 same-word group, χ2 (df = 4) = 5.011, P = 0.286; novel-word group, χ2 (df = 2) = 2.692; P = 0.260; block 2 same-word group, χ2 (df = 4) = 5.694; P = 0.223; novel-word group, χ2 (df = 1) = 1.697; P = 0.193; previous study: block 1 same-word group, χ2 (df = 1) = 1.109, P = 0.292; novel-word group, χ2 (df = 1) = 0.074, P = 0.786. Dotted lines depict connections that were absent in a given block-group model; solid lines highlight the corresponding connections between each pair of areas in the other group. Red circles highlight the regions with noteworthy connections. Fig. S2 depicts the hypothetical starting model.
Fig. S3.
Hemodynamic curves of the same-word and novel-word groups in the RF area during the first block of the test phase. The x axis shows time in seconds from the start of stimulation, and the gray rectangle indicates the stimulation period. Asterisks indicate Bonferroni-corrected P < 0.01.
The hemodynamic analysis also showed differences between groups in the second block of the test phase in the RP region (permutation tests, P = 0.001; Bonferroni-corrected, P = 0.012) and a statistical tendency in the LT ROI (P = 0.01; Bonferroni-corrected, P = 0.12). Consistently with the pattern observed in the RF region and in previous memory studies in newborns (10, 11), the response of the novel-word group was greater than the response of the same-word group.
The connectivity analysis also showed distinct functional configurations in the same-word and the novel-word groups (all pathways differed between groups, χ2 = 1,365.39, df = 14, P < 0.0001). Similar to what was observed in the first block, the region showing significant hemodynamic differences between groups (RP) showed effective connections with the RT region in the same-word group only (path coefficient, 0.53). Moreover, critical connections from the LF region were observed in the novel-word group only: LF–LT path coefficient, 0.416; LF–LP path coefficient, 0.067; LF–RP path coefficient, 0.738 (Fig. 3C).
To further specify the functional connections that distinguish the newborns’ brain responses in the recognition test, we implemented the same connectivity analysis on a previous dataset (experiment 3 in ref. 10). (We contrasted the results of the test phase where the quantity and quality of information is equivalent and thus comparable across studies.) Because this appears to be one of the earliest implementations of the SEM approach to fNIRS data, this analysis allowed us to additionally validate such implementation. In line with the results of the current study, there was a strong coupling between the LF and LT areas (path coefficient, 0.62) and the RT and the RP areas (path coefficient, 0.57) but no connection between the RT and the RF areas (path coefficient, 0) associated with the presentation of novel sounds (Fig. 3D). The critical connections—in the left hemisphere—were significantly stronger in the previous dataset than in the current one (LF to LT ∆ = 0.281, P = 0.001; RT to RP ∆ = 0.16, P = 0.058). Furthermore, like in the current study, we observed strong connections from the RT to the RF areas (path coefficient, 0.73) and no connections from the LF to the LT areas (path coefficient, 0) in the same-word group. Again, the critical connections—in the right hemisphere—were significantly stronger in the previous dataset than in the current one (RT to RF ∆ = –0.132, P = 0.035). A strong connection from the RT to the RP areas (path coefficient, 0.84) was present in the same-word group of the previous study but not in the present one.
Discussion
In habituation-based studies, newborns are able to retain the sounds of specific words for some minutes only if no interfering speech information is presented after the habituation phase and before the test (10). This suggests a strong limitation of the newborns’ verbal memory that might constrain the acquisition of specific sounds from the surrounding language. As opposed to what could have been expected from those previous studies, the present work reports a characteristic recognition response to a familiar word that was presented to newborns in concomitance with other words. Differently from previous works, the various words were presented during—rather than after—habituation. The results corroborated our hypothesis that, in newborns, word-sound representations become more robust with increased input variability and also that, similar to adults (15–22), newborns’ word memories seem to benefit from spaced rather than massive repetitions of a single sound.
The differential brain activations between the same-word group and the novel-word group in the recognition test (firstly and most strongly in RF areas) closely resemble those observed in previous studies addressing word memory in newborns (10, 11). It is hard to disentangle, from these findings, whether the RF region is implicated in recognizing “novel features” (thus the increment of the OxyHb in the novel-word group) or “familiar features” (reflected in the decrement of the OxyHb in the same-word group). [Some studies, particularly with infants and newborns, have reported decrements of OxyHb in response to various experimental stimuli (see ref. 46 for a review).] The connectivity measure contributes information to clarify this issue by showing a strong coupling of the RF region in the same-word group but not the novel-word one. This suggests that the RF region is strongly implicated in the network supporting the recognition of familiar sounds. The connectivity profile in the same-word group was additionally characterized by the presence of strong effective connections between the RT regions and the regions showing significant hemodynamic differences (RF in the first block; RP in the second block). These connections were, by contrast, absent in the novel-word group. This pattern indicates a fundamental role of the RT region [known to process the slow information associated with speech prosody (28, 47) and the prosodic information carried by vowels in single words (11)] as a hub modulating the recognition response. Altogether, this pattern reflects a precocious functional specialization of a network including the temporal to the parietal and frontal connections in the right hemisphere [associated with retrieval in adults (37, 48–51)] supporting the recognition of particular features of word sounds at birth.
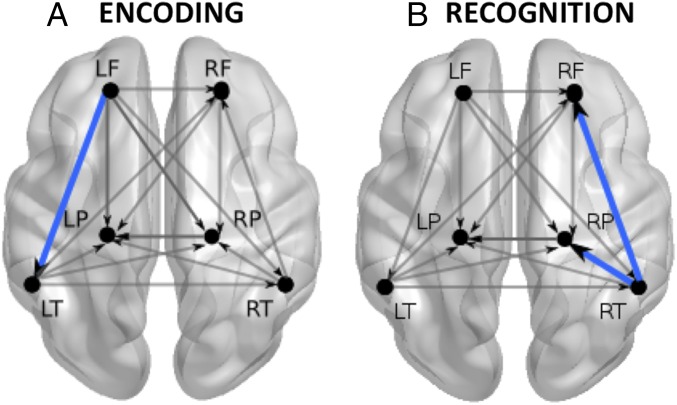
Our results also suggest the involvement of different brain regions on different memory stages (Fig. 4). In particular, although the recognition response included mainly the regions of the right hemisphere described above, encoding recruited predominantly the LF region, as evidenced by the habituation-like function in this region in the first three blocks of the encoding phase (Fig. 2). This localization of the habituation response is consistent with a previous study examining the time evolution of auditory habituation in 3-mo-old infants (33) and learning in newborns (32, 52). These results are also compatible with studies showing the involvement of the LF cortex while adults retrieve previously learned information and simultaneously encode novel aspects of the retrieved information (49–51). This appears highly congruent with the structure of our paradigm that intermixed various sounds during encoding. Such alternation enables the system to contrast the various words several times, highlighting the distinctive aspects of each of them. This should contribute, in turn, to a more accurate representation of these sounds.
Fig. 4.
Verbal memory network at birth and its interactions, mainly characterized by (A) the interplay between temporal and LF regions during encoding and (B) the interaction between temporal, parietal, and RF regions during recognition of words. Blue lines depict the most significant connections characterizing the two memory stages.
The reduction of functional connections from and to the LF region in concomitance with the habituation response further indicates that encoding information at birth is selectively modulated by left-lateralized fronto-temporal and fronto-parietal interactions. The former are likely contingent upon the nature of the stimuli presented to infants (language in the case of our study), whereas the latter might reflect the activation of attentional networks involved in monitoring the invariance or salience of the surrounding stimuli (33, 53). Noticeably, the results of both the connectivity and the hemodynamic activation patterns in the LF region during familiarization (i.e., significant decrement of oxyHb across blocks) are coherent with those observed for the novel words in the test phase.
Even though the models obtained with the SEM approach do not disentangle coactivation of areas in response to a given stimulus from effective connectivity (i.e., directional coupling), the fact that specific connections differentiate the presentation of novel and familiar words suggests that the areas do not simply coactivate in response to speech in newborns but rather interact with each other differently depending on the context.
The results of the connectivity analysis of a previous dataset (experiment 3 in ref. 10) served to document the reliability of the identified connections. In particular, the analysis confirmed that LF–LT connections in the test phase distinguish the exposure to novel sounds even when interfering words appear before testing. The main connection distinguishing the presentation of familiar sounds in the test phase (RT–RF) was also observed in this dataset. These critical connections were significantly stronger in the previous experiment (where interfering sounds presented before the test hindered the differential hemodynamic responses in the test phase) than in the current one (where differential hemodynamic responses between the novel- and same-word groups were observed). Taken together, these findings suggest, on the one hand, that specific connectivity profiles—that is, presence/absence of specific connections—might be informative for generally identifying distinctive memory states: encoding new sounds versus recognition of familiar sounds across studies. On the other hand, the statistical differences suggest a closer link with the results obtained in the traditional hemodynamic activation analysis indicating that the strength of the effective connections might be mostly related to the difficulty of the learning context or increasing cognitive load, as already proposed in the adult literature (54, 55).
To summarize, the present results provide evidence for a functional verbal memory network at birth, mainly characterized by the interplay between temporal and LF regions during encoding and between temporo-parietal and right-frontal regions during recognition of words (Fig. 4). This coordinated activity supports the first and most fundamental stages of verbal learning, enabling human infants to retain the sound of specific words even in the presence of other heterogeneous stimuli.
Although one could believe that encoding and recognition responses rely on similar brain structures, the precocious indication of hemispheric specialization reported here is far from unprecedented. There is a wealth of adult studies showing the consistent involvement of the left prefrontal cortex at encoding as well as activation of the right prefrontal cortex during memory retrieval in a variety of psychological paradigms and test modalities (37, 48–51, 56, 57). The results of the current study suggest that this widely reported encoding–retrieval lateralization pattern between frontal cortices might be rooted very early in development and that this elementary organization might be easily observable in habituation paradigms with variable exposure.
Since the first studies of habituation in infants showing that very young babies memorize a single stimulus after many repetitions (58, 59), it was assumed that presenting various stimuli was detrimental to recognition. Our investigation opens up perspectives on the study of language acquisition and memory development by showing that newborns are able to recognize specific words they heard before even when other words are also presented during the encoding phase.
Materials and Methods
Participants.
Forty healthy full-term newborns (14 males; mean age, 2.7 d; range, 2–5 d) participated in the study. Five additional neonates were tested but not included in the analyses because they failed to complete the experiment due to fussiness. Selection criteria included gestational age between 38 and 42 wk, Apgar scores ≥ 8 in the first and fifth minutes, absence of cephalohematoma, and intact hearing. Moreover, to maximize the likelihood of monitoring the same areas of the brain across different infants, we recruited only babies whose head diameter falls within the 33.5–36.0 cm range (see also ref. 12). Neonates were recruited from the newborn nursery at the Azienda Ospedaliera Universitaria Santa Maria della Misericordia in Udine, Italy between September 2009 and November 2010. All parents gave informed consent for the experiment. The Ethics Committee of the International School for Advanced Studies approved the study.
Stimuli.
Three pseudowords were used in the study (/mita/ /pelu/ /noke/). A female speaker recorded each word in isolation. The words carried stress on the first syllable and were edited to have a mean intensity of 70 dB and a duration of 700 ms. None of the pseudowords have meaning in Italian, the language spoken by the parents of the infants.
Procedure.
The experiment was run inside a dimly lit sound-attenuated booth. Neonates were tested while lying in their cribs either in a state of quiet rest or sleeping. A medical doctor, who was blind to the purpose of the study, assisted the participants during the experiment and contributed to reducing babies’ head movements. Sound stimuli were presented via two loudspeakers. A Mac power PC G5 operated the fNIRS machine and presented the auditory stimuli using the software PsyScope X (psy.ck.sissa.it/). An infrared video camera was used to monitor infants’ behavior and to ensure the correct and stable placement of the probes throughout the experiment.
We implemented a modified version of previous experimental designs to assess memory for words in neonates (10–12), consisting of an encoding phase, a 2-min silent interval, and a test phase (Fig. 1B). In the encoding phase, 10 blocks contained eight words each: six repetitions of a target word (mita for half of the participants and pelu for the other half) and two repetitions of another word (noke). Target and intervening words were presented in random order within blocks. In the test phase, participants heard five blocks containing eight repetitions of a given word: Half of the participants heard again the target word (same-word group), and half heard a completely novel one (novel-word group). All words were separated by pauses of randomized length (0.5 s or 1.5 s). Blocks were spaced at time intervals of varying duration (25 s or 30 s) to avoid synchronization between stimuli occurrences and spontaneous oscillations.
Information about data acquisition, data processing, and probe placement can be found in SI Materials and Methods.
Hemodynamic Response Analysis.
The hemodynamic changes over the encoding phase were assessed by means of robust linear regressions. Regressions were computed both at the group and the individual level. As a means to evaluate the robustness of the results across subjects, slope values for each individual were pooled together and compared against zero with one-sample t tests. To assess whether the same-word and novel-word groups differed in the test, we computed the maximum difference between the mean activation for each condition, as described in previous works (10–12). We evaluated significance using approximate permutation tests (60) by randomly assigning a group label (i.e., “same word” or “novel word”) to each infant. A permutation test was run for each ROI, each of them using 10,000 reassignments. Bonferroni correction for multiple comparisons was applied, when appropriate, across six ROIs × 1 (for the first block) or two test blocks. For additional details, see previously published work (10).
Directional Brain Network Analysis Using SEM.
Effective connectivity analysis was performed with SEM using the AMOS software. We followed the standard procedure to study neural systems connectivity modeling (61), recently implemented in fMRI (that provides comparable neural signals to those measured with fNIRS) (62–64). This approach included the following steps (described in detail in SI Materials and Methods): specification of the theoretical/hypothesized anatomical model, generation of the covariance matrices and structural equations, goodness of fit evaluation, and comparison between models.
Individual models in the test phase were first fitted and then compared using the “stacked model” approach (61). The stacked model implies that when two conditions are compared, they are simultaneously fit to the same model, with the null hypothesis that there are no differences between conditions. In addition, to test for differences in pathways between models, we used bootstrapping with 2,000 samples to estimate 90% bias-corrected confidence intervals. No correction for multiple comparisons is needed for this analysis because the significance of all paths is evaluated at once in model comparisons.
SI Materials and Methods
Data Acquisition.
Brains’ hemodynamic responses were measured with fNIRS using the ETG-4000 machine (Hitachi), allowing for simultaneous recording from 24 channels. The separation between emitters and detectors was 3 cm, the sampling rate 10 Hz, and the total laser power 0.75 mW.
Probe Placement.
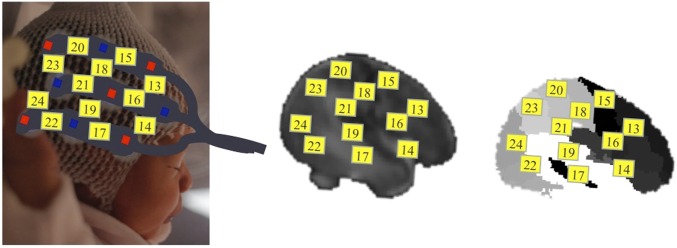
Two silicon chevron-shaped probes provided by the manufacturer were horizontally placed on the neonate’s head (one probe over the left and one over the right hemisphere) using skull landmarks, as reported in previous studies (10–12, 52). Specifically, the probes were placed directly above the ear using the bilateral preauricular points as the reference to align the bottom finger of the probe (channels 3, 6, 8, and 11 in the left hemisphere; channels 17, 19, 22, and 24 in the right hemisphere) with the temporal areas (T3 to T5 and T4 to T6 lines in the left and right hemispheres, respectively). Thereafter, the fibers exiting the silicon holders were oriented in such a way that they crossed symmetrically above the glabella (midpoint between the eyebrows) in the center of the forehead. A stretchy cap was used to keep the probes in place.
The location of the frontal and parietal channels and its correspondence with referential 10–20 system positions was approximately calculated as the optodes were held within fixed silicon arrays ensuring that the source-detector distances were kept equal across infants. In the parietal lobe, P3 was roughly located in the middle of channels 7, 9, and 10 (left hemisphere) and P4 in the middle of channels 8, 21, and 20 (right hemisphere). F3 and F4 in the frontal lobes corresponded to the location of channels 2 (left hemisphere) and 13 (right hemisphere) (Fig. 1A and Fig. S1).
Fig. S1.
Photograph of a neonate with the probe set on the head and a projection from the scalp surface to the intensity model and label map of the neonate MRI templates (templates from ref. 66 are publicly available at http://bric.unc.edu/ideagroup/free-softwares/). Red diamonds correspond to emitters/sources and blue diamonds to detectors. The yellow numbered squares, between adjacent emitter–detector pairs, correspond to the channels.
Given the variability of newborns’ head sizes and shapes, we chose to refer to broad regions (i.e., lobes) when referring to the ROIs. Still, like other recent neonate fNIRS studies (65), we approximately localized the cortical regions underlying the channels by projecting the optode configuration over a neonate MRI template and stereotaxic atlas (66). As depicted in Fig. S1, channels included in the frontal ROIs were positioned over the inferior-middle frontal areas, channels in temporal ROIs were mostly located over the superior and also middle temporal gyrus, whereas channels on the parietal ROIs were placed over the postcentral gyrus, supramarginal gyrus, and the inferior parietal lobule. Note that bordering channels, which might not reliably fall within the same lobes across infants (see ref. 67 referring to this critical issue concerning temporal and inferior frontal areas), or posterior channels that were noisy across participants were not included in the ROIs.
Data Processing.
The signal was bandpass-filtered between 0.02 and 1.00 Hz. Single channels from specific blocks were eliminated if light absorption was less than 1% of the total light emitted or in case of movement artifacts (defined as changes in the signal greater than 0.1 mmol·mm in an interval of 0.2 s). Blocks with more than 12 rejected channels were excluded. Participants were included in the analysis only if the amount of rejected data was less than 30%. A baseline was linearly fitted between the mean of the 5 s preceding the onset of the block and between the 15th second and the 20th second after the offset of the block. The mean signal over the 9 s following the end of the auditory stimulation was used to carry out the statistical analysis. This analysis period corresponds to the time window in which the maximum amplitudes of the hemodynamic responses are expected based on previous fNIRS studies with newborns. OxyHb concentrations of six ROIs (bilateral frontal, temporal, and parietal regions) were used for the data analysis. The channels included in the ROIs were identical to those used in previous studies on newborns’ memory and speech processing using fNIRS (10–12, 52).
Directional Brain Network Analysis Using SEM.
The connectivity analysis of the fNIRS data using SEM consisted of the following steps:
-
-
Specification of the theoretical/hypothesized anatomical model: The model included the six ROIs and directional connections between them (Fig. S2). Hypothetical connections were defined on the basis of anatomical connections among these areas identified in previous studies (41–43). Based on modification indices, a small number of additional connections were added to improve model fit. AMOS uses an iterative maximum likelihood method to obtain a solution for each path coefficient representing the effective connectivity between each node (i.e., anatomical ROI) of the network. The best solution of the set of equations provided by the software minimizes the difference between the observed and predicted covariance matrices. Adding connections to an a priori theoretically and anatomically defined model is a standard procedure of the SEM approach (61–64). This allows identification of interactions potentially missed by an a priori model and can also be used to improve it, a key feature required for ensuring that no strong connections are missed.
-
-
Generation of the covariance matrices and structural equations: Mean time series were computed for each ROI, condition, and subject to calculate a group covariance matrix. We validated the resulting model by comparing the actual covariance matrices representing the relationships between regions and the estimated covariance matrices of the best fitting model using maximum likelihood estimation (68).
-
-
Goodness of fit evaluation: The model’s goodness of fit was evaluated using chi-square statistics to assess the magnitude of discrepancy between the observed and fitted covariance matrices (69). It is generally considered that a good model fit would provide no significant differences between the predicted and the observed data.
-
-
Comparison between models: The adaptation response was conspicuous when analyzing the first three blocks of the encoding phase. Accordingly, three models (one for each block) were constructed to identify coherent functional changes related to this adaptation. Four additional models (two for each group) were constructed as a means to investigate the connectivity changes associated with the hemodynamic response differences observed in the first two blocks of the test phase, where the recognition response was identified.
Fig. S2.
Hypothetical starting model including the six ROIs and directional connections between them.
Acknowledgments
The research leading to these results received funding from the European Research Council under European Union’s Seventh Framework Programme FP7/2007-2013/European Research Council Grant Agreement 269502 PASCAL (to J.M.). Moreover, the writing of this paper was made possible by the support of CONICYT-Chile Programs PIA/BASAL FB0003 and PAI/Academia 79130029 (to D.M.G.) and the “Progetto strategico NEURAT” from the University of Padua (to S.B-.V.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. E.S. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1617589114/-/DCSupplemental.
References
- 1.Oakes LM. Using habituation of looking time to assess mental processes in infancy. J Cogn Dev. 2010;11:255–268. doi: 10.1080/15248371003699977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nordt M, Hoehl S, Weigelt S. The use of repetition suppression paradigms in developmental cognitive neuroscience. Cortex. 2016;80:61–75. doi: 10.1016/j.cortex.2016.04.002. [DOI] [PubMed] [Google Scholar]
- 3.Turk-Browne NB, Scholl BJ, Chun MM. Babies and brains: Habituation in infant cognition and functional neuroimaging. Front Hum Neurosci. 2008;2:16. doi: 10.3389/neuro.09.016.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jardri R, et al. Fetal cortical activation to sound at 33 weeks of gestation: A functional MRI study. Neuroimage. 2008;42:10–18. doi: 10.1016/j.neuroimage.2008.04.247. [DOI] [PubMed] [Google Scholar]
- 5.Draganova R, et al. Sound frequency change detection in fetuses and newborns, a magnetoencephalographic study. Neuroimage. 2005;28:354–361. doi: 10.1016/j.neuroimage.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 6.Webb AR, Heller HT, Benson CB, Lahav A. Mother’s voice and heartbeat sounds elicit auditory plasticity in the human brain before full gestation. Proc Natl Acad Sci USA. 2015;112:3152–3157. doi: 10.1073/pnas.1414924112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheour-Luhtanen M, et al. The ontogenetically earliest discriminative response of the human brain. Psychophysiology. 1996;33:478–481. doi: 10.1111/j.1469-8986.1996.tb01074.x. [DOI] [PubMed] [Google Scholar]
- 8.Mahmoudzadeh M, et al. Syllabic discrimination in premature human infants prior to complete formation of cortical layers. Proc Natl Acad Sci USA. 2013;110:4846–4851. doi: 10.1073/pnas.1212220110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Partanen E, et al. Learning-induced neural plasticity of speech processing before birth. Proc Natl Acad Sci USA. 2013;110:15145–15150. doi: 10.1073/pnas.1302159110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Benavides-Varela S, et al. Memory in the neonate brain. PLoS One. 2011;6:e27497. doi: 10.1371/journal.pone.0027497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Benavides-Varela S, Hochmann JR, Macagno F, Nespor M, Mehler J. Newborn’s brain activity signals the origin of word memories. Proc Natl Acad Sci USA. 2012;109:17908–17913. doi: 10.1073/pnas.1205413109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Benavides-Varela S, Gómez DM, Mehler J. Studying neonates’ language and memory capacities with functional near-infrared spectroscopy. Front Psychol. 2011;2:64. doi: 10.3389/fpsyg.2011.00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Valiante AG, Barr RG, Zelazo PR, Papageorgiou AN, Young SN. A typical feeding enhances memory for spoken words in healthy 2- to 3-day-old newborns. Pediatrics. 2006;117:e476–e486. doi: 10.1542/peds.2004-2859. [DOI] [PubMed] [Google Scholar]
- 14.Swain IU, Zelazo PR, Clifton RK. Newborn infants’ memory for speech sounds retained over 24 hours. Dev Psychol. 1993;29:312–323. [Google Scholar]
- 15.Ebbinghaus H. 1964. Memory: A Contribution to Experimental Psychology (Dover, New York), originally published 1885.
- 16.Xue G, et al. Facilitating memory for novel characters by reducing neural repetition suppression in the left fusiform cortex. PLoS One. 2010;5:e13204. doi: 10.1371/journal.pone.0013204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xue G, et al. Spaced learning enhances subsequent recognition memory by reducing neural repetition suppression. J Cogn Neurosci. 2011;23:1624–1633. doi: 10.1162/jocn.2010.21532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Litman L, Davachi L. Distributed learning enhances relational memory consolidation. Learn Mem. 2008;15:711–716. doi: 10.1101/lm.1132008. [DOI] [PubMed] [Google Scholar]
- 19.Zhao X, et al. Neural mechanisms of the spacing effect in episodic memory: A parallel EEG and fMRI study. Cortex. 2015;69:76–92. doi: 10.1016/j.cortex.2015.04.002. [DOI] [PubMed] [Google Scholar]
- 20.Callan DE, Schweighofer N. Neural correlates of the spacing effect in explicit verbal semantic encoding support the deficient-processing theory. Hum Brain Mapp. 2010;31:645–659. doi: 10.1002/hbm.20894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Russo R, Parkin AJ, Taylor SR, Wilks J. Revising current two-process accounts of spacing effects in memory. J Exp Psychol Learn Mem Cogn. 1998;24:161–172. doi: 10.1037//0278-7393.24.1.161. [DOI] [PubMed] [Google Scholar]
- 22.Challis BH. Spacing effects on cued-memory tests depend on level of processing. J Exp Psychol Learn Mem Cogn. 1993;19:389. [Google Scholar]
- 23.Singh L. Influences of high and low variability on infant word recognition. Cognition. 2008;106:833–870. doi: 10.1016/j.cognition.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rost GC, McMurray B. Speaker variability augments phonological processing in early word learning. Dev Sci. 2009;12:339–349. doi: 10.1111/j.1467-7687.2008.00786.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rost GC, McMurray B. Finding the signal by adding noise: The role of noncontrastive phonetic variability in early word learning. Infancy. 2010;15:608–635. doi: 10.1111/j.1532-7078.2010.00033.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Estes KG, Lew-Williams C. Listening through voices: Infant statistical word segmentation across multiple speakers. Dev Psychol. 2015;51:1517–1528. doi: 10.1037/a0039725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gómez RL. Variability and detection of invariant structure. Psychol Sci. 2002;13:431–436. doi: 10.1111/1467-9280.00476. [DOI] [PubMed] [Google Scholar]
- 28.Telkemeyer S, et al. Sensitivity of newborn auditory cortex to the temporal structure of sounds. J Neurosci. 2009;29:14726–14733. doi: 10.1523/JNEUROSCI.1246-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grossmann T. Mapping prefrontal cortex functions in human infancy. Infancy. 2013;18:303–324. [Google Scholar]
- 30.Baird AA, et al. Frontal lobe activation during object permanence: Data from near-infrared spectroscopy. Neuroimage. 2002;16:1120–1125. doi: 10.1006/nimg.2002.1170. [DOI] [PubMed] [Google Scholar]
- 31.Dehaene-Lambertz G, Dehaene S, Hertz-Pannier L. Functional neuroimaging of speech perception in infants. Science. 2002;298:2013–2015. doi: 10.1126/science.1077066. [DOI] [PubMed] [Google Scholar]
- 32.Bouchon C, Nazzi T, Gervain J. Hemispheric asymmetries in repetition enhancement and suppression effects in the newborn brain. PLoS One. 2015;10:e0140160. doi: 10.1371/journal.pone.0140160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nakano T, Watanabe H, Homae F, Taga G. Prefrontal cortical involvement in young infants’ analysis of novelty. Cereb Cortex. 2009;19:455–463. doi: 10.1093/cercor/bhn096. [DOI] [PubMed] [Google Scholar]
- 34.Watanabe H, Homae F, Nakano T, Taga G. Functional activation in diverse regions of the developing brain of human infants. Neuroimage. 2008;43:346–357. doi: 10.1016/j.neuroimage.2008.07.014. [DOI] [PubMed] [Google Scholar]
- 35.Watanabe H, et al. Effect of auditory input on activations in infant diverse cortical regions during audiovisual processing. Hum Brain Mapp. 2013;34(3):543–565. doi: 10.1002/hbm.21453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cabeza R, Ciaramelli E, Olson IR, Moscovitch M. The parietal cortex and episodic memory: An attentional account. Nat Rev Neurosci. 2008;9:613–625. doi: 10.1038/nrn2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shallice T, et al. Brain regions associated with acquisition and retrieval of verbal episodic memory. Nature. 1994;368:633–635. doi: 10.1038/368633a0. [DOI] [PubMed] [Google Scholar]
- 38.Behrmann M, Geng JJ, Shomstein S. Parietal cortex and attention. Curr Opin Neurobiol. 2004;14:212–217. doi: 10.1016/j.conb.2004.03.012. [DOI] [PubMed] [Google Scholar]
- 39.Jonides J, et al. The role of parietal cortex in verbal working memory. J Neurosci. 1998;18:5026–5034. doi: 10.1523/JNEUROSCI.18-13-05026.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gruber O. Effects of domain-specific interference on brain activation associated with verbal working memory task performance. Cereb Cortex. 2001;11:1047–1055. doi: 10.1093/cercor/11.11.1047. [DOI] [PubMed] [Google Scholar]
- 41.Perani D, et al. Neural language networks at birth. Proc Natl Acad Sci USA. 2011;108:16056–16061. doi: 10.1073/pnas.1102991108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brauer J, Anwander A, Perani D, Friederici AD. Dorsal and ventral pathways in language development. Brain Lang. 2013;127:289–295. doi: 10.1016/j.bandl.2013.03.001. [DOI] [PubMed] [Google Scholar]
- 43.Dubois J, et al. Exploring the early organization and maturation of linguistic pathways in the human infant brain. Cereb Cortex. 2016;26:2283–2298. doi: 10.1093/cercor/bhv082. [DOI] [PubMed] [Google Scholar]
- 44.Homae F, Watanabe H, Nakano T, Taga G. Large-scale brain networks underlying language acquisition in early infancy. Front Psychol. 2011;2:93. doi: 10.3389/fpsyg.2011.00093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Molavi B, et al. Analyzing the resting state functional connectivity in the human language system using near infrared spectroscopy. Front Hum Neurosci. 2014;7:921. doi: 10.3389/fnhum.2013.00921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lloyd-Fox S, Blasi A, Elwell CE. Illuminating the developing brain: The past, present and future of functional near infrared spectroscopy. Neurosci Biobehav Rev. 2010;34:269–284. doi: 10.1016/j.neubiorev.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 47.Homae F, Watanabe H, Nakano T, Asakawa K, Taga G. The right hemisphere of sleeping infant perceives sentential prosody. Neurosci Res. 2006;54:276–280. doi: 10.1016/j.neures.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 48.Tulving E, Kapur S, Craik FI, Moscovitch M, Houle S. Hemispheric encoding/retrieval asymmetry in episodic memory: Positron emission tomography findings. Proc Natl Acad Sci USA. 1994;91:2016–2020. doi: 10.1073/pnas.91.6.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nyberg L, Cabeza R, Tulving E. PET studies of encoding and retrieval: The HERA model. Psychon Bull Rev. 1996;3:135–148. doi: 10.3758/BF03212412. [DOI] [PubMed] [Google Scholar]
- 50.Fletcher PC, Henson RNA. Frontal lobes and human memory: Insights from functional neuroimaging. Brain. 2001;124:849–881. doi: 10.1093/brain/124.5.849. [DOI] [PubMed] [Google Scholar]
- 51.Habib R, Nyberg L, Tulving E. Hemispheric asymmetries of memory: The HERA model revisited. Trends Cogn Sci. 2003;7:241–245. doi: 10.1016/s1364-6613(03)00110-4. [DOI] [PubMed] [Google Scholar]
- 52.Gervain J, Macagno F, Cogoi S, Peña M, Mehler J. The neonate brain detects speech structure. Proc Natl Acad Sci USA. 2008;105:14222–14227. doi: 10.1073/pnas.0806530105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Naghavi HR, Nyberg L. Common fronto-parietal activity in attention, memory, and consciousness: Shared demands on integration? Conscious Cogn. 2005;14:390–425. doi: 10.1016/j.concog.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 54.Newton AT, Morgan VL, Rogers BP, Gore JC. Modulation of steady state functional connectivity in the default mode and working memory networks by cognitive load. Hum Brain Mapp. 2011;32:1649–1659. doi: 10.1002/hbm.21138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fu CH, et al. Modulation of effective connectivity by cognitive demand in phonological verbal fluency. Neuroimage. 2006;30:266–271. doi: 10.1016/j.neuroimage.2005.09.035. [DOI] [PubMed] [Google Scholar]
- 56.Vallesi A, Shallice T. Prefrontal involvement in source memory: An electrophysiological investigation of accounts concerning confidence and accuracy. Brain Res. 2006;1124:111–125. doi: 10.1016/j.brainres.2006.09.076. [DOI] [PubMed] [Google Scholar]
- 57.Kim AS, Vallesi A, Picton TW, Tulving E. Cognitive association formation in episodic memory: Evidence from event-related potentials. Neuropsychologia. 2009;47:3162–3173. doi: 10.1016/j.neuropsychologia.2009.07.015. [DOI] [PubMed] [Google Scholar]
- 58.Fagan JF., 3rd Memory in the infant. J Exp Child Psychol. 1970;9:217–226. doi: 10.1016/0022-0965(70)90087-1. [DOI] [PubMed] [Google Scholar]
- 59.Fantz RL. Visual experience in infants: Decreased attention familiar patterns relative to novel ones. Science. 1964;146:668–670. doi: 10.1126/science.146.3644.668. [DOI] [PubMed] [Google Scholar]
- 60.Nichols TE, Holmes AP. Nonparametric permutation tests for functional neuroimaging: A primer with examples. Hum Brain Mapp. 2002;15:1–25. doi: 10.1002/hbm.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mclntosh AR, Gonzalez-Lima F. Structural equation modeling and its application to network analysis in functional brain imaging. Hum Brain Mapp. 1994;2:2–22. [Google Scholar]
- 62.Dick AS, Goldin-Meadow S, Solodkin A, Small SL. Gesture in the developing brain. Dev Sci. 2012;15:165–180. doi: 10.1111/j.1467-7687.2011.01100.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dick AS, Solodkin A, Small SL. Neural development of networks for audiovisual speech comprehension. Brain Lang. 2010;114:101–114. doi: 10.1016/j.bandl.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mashal N, et al. A network model of observation and imitation of speech. Front Psych. 2012;3:84. doi: 10.3389/fpsyg.2012.00084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Abboub N, Nazzi T, Gervain J. Prosodic grouping at birth. Brain Lang. 2016;162:46–59. doi: 10.1016/j.bandl.2016.08.002. [DOI] [PubMed] [Google Scholar]
- 66.Shi F, et al. Infant brain atlases from neonates to 1- and 2-year-olds. PLoS One. 2011;6:e18746. doi: 10.1371/journal.pone.0018746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lloyd-Fox S, et al. Coregistering functional near-infrared spectroscopy with underlying cortical areas in infants. Neurophotonics. 2014;1:025006. doi: 10.1117/1.NPh.1.2.025006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bollen KA. Structural Equation Modeling with Latent Variables. John Wiley; New York: 1989. [Google Scholar]
- 69.Hu L, Bentler PM. Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Struct Equ Modeling. 1999;6:1–55. [Google Scholar]