Significance
Environments in infancy and childhood influence levels of inflammation in adulthood—an important risk factor for multiple diseases of aging—but the underlying biological mechanisms remain uncertain. Using data from a unique cohort study in the Philippines with a lifetime of information on each participant, we provide evidence that nutritional, microbial, and psychosocial exposures in infancy and childhood predict adult levels of DNA methylation—biochemical marks on the genome that affect gene expression—in genes that regulate inflammation. We also show that DNA methylation in these genes relates to levels of inflammatory biomarkers implicated in cardiovascular and other diseases. These results suggest that epigenetic mechanisms may partially explain how early environments have enduring effects on inflammation and inflammation-related diseases.
Keywords: epigenetics, inflammation, ecological immunology, developmental origins of health, developmental origins of disease
Abstract
Chronic inflammation contributes to a wide range of human diseases, and environments in infancy and childhood are important determinants of inflammatory phenotypes. The underlying biological mechanisms connecting early environments with the regulation of inflammation in adulthood are not known, but epigenetic processes are plausible candidates. We tested the hypothesis that patterns of DNA methylation (DNAm) in inflammatory genes in young adulthood would be predicted by early life nutritional, microbial, and psychosocial exposures previously associated with levels of inflammation. Data come from a population-based longitudinal birth cohort study in metropolitan Cebu, the Philippines, and DNAm was characterized in whole blood samples from 494 participants (age 20–22 y). Analyses focused on probes in 114 target genes involved in the regulation of inflammation, and we identified 10 sites across nine genes where the level of DNAm was significantly predicted by the following variables: household socioeconomic status in childhood, extended absence of a parent in childhood, exposure to animal feces in infancy, birth in the dry season, or duration of exclusive breastfeeding. To evaluate the biological significance of these sites, we tested for associations with a panel of inflammatory biomarkers measured in plasma obtained at the same age as DNAm assessment. Three sites predicted elevated inflammation, and one site predicted lower inflammation, consistent with the interpretation that levels of DNAm at these sites are functionally relevant. This pattern of results points toward DNAm as a potentially important biological mechanism through which developmental environments shape inflammatory phenotypes across the life course.
Inflammation plays a central role in immune defenses against infectious disease (1), but dysregulated or chronic, low-grade inflammatory processes contribute to the pathophysiology of a wide range of diseases, including cardiovascular disease, type 2 diabetes, and autoimmune/atopic conditions (2, 3). Inflammatory activity is also central to normal pregnancy, but dysregulated inflammation can contribute to pregnancy complications and adverse birth outcomes (4, 5). The factors that influence the regulation of inflammation are therefore relevant to clinical practice, and they are of central interest to current research in genomics and immunology as well as epidemiology, demography, and psychobiology (6–11).
Recent empirical work has identified environments in infancy and early childhood as important determinants of inflammatory phenotypes, and concepts from life history theory and ecological immunology have provided a theoretical basis for anticipating these associations (12–14). Individuals born at lower birth weight, and infants who are breastfed for shorter durations, have higher concentrations of C-reactive protein (CRP)—a key biomarker of inflammation—as adults (15–19). Higher levels of microbial exposure in infancy are associated with reduced levels of chronic inflammation in adulthood (18, 20), consistent with broader human and animal model literatures showing that microbial exposures during sensitive periods of immune development have lasting beneficial effects on the regulation of inflammation (21–23). In addition, an emerging body of research in developmental psychobiology reports that major psychosocial stressors (e.g., child neglect, extended parental absence) and socioeconomic adversity in childhood are associated with dysregulated and proinflammatory activity in adulthood (15, 24–26).
Although the underlying biological mechanisms that connect early nutritional, microbial, and psychosocial environments with adult inflammatory phenotypes are not known, epigenetic processes are likely candidates for preserving cellular memories of early experience in the immune system. Indeed, recent human studies suggest that socioeconomic and psychosocial environments in infancy and childhood leave a molecular imprint that has lasting effects on genomic regulation and the developing phenotype (27–30). Of the many marks contributing to the epigenome, DNA methylation (DNAm) has been the major focus of human research and involves the covalent linkage of methyl groups to cytosine residues primarily in the context of CpG dinucleotides. Methylation of sites in gene promoter regions limits access of transcriptional machinery and is typically associated with reduced gene expression, whereas methylation within gene bodies often is associated with enhanced expression (31). The potential biological relevance of these processes is underscored by several studies documenting associations between DNAm and expression of genes involved in the regulation of inflammation as well as biomarkers of inflammation and risk for inflammation-related disorders (30, 32, 33).
Few studies possess the range and time depth of prospectively collected measures needed to test the hypothesis that nutritional, microbial, and psychosocial environments early in development predict patterns of DNAm in inflammatory genes in adulthood. In addition, current understandings of the regulation of inflammation are based almost exclusively on studies with animal models and with human participants residing in affluent, industrialized settings like the United States (34, 35). A broader perspective is important, as 81% of the world’s population resides in low- and middle-income nations (36), where rates of inflammation-related diseases—including cardiovascular, metabolic, atopic, and autoimmune diseases—are rapidly rising (37). The limited variation in microbial, nutritional, and socioeconomic environments in samples drawn from affluent, industrialized settings may constrain investigations into important developmental determinants of inflammatory phenotypes (35).
We use data from a long-term birth cohort study in the Philippines to address both these limitations. Data collection began in 1983, and DNAm as well as concentrations of inflammatory biomarkers were assessed in blood samples collected in 2005, when participants were ∼21 y old. In addition to the prospective design, an advantage of the study is the relatively wide range of ecological variation represented by the sample: Participants were born into areas that were classified as urban residential, congested urban poor, peri-urban, and remote rural (38). In 1983, approximately half the homes had electricity, more than three-quarters collected water from an open source, and less than half used a flush toilet (18). Furthermore, the study initially focused on detailed and frequent assessment of the contextual determinants of maternal and infant health. Beginning with the first interview of the mother during pregnancy and continuing through the offspring’s infancy and childhood, careful attention was given to detailed measurement of proximate nutritional, microbial, psychosocial, and socioeconomic exposures of relevance to growth and development.
We implemented a hypothesis-driven investigation of the association between DNAm in young adulthood and early life environmental exposures. Analyses focused on methylation sites in 114 target genes shown previously to regulate inflammation, which were identified before statistical analyses. The rationale for our targeted analytic approach was twofold. First, limiting our analysis to genes involved in the regulation of inflammation takes advantage of existing knowledge and increases statistical power for detecting meaningful biological associations. Second, venous blood sampling provides access to the tissue of interest—immune cells that increase and decrease inflammation—where patterns of DNAm in regulatory regions are most likely to have functional relevance for inflammation due to the tissue-specific nature of epigenetic processes (39).
We hypothesized that measures of early life environmental exposures, identified in prior research as important to shaping inflammatory phenotypes, would be significant predictors of DNAm in young adulthood. To evaluate the functional relevance of associations between early environments and DNAm, we considered whether differentially methylated sites predicted concentrations of inflammatory biomarkers, measured concurrently in young adulthood. Our analyses identified 10 differentially methylated sites across nine genes, four of which were associated with a composite measure of inflammation. These results provide support for DNAm as a biological mechanism through which experiences early in life may have lasting effects on the regulation of inflammation.
Results
DNAm was characterized in 494 participants with the Illumina 450K array, using DNA extracted from cells in antecubital whole blood, collected by venipuncture when participants were 20.9 y of age (range, 20–22 y). Analyses focused on 222 variable probes in 114 genes (Tables S1 and S2) (40). Before statistical analysis, DNAm values were corrected for interindividual differences in blood cell type composition, bioinformatically derived from 450K DNAm profiles (41).
Table S1.
List of inflammatory genes considered in the analysis and number of variable probes associated with each gene
| Gene name | Number of variable probes |
| ACOT11 | 4 |
| ACVR1 | 0 |
| ADM | 0 |
| AGXT | 0 |
| AIM2 | 4 |
| ANGPT4 | 1 |
| APBA2 | 4 |
| APCS | 0 |
| AQP10 | 0 |
| AQP7 | 0 |
| AQP9 | 0 |
| ASF1A | 1 |
| ASGR2 | 2 |
| ATF3 | 3 |
| ATP6V1E2 | 0 |
| AVIL | 0 |
| BCL2 | 0 |
| C1QA | 0 |
| C1QB | 1 |
| C1QC | 0 |
| C1R | 2 |
| C1S | 1 |
| C2orf40 | 1 |
| CARD9 | 0 |
| CASP10 | 0 |
| CASP6 | 0 |
| CBFA2T3 | 3 |
| CCL1 | 2 |
| CCL11 | 0 |
| CCL2 | 0 |
| CCL20 | 1 |
| CCL26 | 3 |
| CCND2 | 0 |
| CCR7 | 0 |
| CD14 | 1 |
| CD19 | 0 |
| CD1D | 4 |
| CD27 | 1 |
| CD3D | 0 |
| CD3E | 0 |
| CD4 | 0 |
| CD59 | 1 |
| CD7 | 0 |
| CD82 | 4 |
| CD8A | 3 |
| CDC42EP1 | 1 |
| CEBPA | 0 |
| CEBPB | 1 |
| CFH | 0 |
| CLEC2D | 1 |
| CLEC5A | 0 |
| CREBZF | 0 |
| CRHBP | 0 |
| CSF3 | 0 |
| CSF3R | 2 |
| CSNK1E | 0 |
| CTSG | 0 |
| CXCL2 | 0 |
| CXCR1 | 0 |
| CXCR2 | 2 |
| CXCR5 | 2 |
| CXCR6 | 2 |
| CYLD | 1 |
| CYP27B1 | 0 |
| DUSP2 | 1 |
| EBI3 | 0 |
| EGR4 | 1 |
| ENTPD1 | 0 |
| EPHA2 | 1 |
| EVL | 3 |
| F12 | 0 |
| F2RL2 | 1 |
| F8 | 0 |
| FAM13A | 1 |
| FASLG | 1 |
| FBLN5 | 1 |
| FCAR | 1 |
| FCGR3A | 0 |
| FCGRT | 0 |
| FCN2 | 0 |
| FERMT3 | 0 |
| FGD2 | 3 |
| FKBP1B | 3 |
| FOXP3 | 0 |
| FUT7 | 0 |
| FYB | 1 |
| GABRR1 | 0 |
| GATA3 | 1 |
| GLRX2 | 1 |
| GNA12 | 11 |
| GNA13 | 0 |
| GNB2L1 | 0 |
| GNG2 | 1 |
| GNG7 | 0 |
| GNGT2 | 0 |
| GNLY | 1 |
| GP1BA | 0 |
| GPR132 | 3 |
| GPR171 | 1 |
| GPR21 | 0 |
| GPR65 | 2 |
| GRB7 | 0 |
| GSDMC | 0 |
| GUCY1B2 | 1 |
| GZMM | 3 |
| HCK | 0 |
| HIPK3 | 0 |
| HP | 1 |
| HPGDS | 0 |
| HPS4 | 0 |
| IFI44L | 3 |
| IFNG | 0 |
| IFNGR2 | 0 |
| IGLL1 | 2 |
| IL-10 | 0 |
| IL-10RA | 0 |
| IL-10RB | 0 |
| IL-13 | 0 |
| IL-19 | 0 |
| IL-1A | 1 |
| IL-1B | 0 |
| IL-1R2 | 0 |
| IL-20 | 0 |
| IL-21R | 0 |
| IL-23A | 0 |
| IL-26 | 1 |
| IL-2RA | 1 |
| IL-32 | 1 |
| IL-4 | 0 |
| IL-6 | 1 |
| IL-6R | 0 |
| IL-6ST | 0 |
| IL-8 | 1 |
| IRF7 | 1 |
| ITGAX | 0 |
| ITGB7 | 1 |
| JAK3 | 0 |
| JUNB | 0 |
| KLRG1 | 2 |
| KRT1 | 0 |
| LIMK2 | 0 |
| LMO2 | 1 |
| LTA | 3 |
| LTA4H | 0 |
| LTBR | 0 |
| LTC4S | 0 |
| LY9 | 0 |
| LYZ | 1 |
| MAP2K5 | 1 |
| MAP3K14 | 1 |
| MAP3K5 | 1 |
| MAP3K6 | 1 |
| MAP4K4 | 2 |
| MAPK13 | 0 |
| MAPK8 | 0 |
| MEST | 2 |
| MFAP4 | 0 |
| MFSD10 | 0 |
| MMP9 | 1 |
| MPO | 0 |
| MRGPRF | 2 |
| MS4A1 | 0 |
| NCAM1 | 6 |
| NCF2 | 0 |
| NCF4 | 0 |
| NDUFS2 | 0 |
| NFATC1 | 8 |
| NFE2 | 0 |
| NFKB1 | 2 |
| NFKBIA | 2 |
| NFKBIZ | 0 |
| NINJ1 | 0 |
| NLRP12 | 1 |
| NLRP3 | 1 |
| NOL3 | 0 |
| NR1I2 | 0 |
| NR3C1 | 0 |
| NR4A2 | 2 |
| NR4A3 | 0 |
| OLR1 | 0 |
| OSM | 0 |
| P2RX1 | 1 |
| P2RY2 | 0 |
| PDCD1 | 2 |
| PIK3C2B | 3 |
| PIK3R1 | 1 |
| PLCB2 | 2 |
| POR | 0 |
| PPP1R1B | 1 |
| PRF1 | 0 |
| PRKCA | 0 |
| PRKCH | 4 |
| PTGS1 | 1 |
| PTGS2 | 2 |
| PTPRE | 6 |
| PTX3 | 0 |
| PYDC1 | 1 |
| RALGDS | 2 |
| REL | 0 |
| RELA | 0 |
| RELB | 0 |
| RFFL | 1 |
| RHOH | 2 |
| RIMS2 | 1 |
| RIPK4 | 4 |
| RNASE3 | 0 |
| RORC | 1 |
| RUNX3 | 1 |
| S100A8 | 0 |
| SELE | 0 |
| SERPINF1 | 0 |
| SFTPD | 2 |
| SIT1 | 0 |
| SLA2 | 0 |
| SLAMF7 | 0 |
| SLC19A1 | 5 |
| SLC22A18 | 1 |
| SMPD3 | 3 |
| SOCS3 | 2 |
| SPI1 | 0 |
| SPN | 0 |
| ST6GAL1 | 2 |
| STAB1 | 0 |
| STAT3 | 0 |
| SULT1C2 | 4 |
| SULT1C4 | 0 |
| SYDE1 | 0 |
| TIAM1 | 0 |
| TIE1 | 0 |
| TLR1 | 1 |
| TLR3 | 0 |
| TLR4 | 0 |
| TM4SF4 | 0 |
| TNF | 2 |
| TNFAIP3 | 0 |
| TNFAIP6 | 0 |
| TNFRSF11A | 0 |
| TNFRSF1A | 1 |
| TNFRSF1B | 1 |
| TREM1 | 0 |
| TREM2 | 0 |
| TRPM6 | 0 |
| TXK | 1 |
| TYROBP | 0 |
| UBE2N | 0 |
| VTCN1 | 0 |
| XAF1 | 2 |
| XBP1 | 2 |
| ZBTB32 | 1 |
Table S2.
Distribution of beta-values for variable probes considered in statistical analyses
| Probe | Gene name | 10th percentile | 50th percentile | 90th percentile |
| cg04605287 | ACOT11 | 0.416 | 0.497 | 0.572 |
| cg17085123 | ACOT11 | 0.117 | 0.200 | 0.267 |
| cg18755543 | ACOT11 | 0.198 | 0.252 | 0.309 |
| cg21448423 | ACOT11 | 0.594 | 0.651 | 0.702 |
| cg00490406 | AIM2 | 0.306 | 0.421 | 0.489 |
| cg11003133 | AIM2 | 0.540 | 0.647 | 0.716 |
| cg17217296 | AIM2 | 0.381 | 0.640 | 0.835 |
| cg17515347 | AIM2 | 0.384 | 0.529 | 0.644 |
| cg21575706 | ANGPT4 | 0.710 | 0.835 | 0.871 |
| cg01191259 | APBA2 | 0.665 | 0.738 | 0.790 |
| cg15605858 | APBA2 | 0.195 | 0.248 | 0.309 |
| cg26726006 | APBA2 | 0.629 | 0.677 | 0.729 |
| cg27083329 | APBA2 | 0.786 | 0.848 | 0.891 |
| cg12098441 | ASF1A | 0.485 | 0.554 | 0.618 |
| cg08154612 | ASGR2 | 0.184 | 0.243 | 0.299 |
| cg26955290 | ASGR2 | 0.388 | 0.482 | 0.591 |
| cg00909806 | ATF3 | 0.243 | 0.309 | 0.384 |
| cg11968804 | ATF3 | 0.276 | 0.326 | 0.376 |
| cg14988680 | ATF3 | 0.245 | 0.306 | 0.362 |
| cg02623028 | C1QB | 0.742 | 0.795 | 0.845 |
| cg08799922 | C1R | 0.216 | 0.266 | 0.333 |
| cg11024687 | C1R | 0.615 | 0.675 | 0.722 |
| cg19434937 | C1S | 0.689 | 0.755 | 0.799 |
| cg10885338 | C2orf40 | 0.363 | 0.410 | 0.502 |
| cg00730373 | CBFA2T3 | 0.554 | 0.609 | 0.673 |
| cg02431972 | CBFA2T3 | 0.514 | 0.566 | 0.620 |
| cg27430293 | CBFA2T3 | 0.410 | 0.463 | 0.520 |
| cg09704168 | CCL1 | 0.249 | 0.326 | 0.430 |
| cg25101657 | CCL1 | 0.377 | 0.431 | 0.495 |
| cg08575688 | CCL20 | 0.713 | 0.770 | 0.823 |
| cg05556717 | CCL26 | 0.387 | 0.453 | 0.544 |
| cg11303839 | CCL26 | 0.263 | 0.355 | 0.436 |
| cg12943082 | CCL26 | 0.485 | 0.576 | 0.667 |
| cg08830139 | CD14 | 0.606 | 0.680 | 0.725 |
| cg05843457 | CD1D | 0.428 | 0.505 | 0.583 |
| cg10516993 | CD1D | 0.600 | 0.674 | 0.749 |
| cg10963061 | CD1D | 0.378 | 0.449 | 0.517 |
| cg12460133 | CD1D | 0.454 | 0.515 | 0.570 |
| cg22036538 | CD27 | 0.283 | 0.353 | 0.433 |
| cg09864245 | CD59 | 0.204 | 0.336 | 0.377 |
| cg00233028 | CD82 | 0.438 | 0.486 | 0.538 |
| cg13759632 | CD82 | 0.177 | 0.242 | 0.306 |
| cg23664926 | CD82 | 0.296 | 0.365 | 0.428 |
| cg25317631 | CD82 | 0.571 | 0.618 | 0.676 |
| cg06804210 | CD8A | 0.260 | 0.310 | 0.381 |
| cg12606911 | CD8A | 0.155 | 0.218 | 0.327 |
| cg13847640 | CD8A | 0.261 | 0.322 | 0.386 |
| cg03419948 | CDC42EP1 | 0.358 | 0.413 | 0.482 |
| cg05232694 | CEBPB | 0.431 | 0.482 | 0.536 |
| cg27138901 | CLEC2D | 0.622 | 0.680 | 0.736 |
| cg02483735 | CSF3R | 0.701 | 0.759 | 0.804 |
| cg04012535 | CSF3R | 0.348 | 0.414 | 0.474 |
| cg14150666 | CXCR2 | 0.637 | 0.688 | 0.748 |
| cg19225688 | CXCR2 | 0.420 | 0.470 | 0.526 |
| cg19308663 | CXCR5 | 0.225 | 0.267 | 0.361 |
| cg19791714 | CXCR5 | 0.432 | 0.494 | 0.534 |
| cg08033130 | CXCR6 | 0.638 | 0.816 | 0.854 |
| cg19145607 | CXCR6 | 0.639 | 0.750 | 0.796 |
| cg08785784 | CYLD | 0.260 | 0.308 | 0.366 |
| cg01148741 | DUSP2 | 0.303 | 0.360 | 0.412 |
| cg10014308 | EGR4 | 0.411 | 0.472 | 0.518 |
| cg26371327 | EPHA2 | 0.711 | 0.773 | 0.812 |
| cg03555502 | EVL | 0.080 | 0.132 | 0.207 |
| cg10115368 | EVL | 0.741 | 0.795 | 0.842 |
| cg24683041 | EVL | 0.098 | 0.145 | 0.207 |
| cg22828110 | F2RL2 | 0.241 | 0.287 | 0.343 |
| cg17769793 | FAM13A | 0.268 | 0.338 | 0.400 |
| cg17461271 | FASLG | 0.647 | 0.696 | 0.753 |
| cg24074253 | FBLN5 | 0.642 | 0.809 | 0.858 |
| cg14055835 | FCAR | 0.684 | 0.747 | 0.797 |
| cg09048334 | FGD2 | 0.222 | 0.294 | 0.356 |
| cg16773067 | FGD2 | 0.522 | 0.569 | 0.715 |
| cg25569687 | FGD2 | 0.751 | 0.805 | 0.864 |
| cg00901401 | FKBP1B | 0.322 | 0.380 | 0.452 |
| cg03687700 | FKBP1B | 0.251 | 0.305 | 0.354 |
| cg23065097 | FKBP1B | 0.485 | 0.541 | 0.600 |
| cg20380468 | FYB | 0.284 | 0.343 | 0.394 |
| cg12181459 | GATA3 | 0.320 | 0.380 | 0.453 |
| cg21815704 | GLRX2 | 0.485 | 0.539 | 0.594 |
| cg00947599 | GNA12 | 0.486 | 0.587 | 0.689 |
| cg05793240 | GNA12 | 0.723 | 0.779 | 0.838 |
| cg09658497 | GNA12 | 0.562 | 0.666 | 0.798 |
| cg12444411 | GNA12 | 0.476 | 0.572 | 0.673 |
| cg16688681 | GNA12 | 0.803 | 0.862 | 0.917 |
| cg18446336 | GNA12 | 0.439 | 0.516 | 0.606 |
| cg19524238 | GNA12 | 0.806 | 0.870 | 0.925 |
| cg19717773 | GNA12 | 0.518 | 0.594 | 0.720 |
| cg21143896 | GNA12 | 0.463 | 0.547 | 0.636 |
| cg22364890 | GNA12 | 0.435 | 0.891 | 0.947 |
| cg25397054 | GNA12 | 0.418 | 0.469 | 0.544 |
| cg08001559 | GNG2 | 0.395 | 0.457 | 0.518 |
| cg19805943 | GNLY | 0.531 | 0.626 | 0.680 |
| cg16222062 | GPR132 | 0.731 | 0.829 | 0.924 |
| cg16310077 | GPR132 | 0.082 | 0.163 | 0.230 |
| cg16481365 | GPR132 | 0.632 | 0.727 | 0.815 |
| cg11630392 | GPR171 | 0.489 | 0.558 | 0.629 |
| cg13810673 | GPR65 | 0.081 | 0.111 | 0.190 |
| cg19755435 | GPR65 | 0.179 | 0.238 | 0.339 |
| cg13928490 | GUCY1B2 | 0.191 | 0.240 | 0.303 |
| cg06423211 | GZMM | 0.459 | 0.595 | 0.708 |
| cg22603450 | GZMM | 0.453 | 0.518 | 0.586 |
| cg25780551 | GZMM | 0.588 | 0.645 | 0.687 |
| cg23815491 | HP | 0.370 | 0.425 | 0.547 |
| cg03607951 | IFI44L | 0.465 | 0.569 | 0.643 |
| cg05696877 | IFI44L | 0.454 | 0.616 | 0.730 |
| cg06872964 | IFI44L | 0.405 | 0.482 | 0.553 |
| cg10494770 | IGLL1 | 0.302 | 0.346 | 0.471 |
| cg19558972 | IGLL1 | 0.241 | 0.289 | 0.462 |
| cg27606396 | IL-1A | 0.607 | 0.654 | 0.713 |
| cg01810623 | IL-26 | 0.710 | 0.791 | 0.856 |
| cg26316423 | IL-2RA | 0.537 | 0.618 | 0.650 |
| cg03126765 | IL-32 | 0.544 | 0.596 | 0.685 |
| cg01770232 | IL-6 | 0.152 | 0.242 | 0.312 |
| cg18007641 | IL-8 | 0.659 | 0.734 | 0.791 |
| cg17114584 | IRF7 | 0.432 | 0.505 | 0.547 |
| cg20135711 | ITGB7 | 0.787 | 0.887 | 0.911 |
| cg00443307 | KLRG1 | 0.533 | 0.587 | 0.637 |
| cg04548204 | KLRG1 | 0.482 | 0.593 | 0.701 |
| cg09554856 | LMO2 | 0.278 | 0.308 | 0.378 |
| cg06667961 | LTA | 0.413 | 0.470 | 0.528 |
| cg10476003 | LTA | 0.604 | 0.671 | 0.718 |
| cg17709873 | LTA | 0.641 | 0.694 | 0.753 |
| cg22375663 | LYZ | 0.296 | 0.348 | 0.399 |
| cg24247231 | MAP2K5 | 0.622 | 0.675 | 0.791 |
| cg25105522 | MAP3K14 | 0.387 | 0.480 | 0.566 |
| cg15804973 | MAP3K5 | 0.423 | 0.482 | 0.531 |
| cg09230763 | MAP3K6 | 0.659 | 0.712 | 0.765 |
| cg12682972 | MAP4K4 | 0.639 | 0.703 | 0.770 |
| cg13504055 | MAP4K4 | 0.654 | 0.729 | 0.770 |
| cg01888566 | MEST | 0.022 | 0.047 | 0.124 |
| cg13986840 | MEST | 0.477 | 0.538 | 0.590 |
| cg20925811 | MMP9 | 0.665 | 0.731 | 0.792 |
| cg05771342 | MRGPRF | 0.730 | 0.810 | 0.862 |
| cg22986999 | MRGPRF | 0.410 | 0.455 | 0.518 |
| cg04102296 | NCAM1 | 0.693 | 0.751 | 0.813 |
| cg08743050 | NCAM1 | 0.435 | 0.485 | 0.595 |
| cg19624354 | NCAM1 | 0.332 | 0.393 | 0.527 |
| cg20783319 | NCAM1 | 0.253 | 0.308 | 0.361 |
| cg21727486 | NCAM1 | 0.351 | 0.416 | 0.485 |
| cg22173837 | NCAM1 | 0.380 | 0.422 | 0.483 |
| cg03511628 | NFATC1 | 0.598 | 0.679 | 0.793 |
| cg05453001 | NFATC1 | 0.753 | 0.819 | 0.872 |
| cg15773974 | NFATC1 | 0.561 | 0.653 | 0.720 |
| cg16308790 | NFATC1 | 0.569 | 0.676 | 0.754 |
| cg16900964 | NFATC1 | 0.419 | 0.463 | 0.555 |
| cg19316407 | NFATC1 | 0.423 | 0.474 | 0.558 |
| cg19375418 | NFATC1 | 0.663 | 0.804 | 0.926 |
| cg27624471 | NFATC1 | 0.406 | 0.471 | 0.599 |
| cg15409712 | NFKB1 | 0.712 | 0.775 | 0.828 |
| cg17692230 | NFKB1 | 0.563 | 0.611 | 0.664 |
| cg03549618 | NFKBIA | 0.748 | 0.819 | 0.887 |
| cg16518861 | NFKBIA | 0.323 | 0.405 | 0.474 |
| cg04695373 | NLRP12 | 0.350 | 0.412 | 0.493 |
| cg27305735 | NLRP3 | 0.136 | 0.180 | 0.237 |
| cg10089963 | NR4A2 | 0.497 | 0.547 | 0.601 |
| cg16058600 | NR4A2 | 0.489 | 0.565 | 0.641 |
| cg00161225 | P2RX1 | 0.123 | 0.183 | 0.236 |
| cg14247008 | PDCD1 | 0.136 | 0.196 | 0.275 |
| cg21855211 | PDCD1 | 0.135 | 0.204 | 0.275 |
| cg17271974 | PIK3C2B | 0.434 | 0.497 | 0.558 |
| cg20240347 | PIK3C2B | 0.544 | 0.596 | 0.671 |
| cg26064794 | PIK3C2B | 0.520 | 0.585 | 0.628 |
| cg18405544 | PIK3R1 | 0.756 | 0.816 | 0.870 |
| cg09233429 | PLCB2 | 0.861 | 0.921 | 0.962 |
| cg23120601 | PLCB2 | 0.814 | 0.878 | 0.916 |
| cg15070513 | PPP1R1B | 0.569 | 0.635 | 0.699 |
| cg09254210 | PRKCH | 0.657 | 0.746 | 0.827 |
| cg13564459 | PRKCH | 0.064 | 0.120 | 0.191 |
| cg14272084 | PRKCH | 0.608 | 0.658 | 0.709 |
| cg14440640 | PRKCH | 0.454 | 0.506 | 0.567 |
| cg13655082 | PTGS1 | 0.455 | 0.511 | 0.560 |
| cg16101346 | PTGS2 | 0.308 | 0.366 | 0.439 |
| cg25147026 | PTGS2 | 0.418 | 0.469 | 0.531 |
| cg05524354 | PTPRE | 0.424 | 0.507 | 0.588 |
| cg11659652 | PTPRE | 0.661 | 0.752 | 0.829 |
| cg16750801 | PTPRE | 0.201 | 0.253 | 0.306 |
| cg18166144 | PTPRE | 0.668 | 0.721 | 0.772 |
| cg22891191 | PTPRE | 0.536 | 0.602 | 0.663 |
| cg24824686 | PTPRE | 0.571 | 0.660 | 0.739 |
| cg16422492 | PYDC1 | 0.404 | 0.463 | 0.526 |
| cg01413354 | RALGDS | 0.253 | 0.346 | 0.438 |
| cg13753488 | RALGDS | 0.336 | 0.420 | 0.507 |
| cg16662477 | RFFL | 0.313 | 0.362 | 0.416 |
| cg25243082 | RHOH | 0.103 | 0.325 | 0.522 |
| cg26137103 | RHOH | 0.659 | 0.707 | 0.759 |
| cg04852977 | RIMS2 | 0.221 | 0.286 | 0.374 |
| cg03540028 | RIPK4 | 0.667 | 0.722 | 0.769 |
| cg06230839 | RIPK4 | 0.612 | 0.687 | 0.746 |
| cg14897238 | RIPK4 | 0.396 | 0.454 | 0.522 |
| cg21863949 | RIPK4 | 0.215 | 0.261 | 0.319 |
| cg25112191 | RORC | 0.598 | 0.657 | 0.704 |
| cg20670361 | RUNX3 | 0.463 | 0.516 | 0.570 |
| cg12667595 | SFTPD | 0.480 | 0.567 | 0.636 |
| cg17004975 | SFTPD | 0.366 | 0.416 | 0.477 |
| cg02119792 | SLC19A1 | 0.078 | 0.152 | 0.299 |
| cg04574459 | SLC19A1 | 0.061 | 0.096 | 0.176 |
| cg21139150 | SLC19A1 | 0.081 | 0.397 | 0.903 |
| cg22240348 | SLC19A1 | 0.041 | 0.163 | 0.302 |
| cg25211403 | SLC19A1 | 0.047 | 0.122 | 0.193 |
| cg21599100 | SLC22A18 | 0.611 | 0.693 | 0.736 |
| cg00891541 | SMPD3 | 0.493 | 0.547 | 0.604 |
| cg05144928 | SMPD3 | 0.457 | 0.522 | 0.593 |
| cg19297232 | SMPD3 | 0.705 | 0.758 | 0.819 |
| cg23378546 | SOCS3 | 0.287 | 0.345 | 0.389 |
| cg27206407 | SOCS3 | 0.099 | 0.183 | 0.242 |
| cg15928398 | ST6GAL1 | 0.100 | 0.222 | 0.308 |
| cg17448192 | ST6GAL1 | 0.519 | 0.608 | 0.669 |
| cg06795125 | SULT1C2 | 0.238 | 0.308 | 0.451 |
| cg13968390 | SULT1C2 | 0.450 | 0.554 | 0.711 |
| cg23163573 | SULT1C2 | 0.473 | 0.542 | 0.629 |
| cg25838818 | SULT1C2 | 0.090 | 0.153 | 0.407 |
| cg02016764 | TLR1 | 0.281 | 0.354 | 0.428 |
| cg11484872 | TNF | 0.447 | 0.519 | 0.593 |
| cg27531490 | TNF | 0.620 | 0.672 | 0.726 |
| cg09043214 | TNFRSF1A | 0.509 | 0.559 | 0.610 |
| cg00566331 | TNFRSF1B | 0.220 | 0.280 | 0.329 |
| cg13469425 | TXK | 0.816 | 0.932 | 0.948 |
| cg07142009 | XAF1 | 0.178 | 0.291 | 0.399 |
| cg23571857 | XAF1 | 0.531 | 0.579 | 0.632 |
| cg00742649 | XBP1 | 0.350 | 0.403 | 0.465 |
| cg18940763 | XBP1 | 0.227 | 0.275 | 0.330 |
| cg09231418 | ZBTB32 | 0.568 | 0.628 | 0.689 |
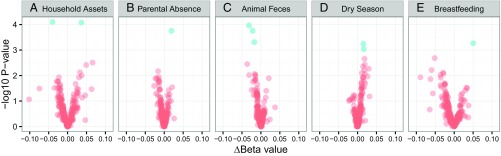
We hypothesized that measures of nutritional, microbial, psychosocial, and socioeconomic exposures early in life—selected before statistical analyses—would predict DNAm in inflammatory genes in adulthood (Table 1). For each probe, we fit linear regression models with parametric Bayes smoothing and included participant sex as a covariate. Multiple comparisons were accounted for by controlling false discovery rate (FDR) with the Benjamini and Hochberg step-up procedure (42), and probes with adjusted P values less than 0.15 are reported in Table 2. Overall, we identified 10 sites across nine genes where DNAm was associated with early life exposures (Fig. 1) (coefficients for all 222 probes are presented in Dataset S1).
Table 1.
Distribution of independent variables for female and male participants
| Variable | Female, n = 395 | Male, n = 99 | Total, n = 494 |
| Birth weight, kg | 3.003 (0.406) | 3.075 (0.431) | 3.017 (0.412) |
| Exclusively breastfed, d | 62.1 (38.0) | 61.9 (39.9) | 62.0 (38.3) |
| Diarrhea, infancy, episodes | 2.2 (1.6) | 2.4 (1.8) | 2.2 (1.7) |
| Fecal exposure, intervals | 1.1 (1.2) | 1.2 (1.2) | 1.2 (1.2) |
| Born in dry season, % | 20.8 | 22.2 | 21.1 |
| Household assets, number | 3.1 (1.5) | 3.1 (1.8) | 3.1 (1.6) |
| Parental absence, % | 16.5 | 16.2 | 16.4 |
Mean (SD) values are presented for continuous variables. n = 494 for all variables except birth weight (n = 487) and gestational length (n = 491).
Table 2.
Methylation sites significantly associated with exposures in infancy/childhood
| Probe | Symbol | Gene region | Exposure | logFC | Adjusted P value |
| cg19434937 | C1S | Body | Household assets (number of items) | 0.0352 | 0.0093 |
| cg08001559 | GNG2 | TSS200 | Household assets (number of items) | −0.0303 | 0.0093 |
| cg06804210 | CD8A | TSS1500 | Animal feces (number of intervals) | −0.0481 | 0.0196 |
| cg12606911 | CD8A | TSS1500 | Animal feces (number of intervals) | −0.0835 | 0.0196 |
| cg27083329 | APBA2 | Upstream | Animal feces (number of intervals) | −0.0630 | 0.0360 |
| cg10014308 | EGR4 | 3′ UTR | Parental absence (1, 0) | 0.1110 | 0.0391 |
| cg02016764 | TLR1 | 5′ UTR | Breastfeeding duration (days) | 0.0014 | 0.0702 |
| cg27606396 | IL-1A | 5′ UTR | Birth in dry season (1, 0) | 0.0994 | 0.1020 |
| cg26064794 | PIK3C2B | Body | Birth in dry season (1, 0) | 0.1026 | 0.1020 |
| cg23163573 | SULT1C2 | 5′ UTR | Breastfeeding duration (days) | −0.0014 | 0.1162 |
Fig. 1.
Volcano plots of the associations between DNAm in young adulthood and variables measured early in development, including (A) household assets in childhood, (B) extended parental absence in childhood, (C) exposure to animal feces in infancy, (D) birth in the dry season, and (E) duration of exclusive breastfeeding. Each point represents a CpG site (n = 222), with the magnitude of association between each variable and DNAm on the x axis (delta beta) and –log10 of the uncorrected P value from the linear regression models on the y axis. Blue dots represent sites with adjusted P values < 0.15.
Household socioeconomic status was indicated by the number of physical assets owned by the family in infancy and childhood, which provides a more stable measure of socioeconomic adversity than income reports in low-income settings (43). Having fewer household assets was associated with lower DNAm for a probe located in the first intron of C1S and higher DNAm for a probe in the promoter region of GNG2. Another measure of adversity in childhood—extended absence of the participant’s mother or father (24)—was also associated with DNAm. Parental absence predicted higher DNAm in EGR4, in a site located in the 3′ untranslated region in the second exon. Parental absence was not significantly associated with household assets (3.3 assets with parental absence vs. 3.0 without; t = –1.47, P = 0.14).
We considered the hypothesis that three measures of microbial exposures in infancy—frequency of infectious diarrhea, exposure to animal feces in the home, and season of birth—would predict DNAm. Previously, all three measures were shown to predict adult CRP in this population (18), and they are only moderately intercorrelated. The Philippines is a highly seasonal tropical environment, and the frequency of infectious diarrhea in infancy was positively correlated with birth in the dry season (Pearson R = 0.14, P = 0.002) and frequency of exposure to animal feces (R = 0.11, P = 0.02). Season of birth and exposure to animal feces were not correlated (R = 0.01, P = 0.78).
Exposure to animal feces significantly predicted DNAm for three sites across two genes: CD8A and APBA2. For each probe, increased frequency of fecal exposure was associated with less methylation. Both probes for CD8A are located in a CpG island in the promoter region, whereas the APBA2 probe is located in the second intron, in a high-density CpG shore. Birth in the dry season predicted higher DNAm for a probe in the first exon, 5′ UTR of IL-1A, and for another probe in the third intron of PIK3C2B. Frequency of infectious diarrhea in infancy was not associated with DNAm in any of the target genes.
Mode of feeding is a defining component of the postnatal nutritional environment, and duration of exclusive breastfeeding predicted DNAm for two sites associated with TLR1 and SULT1C2. Breastfeeding duration was positively associated with DNAm in the second intron 5′ UTR of TLR1 and negatively associated with DNAm in the first exon, 5′ UTR of SULT1C2. There were no significant associations between participant birth weight (adjusted for gestational age) and DNAm in young adulthood at any of the sites considered.
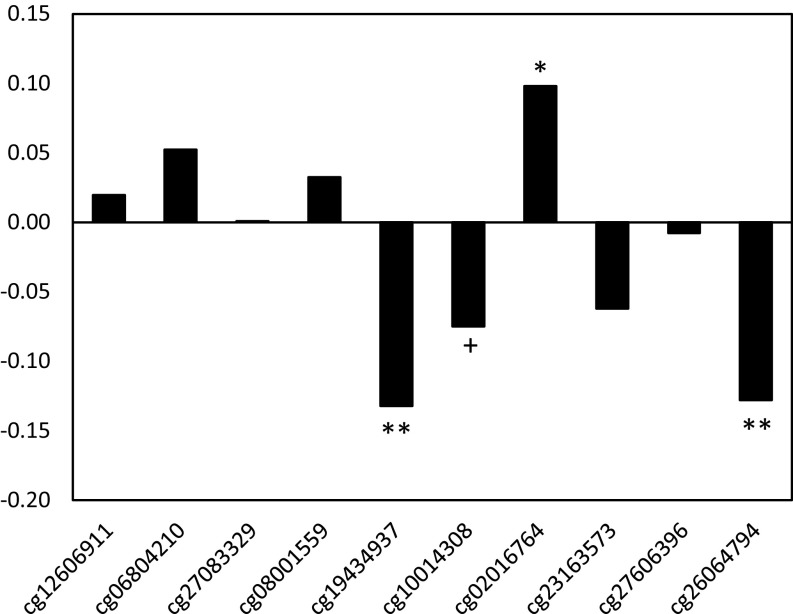
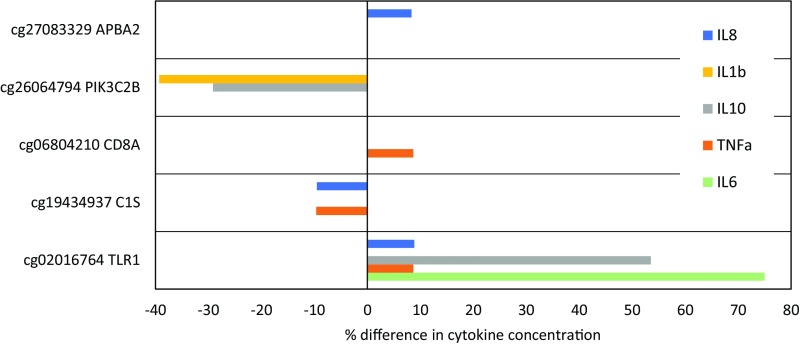
To evaluate functional biological relevance, we tested for associations between DNAm at sites identified in Table 2 and inflammatory biomarkers measured in plasma collected during the same blood draw as the methylation analysis: CRP, IL-6, TNFα, IL-10, IFNγ, IL-1β, and IL-8. Due to the level of intercorrelation across the markers (Table S3), we constructed a summary index averaging standardized values; this approach also reduced the probability of type 1 error. Higher DNAm at sites in C1S and PIK3C2B predicted significantly lower overall levels of inflammation, with a trend toward lower inflammation with higher DNAm in EGR4 (Fig. 2). Inflammation was positively associated with DNAm in TLR1. Patterns of association between DNAm and each of the inflammatory markers in the index are presented as supplementary material (Table S4). The magnitude of many associations was substantial, with cytokine concentrations that differed by 8.3–75.0% for individuals with low versus high levels of DNAm at the identified sites (Fig. S1).
Table S3.
Bivariate correlations (Pearson R; P value) between inflammatory biomarkers (n = 478)
| Inflammatory biomarker | CRP | IL-6 | IL-10 | TNFa | IL-1b | IFNg |
| IL-6 | 0.225 | |||||
| 0.000 | ||||||
| IL-10 | 0.115 | 0.393 | ||||
| 0.012 | 0.000 | |||||
| TNFa | 0.162 | 0.287 | 0.300 | |||
| 0.000 | 0.000 | 0.000 | ||||
| IL-1b | −0.012 | 0.207 | 0.235 | 0.102 | ||
| 0.794 | 0.000 | 0.000 | 0.026 | |||
| IFNg | −0.029 | 0.196 | 0.354 | 0.258 | 0.159 | |
| 0.534 | 0.000 | 0.000 | 0.000 | 0.001 | ||
| IL-8 | 0.134 | 0.192 | 0.257 | 0.340 | 0.082 | 0.136 |
| 0.003 | 0.000 | 0.000 | 0.000 | 0.074 | 0.003 |
P values are presented in italics.
Fig. 2.
Correlation between DNAm and inflammation index for probes identified in Table 2. Partial correlation coefficients are adjusted for participant sex and the presence of infectious symptoms at the time of blood collection. +P = 0.1; *P < 0.05; **P < 0.01.
Table S4.
Associations between DNAm for probes identified in Table 2 and circulating concentrations of CRP and inflammatory cytokines
| CRP | IL-6 | TNFα | IL-10 | IFNγ | IL-1β | IL-8 | |||||||||
| Probe | Symbol | β (SE) | P | β (SE) | P | β (SE) | P | β (SE) | P | β (SE) | P | β (SE) | P | β (SE) | P |
| cg19434937 | C1S | −0.374 (0.141) | 0.009 | −0.383 (0.141) | 0.007 | ||||||||||
| cg08001559 | GNG2 | −0.353 (0.190) | 0.064 | 0.309 (0.168) | 0.066 | ||||||||||
| cg06804210 | CD8A | 0.279 (0.142) | 0.049 | ||||||||||||
| cg12606911 | CD8A | ||||||||||||||
| cg27083329 | APBA2 | −0.168 (0.100) | 0.094 | 0.228 (0.100) | 0.023 | ||||||||||
| cg10014308 | EGR4 | ||||||||||||||
| cg02016764 | TLR1 | 0.313 (0.151) | 0.038 | 0.261 (0.129) | 0.044 | 0.278 (0.129) | 0.031 | 0.284 (0.129) | 0.028 | ||||||
| cg27606396 | IL1A | −0.448 (0.265) | 0.091 | −0.361 (0.196) | 0.066 | ||||||||||
| cg26064794 | PIK3C2B | −0.323 (0.160) | 0.044 | −0.529 (0.180) | 0.003 | −0.300 (0.160) | 0.061 | ||||||||
| cg23163573 | SULT1C2 | −0.216 (0.119) | 0.071 | ||||||||||||
n = 492 for CRP; n = 478 for all cytokines; all values were log10-transformed and standardized (mean = 0, SD = 1) before analysis. P values are not adjusted for multiple comparisons. P values are presented in italics.
Fig. S1.
Percent differences in cytokine concentrations associated with low and high levels of DNAm. Cytokine concentrations and percent differences were determined as follows: (i) Regression models were run predicting log-transformed cytokine concentration as a function of probe M-value, adjusting for participant sex and the presence of infectious symptoms at the time of blood collection (Table S4); (ii) low and high levels of DNAm were defined as probe-specific M-values at the 25th percentile and 75th percentile, respectively; (iii) cytokine concentrations corresponding to low and high M-values were predicted based on coefficients in regression models; and (iv) predicted values were anti–log-transformed to calculate % difference [100 × (high – low)/low] in cytokine concentration.
In these analyses, we inferred that differential methylation was contributing to differences in inflammatory biomarker production within our sample. However, the reverse process is possible, in which increases in inflammation can affect DNAm in white blood cells (44). To evaluate this scenario, we reran our models with early life exposures and DNAm for the probes identified in Table 2, adding the inflammation index as a covariate. If inflammatory processes are more causally proximate to early life exposures, then associations between these exposures and DNAm should be attenuated in the presence of adjustment for inflammatory biomarkers. Coefficients were virtually identical, suggesting that inflammation does not account for the association between early exposures and DNAm (Table S5).
Table S5.
Association between methylation sites and exposures in infancy/childhood, before and after adjusting for inflammation in adulthood
| Probe | Exposure | logFC unadjusted | logFC adjusted |
| cg19434937 | Household assets (number of items) | 0.0389 | 0.0365 |
| cg08001559 | Household assets (number of items) | −0.0282 | −0.0281 |
| cg06804210 | Animal feces (number of intervals) | −0.0463 | −0.0455 |
| cg12606911 | Animal feces (number of intervals) | −0.0785 | −0.0783 |
| cg27083329 | Animal feces (number of intervals) | −0.0684 | −0.0687 |
| cg10014308 | Parental absence (1, 0) | 0.1043 | 0.1035 |
| cg02016764 | Breastfeeding duration (days) | 0.0013 | 0.0012 |
| cg27606396 | Birth in dry season (1, 0) | 0.0916 | 0.0918 |
| cg26064794 | Birth in dry season (1, 0) | 0.1023 | 0.1048 |
| cg23163573 | Breastfeeding duration (days) | −0.0014 | −0.0014 |
n = 478 for all models. All models include participant sex as covariate. Adjusted models include summary inflammation index as covariate.
Individual genetic differences can contribute to patterns of DNAm and may confound associations with early environmental exposures (45). To determine whether this was a factor in our analysis, we reran our models including the top three principal components of genetic variation, as previously derived for this population (46). Adjusting for the principal components did not alter the magnitude or significance of associations reported in Table 2, indicating that our results are not likely to be confounded by genetic factors (Table S6).
Table S6.
Associations between methylation sites and exposures in infancy/childhood before and after adjustment for individual genetic variation
| Not adjusted for genetic variation | Adjusted for genetic variation | ||||
| Probe | Exposure | logFC | Adjusted P value | logFC | Adjusted P value |
| cg19434937 | Household assets (number of items) | 0.0352 | 0.0094 | 0.0363 | 0.0136 |
| cg08001559 | Household assets (number of items) | −0.0303 | 0.0094 | −0.0297 | 0.0152 |
| cg06804210 | Animal feces (number of intervals) | −0.0481 | 0.0197 | −0.0492 | 0.0143 |
| cg12606911 | Animal feces (number of intervals) | −0.0835 | 0.0197 | −0.0848 | 0.0143 |
| cg27083329 | Animal feces (number of intervals) | −0.0630 | 0.0364 | −0.0630 | 0.0380 |
| cg10014308 | Parental absence (1, 0) | 0.1110 | 0.0395 | 0.112 | 0.0362 |
| cg02016764 | Breastfeeding duration (days) | 0.0014 | 0.0702 | 0.0014 | 0.0712 |
| cg27606396 | Birth in dry season (1, 0) | 0.0994 | 0.1030 | 0.0964 | 0.1090 |
| cg26064794 | Birth in dry season (1, 0) | 0.1026 | 0.1030 | 0.1026 | 0.1090 |
| cg23163573 | Breastfeeding duration (days) | −0.0014 | 0.1162 | −0.0013 | 0.1436 |
Discussion
Developmental plasticity and ecological sensitivity are defining characteristics of the human immune system (12), but the mechanisms shaping the development and regulation of inflammation are poorly understood. Prior research, in a wide range of ecological settings, suggests that nutritional, microbial, and psychosocial exposures during sensitive periods of immune development have lasting effects on inflammation (10, 16, 18, 19). In this study, we use a prospective design to link these exposures with patterns of DNAm in inflammatory genes in young adulthood, providing evidence that DNAm may be an important epigenetic mechanism through which early environments have enduring effects on inflammatory phenotypes. We also show that variations in DNAm relate to expression of inflammatory biomarkers implicated in a host of prevalent chronic diseases, underscoring the potential public health significance of these results.
Our analyses focused on target genes known to be involved in the regulation of inflammation, and we identified 10 CpG sites across nine genes that were predicted by developmental environments. The most statistically significant associations were for sites in the C1S and GNG2 genes, which were predicted by household assets, a measure of socioeconomic adversity in infancy/childhood. C1S is a protein-coding gene that contributes to the production of C1, the first component of the classical pathway of the complement system, and GNG2 produces proteins that are involved with transmembrane signaling mechanisms.
Similarly, we identified parental absence—a different type of childhood adversity—as a significant predictor of DNAm in the EGR4 gene. EGR4 is a protein coding gene and transcription factor that regulates cell proliferation and proinflammatory cytokine production. These results are consistent with several studies documenting associations between early life socioeconomic status and DNAm in adulthood, drawing on samples from the United States, United Kingdom, Canada, and Italy (30, 47–49), and with a recent study in the United States showing that higher levels of stress in infancy/early childhood are biologically embedded in the epigenome (27). Our study reports associations between early adversity and DNAm in adulthood in a non-Western, lower income setting. Furthermore, our findings are consistent with experimental animal models documenting durable effects of maternal separation on the regulation of inflammation (50–52).
A unique advantage of our study is the depth of prospectively collected data on more proximate environmental factors, beginning in infancy. As hypothesized, exposure to animal feces and season of birth—two proxy measures of the intensity and diversity of microbial encounters in infancy—predicted DNAm at five sites across four genes. Increased animal feces exposure was associated with reduced DNAm at two promoter sites in CD8A and one upstream site in a high-density CpG shore in APBA2. APBA2 encodes a protein that interacts with amyloid precursor protein and is involved in signal transduction processes.
As part of the T-cell receptor complex, CD8A recognizes antigens and initiates phosphorylation cascades that lead to the activation of transcription factors such as NFAT, NF-κB, and AP-1, all of which play important roles in the regulation of inflammation. We document strong, negative associations between intensity of exposure to animal feces in infancy and DNAm at two sites in the CD8A promoter, ∼300 bp apart, which are associated with a DNase hypersensitivity cluster. The potential significance of this finding is underscored by the consistent pattern of DNAm in two neighboring sites, the likely regulatory role of the gene region, and the established importance of the T-cell receptor complex to immune activity.
Birth in the dry season predicted higher DNAm in the first exon, 5′ UTR region of IL-1A and the third intron of PIK3C2B. Both CpG sites are located in regions marked by increased chromatin accessibility, DNase hypersensitivity, and transcription factor binding sites. IL-1A produces a cytokine in the interleukin 1 family (IL-1α) that is involved in several immune and inflammatory processes. PIK3C2B is a protein coding gene that plays roles in signal pathways related to cell proliferation, survival, and migration.
The pattern of associations between postnatal microbial exposures and methylation of inflammatory genes underscores the relevance of epigenetic processes to the “hygiene” or “old friends” hypotheses, which link the development of immunoregulatory pathways to microbial environments early in development (22, 53). More specifically, our findings are consistent with experimental animal models documenting negative associations between early exposure to microbes/microbial products and inflammatory processes later in life (54–56). Similarly, a relatively recent study in the Gambia has reported an association between season of birth and DNAm of metastable epialleles (57), while our analysis reports an association between season of birth and DNAm of genes involved in the regulation of inflammation.
Longer durations of breastfeeding in infancy have been associated with reduced inflammation in adulthood (19), and we identified sites in two genes where breastfeeding duration predicted levels of DNAm. Breastfeeding duration was negatively associated with DNAm in the 5′ UTR region of SULT1C2, which encodes a protein in the SULT1 subfamily that facilitates sulfate conjugation of phenol-containing compounds. Breastfeeding duration predicted higher DNAm in the 5′ UTR region of TLR1, which is a member of the Toll-like receptor (TLR) family that is centrally involved in pathogen recognition and the activation of innate immune processes, including inflammation. The TLR family is highly conserved and widely expressed on immune cells, and TLR1 forms a cluster with TLR2 and CD14 to recognize bacterial lipoproteins and transduce signals that lead to NF-κB activation, cytokine production, and the inflammatory response (58). Prior research has documented associations between breastfeeding and DNAm in the LEP gene (59), while our study links breastfeeding with DNAm in immunoregulatory genes (19).
The range and time depth of measures in our study are major strengths, but the design does not include direct measures of gene expression, making it more difficult to infer the functional consequences of varying levels of DNAm at the identified sites. Similarly, the pattern of association with inflammatory biomarkers is not consistent for all sites, and we document associations between 4 of 10 DNAm sites and production of inflammatory biomarkers. Prior research has documented stronger associations between DNAm and inflammatory biomarkers in stimulated samples than in basal samples (47), suggesting that our tests of association between DNAm and inflammation in nonstimulated samples may represent a conservative estimate of the functional significance of the identified sites. Additional research is needed to determine the extent to which DNAm directly mediates transcriptional activity at these sites or, alternatively, whether it serves as a marker for other regulatory processes. The use of in vivo or ex vivo experimental protocols (e.g., vaccination, cell culture systems) to stimulate and measure the inflammatory response would provide better indicators of the inflammatory milieu, however the logistics of our field location precluded this approach. Despite these limitations, single measures of baseline concentrations of inflammatory biomarkers have been shown to predict increased risk for the onset of cardiovascular, metabolic, and other diseases linked to inflammation (2, 3), attesting to their biological and clinical significance.
Another potential limitation of our study is the use of methylation data to estimate and adjust for blood cell composition in the absence of direct cell counts. Although this bioinformatic approach has been validated for use with whole blood samples (41, 60), the possibility of residual confounding by cell composition remains. In addition, we measured DNAm and inflammatory cytokines concurrently and conceptualized cytokine production as a downstream consequence of differential methylation in immunoregulatory genes. Because our study design precludes formal mediation analyses, we cannot rule out the possibility that increases in inflammation are causing changes in DNAm. Our tests of reverse causality suggest this is not the case, although they are limited by the cross-sectional measures and the mixed pattern of association between inflammatory biomarkers and DNAm in our sample. Lastly, we rely on proxy—rather than direct—measures of microbial exposure that are only moderately correlated. The distinct pattern of results for season of birth and exposure to animal feces may be attributable to this fact, and additional research is needed to determine the timing of microbial exposure and the types of exposures that impact DNAm in inflammatory genes.
Recent studies demonstrate that genetic polymorphisms can influence patterns of DNAm as well as moderate patterns of association between environmental exposures, DNAm, and phenotypic outcomes (45, 61). In our analyses, we determined that adjusting for principal components of genetic variation did not alter associations between early environmental exposures and DNAm, but this is a relatively limited test of genetic confounding that does not consider the potential role of specific genes in moderating patterns of association. Future research should consider the possibility of interactions between early environmental exposures and allelic variation in shaping DNAm and the regulation of inflammation.
Our results contribute to a rapidly growing body of literature investigating the importance of early life environments to the regulation of inflammation later in life (35). They point to DNAm as a potentially important biological mechanism through which nutritional, microbial, psychosocial, and socioeconomic exposures impact inflammatory phenotypes across the life course. These findings highlight specific genes that may be particularly promising foci for future research, and they contribute to the emerging emphasis on epigenetic processes in the developmental origins of health and disease (62–66).
Materials and Methods
Participants and Study Design.
Analyses were implemented with a subset of participants in the Cebu Longitudinal Health and Nutrition Survey (CLHNS), an ongoing birth cohort study in the Philippines (SI Materials and Methods for additional information on identification of study participants). The study began in 1983 with the recruitment of a community-based sample of 3,327 pregnant women, and home visits were made before birth, immediately following birth, and every 2 mo for 2 y (38). A follow-up survey in 2005 included the collection of venous blood, when the participants were 20–22 y of age. All data were collected and analyzed under conditions of informed consent with institutional review board approvals from the University of North Carolina at Chapel Hill, Northwestern University, and the University of British Columbia.
DNAm.
Overnight fasting blood samples were collected into EDTA-coated vacutainer tubes and kept in coolers on ice packs for no more than 2 h during transport to a central facility, where samples were centrifuged to separate plasma and white blood cells before freezing at –70 °C. Genomic DNA was treated with sodium bisulfite (Zymo Research), and converted DNA was applied to the Illumina HumanMethylation450 Bead Chip using the manufacturer’s standard conditions (Illumina Inc.). Additional details on laboratory procedures, quality control analyses, and data processing are provided in SI Materials and Methods. Proportions of blood cell types were predicted using a previously established algorithm, and variance associated with cell composition was removed before statistical analyses (41, 60).
Data Analysis.
The following independent variables were considered as measures of the early life nutritional, microbial, and psychosocial environment: birth weight (measured in the home immediately after delivery); duration of exclusive breastfeeding (based on maternal reports collected in the home during bimonthly interviews); frequency of infectious diarrhea, birth to 24 mo (maternal report during bimonthly interviews); exposure to animal feces, 6–12 mo (interviewer observation during bimonthly interviews); birth in the dry season (February through April; indicator of higher levels of microbial exposure in early infancy) (18); and household assets in infancy and childhood [sum of items, averaged across four surveys; extended absence of the participant’s mother or father in childhood (up to ∼11 y of age)] (additional details on variable construction and validation are provided in SI Materials and Methods).
Tests of association between early environments and DNAm were limited to highly variable methylation sites in target genes involved in the regulation of inflammation (Table S1). Target genes were identified using a web-based gene network and functional annotation tool (genemania.org/) and review of prior human studies investigating the genetic and epigenetic control of inflammation (30, 48, 67–70) (additional details on target gene selection are provided in SI Materials and Methods). A total of 249 target genes were identified. Probes were matched to target genes based on proximity of the nearest transcription start site (71), resulting in 5,152 probes, which were then filtered to exclude probes for which variability in beta-values between the 10th and 90th percentiles was <10% (72). The final dataset included beta-values for 222 variable probes across 114 target genes (Table S2 and Dataset S2). Beta-values were converted to M-values before statistical analysis (73).
For hypothesis testing, probe-wise variance was determined by fitting linear regression models and applying a parametric empirical Bayes smoothing formula using the R bioconductor package limma (74). All models adjusted for participant sex. We accounted for multiple comparisons using the Benjamini and Hochberg step-up procedure for controlling FDR, with q = 0.05 (42). For exploratory purposes and to capture associations of potential biological relevance, we report all associations with adjusted P values < 0.15. To evaluate the functional relevance of identified sites, we tested for associations between these sites and the following inflammatory biomarkers quantified in the same set of blood samples used to measure DNAm: CRP, IL-1β, IL-6, IL-8, IL-10, IFNγ, and TNFα (SI Materials and Methods for additional information on statistical analyses).
SI Materials and Methods
Participants and Study Design.
The CLHNS study began in 1983 with the recruitment of a community-based sample of 3,327 pregnant women in and around Cebu City, the second largest urban area in the Philippines (38). Home visits were made before birth, immediately following birth, and every 2 mo for 2 y. Follow-up surveys were conducted in 1991–1992, 1994–1995, 1998–1999, and 2002, with a comprehensive survey, including venous blood collection, conducted in 2005 when the participants were 20–22 y of age. Attrition in the CLHNS is due primarily to factors related to out-migration, and rates of refusal during initial recruitment were low (<4%) (38, 75).
Of the 3,327 pregnant women, 3,080 gave birth to a single live infant, comprising a cohort that is representative of births in Metro Cebu in 1983–1984. The 2005 survey included 1,885 participants, 1,759 of whom provided a venipuncture blood sample. From this subsample, we selected 395 female participants for methylation analysis, based on their participation in a pregnancy tracking study initiated in 2009 (76). In addition, we randomly selected 99 male participants for methylation analysis. All data were collected and analyzed under conditions of informed consent with institutional review board approvals from the University of North Carolina at Chapel Hill, Northwestern University, and the University of British Columbia.
The methylation sample did not differ from the rest of the original cohort in household income (260.2 vs. 287.5 pesos, t = 1.05, P = 0.30) as assessed when the study started in 1983. However, maternal education was lower (6.9 vs. 7.7 y, t = 4.01, P < 0.001), household assets were marginally lower (2.36 vs. 2.54 items, t = 1.85, P = 0.06), and the likelihood of home ownership was higher (74.7 vs. 64.1%, Pearson X2 = 20.6, P < 0.001) in the methylation sample. Participants did not differ in birth weight (3,107 vs. 2,984 g, t = 1.55, P = 0.12), season of birth (21.1 vs. 18.1% born in dry season, X2 = 2.40, P = 0.12), or episodes of infectious diarrhea in the first year (1.04 vs. 1.09, t = 0.93, P = 0.35).
DNAm.
Overnight fasting blood samples were collected into EDTA-coated vacutainer tubes in 2005 and kept in coolers on ice packs for no more than 2 h during transport to a central facility where samples were centrifuged to separate plasma and white blood cells before freezing at –70 °C. Samples were express shipped to the United States on dry ice and stored frozen at –80 °C before DNA extraction (Puregene, Gentra). A total of 750 ng of genomic DNA was treated with sodium bisulfite to convert unmethylated cytosines using the Zymo EZDNA methylation kit per the manufacturer’s instructions (Zymo Research). A total of 160 ng of converted DNA was applied to the Illumina HumanMethylation450 Bead Chip using the manufacturer’s standard conditions (Illumina Inc.). Background subtraction and color correction were performed using Illumina Genome Studio with default parameters. Data were then exported into R for further analysis.
Quality control was performed to confirm participant sex and replicate status, and then sex chromosome probes were removed from further analysis. Unreliable probes with a detection P value above 0.01, with fewer than three beads contributing to signal, and those previously shown to bind to multiple genomic regions were also removed, leaving 434,728 probes (77). Data were quantile normalized using the R lumi package, and then probe types were normalized using the SWAN method (78). Next, plate, row, and chip batch variables were assessed using PCA and corrected using the COMBAT function in the sva R package (79). Finally, proportions of blood cell types were predicted using a previously established algorithm, and variance associated with cell composition was removed using a linear regression approach (41, 60).
Independent Variables.
Birth weight was measured in the home immediately after birth using standard procedures (80), and gestational age was recorded based on maternal report. Information on breastfeeding was collected at bimonthly interviews, conducted in the home, during the first 2 y following birth. The duration of exclusive breastfeeding was defined as the number of days of breastmilk consumption before the introduction of any supplementary foods or liquids. Measures of postnatal microbial exposure were collected during in-home interviews in infancy, following prior research in the Philippines and elsewhere (81, 82). These measures included infectious diarrhea, exposure to animal feces, and season of birth, all of which have been associated with inflammation in young adulthood in this cohort (18).
Frequency of infectious diarrhea in the first 2 y was assessed by asking mothers whether their infant had shown symptoms of diarrhea during the week preceding the interview, and a variable was created summing the number of bimonthly intervals when symptoms were present. Complete observations were available for 92.1% of participants, with 5.0% missing one bimonthly interval, 1.8% missing two, and 1.1% missing three or more. In cases with missing intervals, frequency of diarrhea was calculated as a percentage across observed intervals and then adjusted to 12 intervals. The frequency of diarrhea symptoms across intervals ranged from 6.0% to 23.4% and increased with age as expected due to the introduction of complementary food and liquids (82).
Exposure to animal feces was assessed by summing the number of bimonthly intervals between 6 and 12 mo of age, inclusive, that the interviewer observed that the infant was crawling and that animals (e.g., dogs, chickens) were present in the home. The intensity of exposure at each interval was coded as 0 (absent), 1 (some), and 2 (high). The measure captures the period of exposure that is most variable within the sample: At 2 mo and 4 mo, only 0.6% and 3.5% of the sample, respectively, had any level of exposure. By 6 mo, 40.2% of the sample was exposed, with 98.7% exposure at 12 mo. Intensity of exposure to animal feces was positively associated with the frequency of infectious diarrhea during the same period (Pearson R = 0.11, P = 0.02).
Season of birth predicts the intensity of microbial exposure since rainfall, poor drainage, and water supplies contaminated by heavy rains that are associated with the spread of pathogens and infectious morbidity in Cebu (82, 83). Birth in the dry season—previously validated as an indicator of higher levels of microbial exposure postnatally, in early infancy (18)—was defined as birth in the months of February through April, inclusive, based on birth dates recorded immediately after delivery.
Household assets were used as a measure of socioeconomic adversity in childhood, as durable assets provide a more stable measure of socioeconomic status in low-income settings due to volatility or inaccuracy in reports of household income (43). Assets included home ownership, electricity in the home, type of housing material, and ownership of items such as air conditioner, television, refrigerator, or car. Assets were assessed in 1983–1984, 1985–1986, 1991–1992, and 1994–1995, and a simple sum of items was generated and averaged across surveys for a summary measure of household assets in childhood. Pearson correlations for assets across surveys ranged between 0.50 and 0.84, and Cronbach’s alpha for the summary household assets variable was 0.86.
Psychosocial adversity in childhood was defined as extended absence of the participant’s mother or father in childhood. A dichotomous variable was constructed to indicate maternal or paternal absence for an extended period at any point in time before the 1994–1995 survey, when participants were ∼11 y old. Maternal absences were due primarily to divorce/separation with residence in a separate household (∼46%), extended separations for employment outside of Cebu (∼41%), or death (∼13%) (24). Questions regarding the reasons for paternal absence were not included in the survey.
Data Analysis.
Analyses were limited to highly variable methylation sites in target genes involved in the regulation of inflammation (Table S1). Target genes were identified using a web-based gene network and functional annotation tool (genemania.org/) to implement an unbiased search for potential targets. The following genes were entered to initiate the search based on the important roles they (and their protein products) play in chronic inflammation (84): CRP, IL-6, TNFα, and IL-10. Other search parameters included a maximum of 50 resultant genes and “biological process-based” gene ontology weighting.
In addition, we reviewed prior human studies investigating the genetic and epigenetic control of inflammation. A large meta-analysis of prior genome-wide association studies identified 18 genes significantly associated with concentrations of CRP (70). Two studies of socioeconomic status and DNAm identified 28 inflammatory genes (30, 48). Three studies of DNAm and CRP identified 161 genes where methylation status was associated with inflammation (67–69). In total, these studies identified 207 potential targets, 8 of which overlapped with the prior list, resulting in a final set of 249 genes for our analysis.
Using an expanded annotation for the 450K array (71), probes were matched to the 249 target genes based on proximity of the nearest transcription start site. This resulted in 5,152 probes, which were then filtered to exclude those probes for which variability in beta-values between the 10th and 90th percentiles was <10%. Most sites of DNAm are invariable across individuals (72), and removing sites with no underlying variability reduces the burden of multiple test correction and focuses the analyses on sites that can plausibly vary in relation to variability in the independent variables of interest (40). The final dataset included beta-values for 222 variable probes across 114 target genes (Table S2). Beta-values were converted to M-values before statistical analysis (73).
For hypothesis testing, probe-wise variance was determined by fitting linear regression models and applying a parametric empirical Bayes smoothing formula over the entire array dataset that passed quality control (434,728 probes) using the R bioconductor package limma (74). This approach allowed for gene-wise information borrowing to better estimate the variation for each probe. All models were adjusted for participant sex.
We first evaluated measures of the socioeconomic and psychosocial environment (household assets, parental absence) as independent variables predicting M-values for each of the variable CpG sites. We then considered measures of microbial exposure in infancy (frequency of infectious diarrhea, exposure to animal feces, season of birth). Finally, we evaluated models including measures of the prenatal (birth weight, adjusted for gestational age) and postnatal (duration of exclusive breastfeeding) nutritional environment. We accounted for multiple comparisons using the Benjamini and Hochberg step-up procedure for controlling FDR, with q = 0.05 (42). For exploratory purposes and to capture associations of potential biological relevance, we report all associations with adjusted P values < 0.15. Beta and P values for all probes are available in Dataset S1. The genomic context of the DNAm sites and the functions of the associated genes were interpreted with the UCSC Genome Browser (genome.ucsc.edu/), using the Human February 2009 (GRCh37/hg19) Assembly (85).
To evaluate the functional relevance of identified sites, we tested for associations between these sites and the following inflammatory biomarkers quantified in the same set of blood samples used to measure DNAm: CRP, IL-1β, IL-6, IL-8, IL-10, IFNγ, and TNFα. Highly sensitive immunoassay protocols were implemented as described previously (86, 87). All values were log10-transformed before analysis. We also considered a summary inflammation index calculated as follows: Values for CRP and all cytokines were log10-transformed, standardized (mean = 0, SD = 1), and then averaged into a single variable. All models were adjusted for gender as well as the presence of infectious symptoms at the time of blood collection (86).
Complete data were available for 494 participants for all analyses investigating early environments as predictors of DNAm, with the exception of models including birth weight, which were limited to 487 participants. For analyses predicting inflammatory biomarkers, n = 492 for CRP and n = 478 for cytokines and the summary inflammation variable due to limited sample volume for cytokine assays. A de-identified dataset with all observations and variables is available in Dataset S2.
Supplementary Material
Acknowledgments
Research reported in this manuscript was supported by National Institutes of Health Grants RO1 HL085144 and RO1 TW05596 and Biological Anthropology Program at the National Science Foundation Grants BCS-0746320 and BCS-1440564. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the National Science Foundation.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1620661114/-/DCSupplemental.
References
- 1.Kumar R, Clermont G, Vodovotz Y, Chow CC. The dynamics of acute inflammation. J Theor Biol. 2004;230:145–155. doi: 10.1016/j.jtbi.2004.04.044. [DOI] [PubMed] [Google Scholar]
- 2.Pradhan AD, Manson JE, Rifai N, Buring JE, Ridker PM. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA. 2001;286:327–334. doi: 10.1001/jama.286.3.327. [DOI] [PubMed] [Google Scholar]
- 3.Ridker PM, Buring JE, Cook NR, Rifai N. C-reactive protein, the metabolic syndrome, and risk of incident cardiovascular events: An 8-year follow-up of 14 719 initially healthy American women. Circulation. 2003;107:391–397. doi: 10.1161/01.cir.0000055014.62083.05. [DOI] [PubMed] [Google Scholar]
- 4.Challis JR, et al. Inflammation and pregnancy. Reprod Sci. 2009;16:206–215. doi: 10.1177/1933719108329095. [DOI] [PubMed] [Google Scholar]
- 5.Romero R, Gotsch F, Pineles B, Kusanovic JP. Inflammation in pregnancy: Its roles in reproductive physiology, obstetrical complications, and fetal injury. Nutr Rev. 2007;65:S194–S202. doi: 10.1111/j.1753-4887.2007.tb00362.x. [DOI] [PubMed] [Google Scholar]
- 6.Crimmins EM, Finch CE. Infection, inflammation, height, and longevity. Proc Natl Acad Sci USA. 2006;103:498–503. doi: 10.1073/pnas.0501470103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alley DE, et al. Socioeconomic status and C-reactive protein levels in the US population: NHANES IV. Brain Behav Immun. 2006;20:498–504. doi: 10.1016/j.bbi.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 8.Nazmi A, Victora CG. Socioeconomic and racial/ethnic differentials of C-reactive protein levels: A systematic review of population-based studies. BMC Public Health. 2007;7:212. doi: 10.1186/1471-2458-7-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ranjit N, et al. Socioeconomic position, race/ethnicity, and inflammation in the multi-ethnic study of atherosclerosis. Circulation. 2007;116:2383–2390. doi: 10.1161/CIRCULATIONAHA.107.706226. [DOI] [PubMed] [Google Scholar]
- 10.Miller GE, et al. Low early-life social class leaves a biological residue manifested by decreased glucocorticoid and increased proinflammatory signaling. Proc Natl Acad Sci USA. 2009;106:14716–14721. doi: 10.1073/pnas.0902971106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ter Horst R, et al. Host and environmental factors influencing individual human cytokine responses. Cell. 2016;167:1111–1124 e13. doi: 10.1016/j.cell.2016.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McDade TW. Life history theory and the immune system: Steps toward a human ecological immunology. Am J Phys Anthropol. 2003;(Suppl 37):100–125. doi: 10.1002/ajpa.10398. [DOI] [PubMed] [Google Scholar]
- 13.McDade TW, Georgiev AV, Kuzawa CW. Trade-offs between acquired and innate immune defenses in humans. Evol Med Public Health. 2016;2016:1–16. doi: 10.1093/emph/eov033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee KA. Linking immune defenses and life history at the levels of the individual and the species. Integr Comp Biol. 2006;46:1000–1015. doi: 10.1093/icb/icl049. [DOI] [PubMed] [Google Scholar]
- 15.Danese A, Pariante CM, Caspi A, Taylor A, Poulton R. Childhood maltreatment predicts adult inflammation in a life-course study. Proc Natl Acad Sci USA. 2007;104:1319–1324. doi: 10.1073/pnas.0610362104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sattar N, et al. Inverse association between birth weight and C-reactive protein concentrations in the MIDSPAN Family Study. Arterioscler Thromb Vasc Biol. 2004;24:583–587. doi: 10.1161/01.ATV.0000118277.41584.63. [DOI] [PubMed] [Google Scholar]
- 17.Tzoulaki I, et al. Size at birth, weight gain over the life course, and low-grade inflammation in young adulthood: northern Finland 1966 Birth Cohort study. Eur Heart J. 2008;29:1049–1056. doi: 10.1093/eurheartj/ehn105. [DOI] [PubMed] [Google Scholar]
- 18.McDade TW, Rutherford J, Adair L, Kuzawa CW. Early origins of inflammation: Microbial exposures in infancy predict lower levels of C-reactive protein in adulthood. Proc Biol Sci. 2010;277:1129–1137. doi: 10.1098/rspb.2009.1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McDade TW, et al. Long-term effects of birth weight and breastfeeding duration on inflammation in early adulthood. Proc Biol Soc B. 2014;281:20133116. doi: 10.1098/rspb.2013.3116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McDade TW, et al. Analysis of variability of high sensitivity C-reactive protein in lowland Ecuador reveals no evidence of chronic low-grade inflammation. Am J Hum Biol. 2012;24:675–681. doi: 10.1002/ajhb.22296. [DOI] [PubMed] [Google Scholar]
- 21.Rook GAW, Stanford JL. Give us this day our daily germs. Immunol Today. 1998;19:113–116. doi: 10.1016/s0167-5699(97)01204-8. [DOI] [PubMed] [Google Scholar]
- 22.Yazdanbakhsh M, Kremsner PG, van Ree R. Allergy, parasites, and the hygiene hypothesis. Science. 2002;296:490–494. doi: 10.1126/science.296.5567.490. [DOI] [PubMed] [Google Scholar]
- 23.Radon K, et al. Chronische Autoimmunerkrankungen und Kontakt zu Tieren (Chronic Autoimmune Disease and Animal Contact) Study Group Contact with farm animals in early life and juvenile inflammatory bowel disease: A case-control study. Pediatrics. 2007;120:354–361. doi: 10.1542/peds.2006-3624. [DOI] [PubMed] [Google Scholar]
- 24.McDade TW, Hoke M, Borja JB, Adair LS, Kuzawa C. Do environments in infancy moderate the association between stress and inflammation in adulthood? Initial evidence from a birth cohort in the Philippines. Brain Behav Immun. 2012;31:23–30. doi: 10.1016/j.bbi.2012.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miller GE, Chen E. Harsh family climate in early life presages the emergence of a proinflammatory phenotype in adolescence. Psychol Sci. 2010;21:848–856. doi: 10.1177/0956797610370161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Taylor SE, Lehman BJ, Kiefe CI, Seeman TE. Relationship of early life stress and psychological functioning to adult C-reactive protein in the coronary artery risk development in young adults study. Biol Psychiatry. 2006;60:819–824. doi: 10.1016/j.biopsych.2006.03.016. [DOI] [PubMed] [Google Scholar]
- 27.Essex MJ, et al. Epigenetic vestiges of early developmental adversity: Childhood stress exposure and DNA methylation in adolescence. Child Dev. 2013;84:58–75. doi: 10.1111/j.1467-8624.2011.01641.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Esposito EA, et al. Differential DNA methylation in peripheral blood mononuclear cells in adolescents exposed to significant early but not later childhood adversity. Dev Psychopathol. 2016;28:1385–1399. doi: 10.1017/S0954579416000055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boyce WT, Kobor MS. Development and the epigenome: The ‘synapse’ of gene-environment interplay. Dev Sci. 2015;18:1–23. doi: 10.1111/desc.12282. [DOI] [PubMed] [Google Scholar]
- 30.Needham BL, et al. Life course socioeconomic status and DNA methylation in genes related to stress reactivity and inflammation: The multi-ethnic study of atherosclerosis. Epigenetics. 2015;10:958–969. doi: 10.1080/15592294.2015.1085139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kass SU, Landsberger N, Wolffe AP. DNA methylation directs a time-dependent repression of transcription initiation. Curr Biol. 1997;7:157–165. doi: 10.1016/s0960-9822(97)70086-1. [DOI] [PubMed] [Google Scholar]
- 32.Nilsson E, et al. Altered DNA methylation and differential expression of genes influencing metabolism and inflammation in adipose tissue from subjects with type 2 diabetes. Diabetes. 2014;63:2962–2976. doi: 10.2337/db13-1459. [DOI] [PubMed] [Google Scholar]
- 33.Nile CJ, Read RC, Akil M, Duff GW, Wilson AG. Methylation status of a single CpG site in the IL6 promoter is related to IL6 messenger RNA levels and rheumatoid arthritis. Arthritis Rheum. 2008;58:2686–2693. doi: 10.1002/art.23758. [DOI] [PubMed] [Google Scholar]
- 34.Seok J, et al. Inflammation and Host Response to Injury, Large Scale Collaborative Research Program Genomic responses in mouse models poorly mimic human inflammatory diseases. Proc Natl Acad Sci USA. 2013;110:3507–3512. doi: 10.1073/pnas.1222878110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McDade TW. Early environments and the ecology of inflammation. Proc Natl Acad Sci USA. 2012;109:17281–17288. doi: 10.1073/pnas.1202244109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.United Nations DoEaSA, Population Division 2015. World Population Prospects: The 2015 Revision, Volume I: Comprehensive Tables (ST/ESA/SER.A/379) (United Nations, New York)
- 37.Yusuf S, Reddy S, Ounpuu S, Anand S. Global burden of cardiovascular diseases: Part I: General considerations, the epidemiologic transition, risk factors, and impact of urbanization. Circulation. 2001;104:2746–2753. doi: 10.1161/hc4601.099487. [DOI] [PubMed] [Google Scholar]
- 38.Adair LS, et al. Cohort profile: The Cebu longitudinal health and nutrition survey. Int J Epidemiol. 2011;40:619–625. doi: 10.1093/ije/dyq085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jiang R, et al. Discordance of DNA methylation variance between two accessible human tissues. Sci Rep. 2015;5:8257. doi: 10.1038/srep08257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bourgon R, Gentleman R, Huber W. Independent filtering increases detection power for high-throughput experiments. Proc Natl Acad Sci USA. 2010;107:9546–9551. doi: 10.1073/pnas.0914005107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Koestler DC, et al. Blood-based profiles of DNA methylation predict the underlying distribution of cell types: A validation analysis. Epigenetics. 2013;8:816–826. doi: 10.4161/epi.25430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Benjamini Y, Hochberg Y. Controlling the false discovery rate—A practical and powerful approach to multiple testing. J Roy Stat Soc B Met. 1995;57:289–300. [Google Scholar]
- 43.Vyas S, Kumaranayake L. Constructing socio-economic status indices: How to use principal components analysis. Health Policy Plan. 2006;21:459–468. doi: 10.1093/heapol/czl029. [DOI] [PubMed] [Google Scholar]
- 44.Niwa T, et al. Inflammatory processes triggered by Helicobacter pylori infection cause aberrant DNA methylation in gastric epithelial cells. Cancer Res. 2010;70:1430–1440. doi: 10.1158/0008-5472.CAN-09-2755. [DOI] [PubMed] [Google Scholar]
- 45.Galanter JM, et al. Differential methylation between ethnic sub-groups reflects the effect of genetic ancestry and environmental exposures. eLife. 2017;6:6. doi: 10.7554/eLife.20532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wu Y, et al. Genome-wide association with C-reactive protein levels in CLHNS: Evidence for the CRP and HNF1A loci and their interaction with exposure to a pathogenic environment. Inflammation. 2012;35:574–583. doi: 10.1007/s10753-011-9348-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lam LL, et al. Factors underlying variable DNA methylation in a human community cohort. Proc Natl Acad Sci USA. 2012;109:17253–17260. doi: 10.1073/pnas.1121249109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stringhini S, et al. Life-course socioeconomic status and DNA methylation of genes regulating inflammation. Int J Epidemiol. 2015;44:1320–1330. doi: 10.1093/ije/dyv060. [DOI] [PubMed] [Google Scholar]
- 49.Borghol N, et al. Associations with early-life socio-economic position in adult DNA methylation. Int J Epidemiol. 2012;41:62–74. doi: 10.1093/ije/dyr147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cole SW, et al. Transcriptional modulation of the developing immune system by early life social adversity. Proc Natl Acad Sci USA. 2012;109:20578–20583. doi: 10.1073/pnas.1218253109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Loria AS, Pollock DM, Pollock JS. Early life stress sensitizes rats to angiotensin II-induced hypertension and vascular inflammation in adult life. Hypertension. 2010;55:494–499. doi: 10.1161/HYPERTENSIONAHA.109.145391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kruschinski C, et al. Postnatal life events affect the severity of asthmatic airway inflammation in the adult rat. J Immunol. 2008;180:3919–3925. doi: 10.4049/jimmunol.180.6.3919. [DOI] [PubMed] [Google Scholar]
- 53.Rook GA, Lowry CA, Raison CL. Hygiene and other early childhood influences on the subsequent function of the immune system. Brain Res. 2015;1617:47–62. doi: 10.1016/j.brainres.2014.04.004. [DOI] [PubMed] [Google Scholar]
- 54.Mulder IE, et al. Environmentally-acquired bacteria influence microbial diversity and natural innate immune responses at gut surfaces. BMC Biol. 2009;7:79. doi: 10.1186/1741-7007-7-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stein MM, et al. Innate immunity and asthma risk in Amish and Hutterite farm children. N Engl J Med. 2016;375:411–421. doi: 10.1056/NEJMoa1508749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shanks N, et al. Early-life exposure to endotoxin alters hypothalamic-pituitary-adrenal function and predisposition to inflammation. Proc Natl Acad Sci USA. 2000;97:5645–5650. doi: 10.1073/pnas.090571897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Waterland RA, et al. Season of conception in rural gambia affects DNA methylation at putative human metastable epialleles. PLoS Genet. 2010;6:e1001252. doi: 10.1371/journal.pgen.1001252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell. 2010;140:805–820. doi: 10.1016/j.cell.2010.01.022. [DOI] [PubMed] [Google Scholar]
- 59.Obermann-Borst SA, et al. Duration of breastfeeding and gender are associated with methylation of the LEPTIN gene in very young children. Pediatr Res. 2013;74:344–349. doi: 10.1038/pr.2013.95. [DOI] [PubMed] [Google Scholar]
- 60.Jones MJ, Islam SA, Edgar RD, Kobor MS. Adjusting for cell type composition in DNA methylation data using a regression-based approach. Methods Mol Biol. 2017;1589:99–106. doi: 10.1007/7651_2015_262. [DOI] [PubMed] [Google Scholar]
- 61.Klengel T, et al. Allele-specific FKBP5 DNA demethylation mediates gene-childhood trauma interactions. Nat Neurosci. 2013;16:33–41. doi: 10.1038/nn.3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Waterland RA, Michels KB. Epigenetic epidemiology of the developmental origins hypothesis. Annu Rev Nutr. 2007;27:363–388. doi: 10.1146/annurev.nutr.27.061406.093705. [DOI] [PubMed] [Google Scholar]
- 63.Kuzawa CW, Sweet E. Epigenetics and the embodiment of race: Developmental origins of US racial disparities in cardiovascular health. Am J Hum Biol. 2009;21:2–15. doi: 10.1002/ajhb.20822. [DOI] [PubMed] [Google Scholar]
- 64.Hertzman C, Boyce T. How experience gets under the skin to create gradients in developmental health. Annu Rev Public Health. 2010;31:329–347. doi: 10.1146/annurev.publhealth.012809.103538. [DOI] [PubMed] [Google Scholar]
- 65.Wadhwa PD, Buss C, Entringer S, Swanson JM. Developmental origins of health and disease: Brief history of the approach and current focus on epigenetic mechanisms. Semin Reprod Med. 2009;27:358–368. doi: 10.1055/s-0029-1237424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mulligan CJ. Early environments, stress, and the epigenetics of human health. Annu Rev Anthropol. 2016;45:233–249. [Google Scholar]
- 67.Guénard F, et al. Methylation and expression of immune and inflammatory genes in the offspring of bariatric bypass surgery patients. J Obes. 2013;2013:492170. doi: 10.1155/2013/492170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Siedlinski M, et al. Association of cigarette smoking and CRP levels with DNA methylation in α-1 antitrypsin deficiency. Epigenetics. 2012;7:720–728. doi: 10.4161/epi.20319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sun YV, et al. Gene-specific DNA methylation association with serum levels of C-reactive protein in African Americans. PLoS One. 2013;8:e73480. doi: 10.1371/journal.pone.0073480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dehghan A, et al. Meta-analysis of genome-wide association studies in >80 000 subjects identifies multiple loci for C-reactive protein levels. Circulation. 2011;123:731–738. doi: 10.1161/CIRCULATIONAHA.110.948570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Price ME, et al. Additional annotation enhances potential for biologically-relevant analysis of the Illumina Infinium HumanMethylation450 BeadChip array. Epigenetics Chromatin. 2013;6:4. doi: 10.1186/1756-8935-6-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Smith AK, et al. DNA extracted from saliva for methylation studies of psychiatric traits: Evidence tissue specificity and relatedness to brain. Am J Med Genet B Neuropsychiatr Genet. 2015;168B:36–44. doi: 10.1002/ajmg.b.32278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Du P, et al. Comparison of Beta-value and M-value methods for quantifying methylation levels by microarray analysis. BMC Bioinformatics. 2010;11:587. doi: 10.1186/1471-2105-11-587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ritchie ME, et al. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015;43:e47. doi: 10.1093/nar/gkv007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Perez TL. 2015. Attrition in the Cebu Longitudinal Health and Nutrition Survey, report series No. 1 (USC-Office of Population Studies Foundation, Inc., Cebu City, Philippines)
- 76.McDade TW, Borja JB, Largado F, Adair LS, Kuzawa CW. Adiposity and chronic inflammation in young women predict inflammation during normal pregnancy in the Philippines. J Nutr. 2016;146:353–357. doi: 10.3945/jn.115.224279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Price EM, et al. Different measures of “genome-wide” DNA methylation exhibit unique properties in placental and somatic tissues. Epigenetics. 2012;7:652–663. doi: 10.4161/epi.20221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Maksimovic J, Gordon L, Oshlack A. SWAN: Subset-quantile within array normalization for illumina infinium HumanMethylation450 BeadChips. Genome Biol. 2012;13:R44. doi: 10.1186/gb-2012-13-6-r44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Leek JT, Johnson WE, Parker HS, Jaffe AE, Storey JD. The sva package for removing batch effects and other unwanted variation in high-throughput experiments. Bioinformatics. 2012;28:882–883. doi: 10.1093/bioinformatics/bts034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lohman TG, Roche AF, Martorell R. Anthropometric Standardization Reference Manual. Human Kinetics Books; Champaign, IL: 1988. [Google Scholar]
- 81.Nurgalieva ZZ, et al. Helicobacter pylori infection in Kazakhstan: Effect of water source and household hygiene. Am J Trop Med Hyg. 2002;67:201–206. doi: 10.4269/ajtmh.2002.67.201. [DOI] [PubMed] [Google Scholar]
- 82.VanDerslice J, Popkin B, Briscoe J. Drinking-water quality, sanitation, and breast-feeding: Their interactive effects on infant health. Bull World Health Organ. 1994;72:589–601. [PMC free article] [PubMed] [Google Scholar]
- 83.Moe CL, Sobsey MD, Samsa GP, Mesolo V. Bacterial indicators of risk of diarrhoeal disease from drinking-water in the Philippines. Bull World Health Organ. 1991;69:305–317. [PMC free article] [PubMed] [Google Scholar]
- 84.Pearson TA, et al. Centers for Disease Control and Prevention; American Heart Association Markers of inflammation and cardiovascular disease: Application to clinical and public health practice: A statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation. 2003;107:499–511. doi: 10.1161/01.cir.0000052939.59093.45. [DOI] [PubMed] [Google Scholar]
- 85.Speir ML, et al. The UCSC Genome Browser database: 2016 update. Nucleic Acids Res. 2016;44:D717–D725. doi: 10.1093/nar/gkv1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.McDade TW, Rutherford JN, Adair L, Kuzawa C. Population differences in associations between C-reactive protein concentration and adiposity: Comparison of young adults in the Philippines and the United States. Am J Clin Nutr. 2009;89:1237–1245. doi: 10.3945/ajcn.2008.27080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.McDade TW, Tallman PS, Adair LS, Borja J, Kuzawa CW. Comparative insights into the regulation of inflammation: Levels and predictors of interleukin 6 and interleukin 10 in young adults in the Philippines. Am J Phys Anthropol. 2011;146:373–384. doi: 10.1002/ajpa.21586. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.