Significance
We report the significant presence of traffic-originated nanocluster aerosol (NCA) particles in a particle diameter range of 1.3–3.0 nm of urban air, determine the emission factors for the NCA, and evaluate its global importance. Our findings are important because they significantly update the current understanding of atmospheric aerosol in urban areas. They demonstrate that in urban air, extremely small particles form a significant fraction of the total particle number and are a direct result of anthropogenic emissions, that is, the emissions from road traffic. Thus, our findings also imply that in urban areas, an atmospheric nucleation process is not necessary for the formation of a large number of particles that affect population health and climate.
Keywords: nanocluster aerosol, atmospheric aerosol, combustion-derived nanoparticles, air pollution, traffic emission
Abstract
In densely populated areas, traffic is a significant source of atmospheric aerosol particles. Owing to their small size and complicated chemical and physical characteristics, atmospheric particles resulting from traffic emissions pose a significant risk to human health and also contribute to anthropogenic forcing of climate. Previous research has established that vehicles directly emit primary aerosol particles and also contribute to secondary aerosol particle formation by emitting aerosol precursors. Here, we extend the urban atmospheric aerosol characterization to cover nanocluster aerosol (NCA) particles and show that a major fraction of particles emitted by road transportation are in a previously unmeasured size range of 1.3–3.0 nm. For instance, in a semiurban roadside environment, the NCA represented 20–54% of the total particle concentration in ambient air. The observed NCA concentrations varied significantly depending on the traffic rate and wind direction. The emission factors of NCA for traffic were 2.4·1015 (kgfuel)−1 in a roadside environment, 2.6·1015 (kgfuel)−1 in a street canyon, and 2.9·1015 (kgfuel)−1 in an on-road study throughout Europe. Interestingly, these emissions were not associated with all vehicles. In engine laboratory experiments, the emission factor of exhaust NCA varied from a relatively low value of 1.6·1012 (kgfuel)−1 to a high value of 4.3·1015 (kgfuel)−1. These NCA emissions directly affect particle concentrations and human exposure to nanosized aerosol in urban areas, and potentially may act as nanosized condensation nuclei for the condensation of atmospheric low-volatile organic compounds.
Detailed characterization of aerosol sources is required to understand climate impacts and health effects of atmospheric aerosols, as well as to develop technologies and policies capable of mitigating air pollution in urbanized areas. In densely populated areas, one of the most significant source of particles is traffic (1, 2). Owing to their small size and complicated chemical and physical characteristics (3–6), atmospheric particles resulting from traffic emissions pose a significant risk to human health (7–12), and also contribute to anthropogenic forcing of climate (13, 14). Previous research on vehicular emissions has demonstrated the presence of soot and ash (3, 15) and solid sub–10-nm core particles (4–6) in primary emissions from vehicles and engines and their variation, depending on vehicle technologies (4, 6), the properties of fuels and lubricant oils (15, 16), and driving conditions (15–17). In addition to particles, exhaust typically contains species that reside in the gaseous phase in the undiluted high-temperature exhaust (5, 18, 19) but condense or even nucleate to the particle phase immediately after the exhaust is released to the atmosphere. Here, we term such aerosols delayed primary aerosols, because particle precursors exist already in the undiluted exhaust and the amounts and characteristics of the resulting atmospheric particulate matter do not depend significantly on atmospheric processing or atmospheric photochemistry. In the secondary aerosol formation process driven by atmospheric photochemistry, volatile gaseous compounds emitted by traffic are chemically transformed in the atmosphere to less volatile species, enabling and enhancing secondary aerosol particle formation via condensation, as well as new particle formation (20–22) Whereas the primary and delayed primary particle emissions affect mostly the air quality near the emission source, the effects of the secondary processes are more important on a regional scale.
Previous research has demonstrated the importance of nanosized clusters in atmospheric processes, especially in the formation of ultrafine particles (23, 24). Some studies have also shown that nanosized clusters have a role in engine emission formation (4, 25). Research on particle number concentrations near traffic has shown highly elevated concentrations (of the order of 105–106) of ultrafine particles near roadways (2, 26), also indicating the likelihood of high concentrations of nanosized particles and clusters existing in such environments. However, up to now the measurement techniques suitable for their detection have not been applied in such environments, leaving the direct observation, formation, and effects of particles smaller than 3 nm unexplored.
Here we report the significant presence of nanocluster aerosol (NCA) particles in a particle diameter range of 1.3–3.0 nm in urban air. Our findings strongly indicate that the source of this NCA is traffic and, more specifically, the exhaust of vehicles. We also show that the significance of NCA in vehicular emissions has been previously underestimated owing to a lack of knowledge and unavailability of suitable measurement techniques. The observations reported here are based on the results from three atmospheric measurements covering a wide range of environments: stationary measurements at the roadside of a main road in a semiurban area, stationary measurements in a street canyon in an urban area, and a long-distance on-road study with a mobile aerosol laboratory. Further evidence for the existence of the NCA in traffic emissions was obtained in a laboratory study in which emissions of a modern diesel engine were characterized. The NCA measurements were performed with a particle size magnifier (PSM) (27), which uses the method for NCA detection suggested by Iida et al. (28) and enables the detection of extremely small nanoparticles. This method is currently widely used in nanoparticle formation studies (24).
Results and Discussion
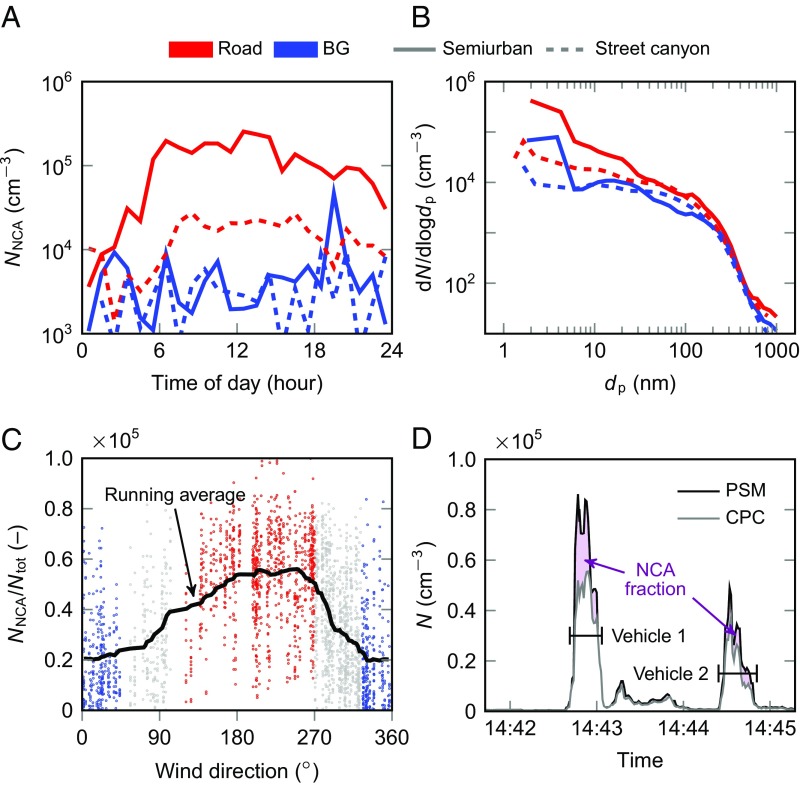
In traffic environments, high NCA concentrations were observed when the wind was blowing from the road to the measurement site. Fig. 1A shows the average diurnal variation of NCA concentrations measured in a semiurban roadside location (solid lines) and in a street canyon (dashed lines). The concentrations are shown separately for two prevailing wind sectors: “BG” refers to the situations when the wind was blowing from the urban background area toward the monitoring station, whereas “road” refers to the situation when the wind direction was from the street or road toward the monitoring station. Data for all wind directions are provided in Fig. S1. In general, when the wind was blowing from the road toward the monitoring station, the NCA concentrations exceeded 105 cm−3 in the semiurban environment and 104 cm−3 in the street canyon environment. The diurnal variation in NCA number concentration was similar in both measurements, increasing rapidly in the early morning and decreasing to very low values in the evening.
Fig. 1.
NCA number concentrations in two different roadside environments, semiurban (solid line, speed limit 80 km/h) and street canyon (dashed line, speed limit 40 km/h). (A) Diurnal variation in NCA concentration. Two sectors, road (red; n = 926) and urban background (blue; n = 548) are distinguished from the dataset based on the wind direction. (B) Particle number size distributions, including the NCA of fresh exhaust aerosol measured in the roadside environments, showing the high contribution of nanoclusters to total particle numbers. The road-influenced average distribution (red) and the average distribution from the clean sector (blue) representing the urban background are shown. (C) Ratio of NCA number concentration to the total aerosol number concentration as a function of the wind direction in the semiurban environment. The nearest road is between the angles 110–270°. (D) An example of the time series measured with the PSM and a CPC, showing the effect of two passing vehicles on the particle concentration in the street canyon.
Fig. S1.
NCA number concentrations in two different roadside environments, semiurban (solid line) and street canyon (dashed line). In addition to the data in Fig. 1 A and B, the data for all wind directions are shown (black). The sectors of road (red) and urban background (blue) are distinguished based on the wind direction. (A) Diurnal variation in NCA concentration. (B) Particle number size distributions.
The particle size distribution analysis (Fig. 1B) indicated that in the semiurban roadside environment, the observed NCA formed a separate mode with high number concentrations, but a clearly distinguishable NCA mode was not observed in the street canyon. Naturally, the loading of particles larger than 3 nm was also higher in the road-originated air;, the median number concentration was 1.3·105 cm−3 in the semiurban roadside environment, compared with 7.6·104 cm−3 for the background sector. Like the NCA number concentration, the fraction of NCA in the total particle concentration also depended on the wind direction. In the semiurban roadside environment, on average 38% of measured aerosol particles in number were attributed to NCA, i.e., the size range 1.3–3 nm, whereas when the wind direction was from the road to the monitoring station, the NCA fraction increased to 54 ± 18% (n = 926). This value is the mean of the red dots shown in Fig. 1C, each of which corresponds to a 5-min average of the measured concentrations. The SD and the number of data points are shown as well. In the background aerosol, the NCA fraction was lower, averaging 20 ± 17% (n = 548). Based on this evidence (i.e., diurnal variation and the effect of wind direction on the NCA concentration and fraction), we conclude that road traffic is a major source of NCA.
Interestingly, not all vehicles emit high amounts of NCA, as demonstrated by Fig. 1D, which shows the concentration time series measured with a PSM and a condensation particle counter (CPC). The NCA concentration was obtained as the concentration difference of the two readings; as shown, the passing of vehicle 1 resulted in a high amount of NCA (∼30% of the total concentration), whereas a much lower fraction of NCA was measured after the passing of vehicle 2.
For a more regional evaluation, we conducted an on-road NCA experiment across western Europe, from northern Spain to Finland (Fig. 2B). This experiment was conducted mainly on motorways (71% of all en route observations) using a mobile laboratory equipped with similar instrumentation for the measurement of NCA as in the previous roadside studies. The NCA concentrations were particularly elevated in high-speed traffic environments and lower when traffic influence was reduced, e.g., during stoppages and on minor roads (Fig. 2A). The influence of traffic is clearly demonstrated during the bridge crossing the Danish Straits (Fig. 2C). The crossing was performed during heavy cross-wind conditions, which in practice caused the instruments to sample only marine background. At this time, both the concentration and the fraction of NCA were drastically reduced. On-road observations also showed that different road environments resulted in different NCA loadings (Fig. 2A); high-speed motorways exhibited higher NCA concentrations than minor roads with lower speeds, and the highest NCA concentrations were measured in traffic tunnels, where the dilution of exhaust emissions is limited.
Fig. 2.
NCA concentrations measured during the on-road experiment. (A) Geometric means, means, and medians of NCA concentrations shown in different experimental situations. (B) The entire studied route, indicated by a black line. Locations of the measurements shown in C are indicated in red. (C) Excerpt of NCA and total particle concentration time series and speed profile of the mobile laboratory van. Longer time series for the experiment are shown in Figs. S7 and S8.
The foregoing observations strongly indicate that NCA observed in urban and traffic environments is emitted by traffic and not derived from atmospheric particle formation. To provide evidence of NCA as a byproduct of combustion, we performed three laboratory experiments with a modern diesel engine. We found that, the exhaust contained significant amounts of NCA in addition to soot and nucleation mode particles larger than 3 nm (Fig. 3). The NCA concentration depended on the engine load in a similar way as the concentration of particles larger than 3 nm. It should be noted that in the engine laboratory experiment, the exhaust dilution and sampling system enables real world-like nanoparticle formation (29), but does not emulate atmospheric oxidation. As discussed previously, secondary aerosol formation requires the oxidation of gaseous emissions to low-volatility compounds. Our experiments show that fresh exhaust can contain sufficient low-volatility compounds to enable NCA formation in vehicle exhaust without atmospheric oxidation. We also found that on application of thermal treatment to the exhaust sample, the NCA disappeared at an engine load of 75% and nearly disappeared at a load of 100% (Fig. S2). These findings indicate that the NCA should be considered delayed primary aerosol, composed of species not in the particle phase in hot undiluted exhaust. However, the total NCA emission of traffic is a result of emissions from a large number of vehicles with varying technologies, which could cause significant variation in the mechanism of formation and composition of emitted NCA.
Fig. 3.
Particle number size distributions of fresh exhaust aerosol measured in an engine laboratory, showing the high contribution of nanoclusters in total particle number for a diesel engine with three engine loads (50%, 75%, and 100%). The previously undetected particles are shadowed in gray. The PSM (solid lines) and the SMPS (dashed lines) were used after the exhaust sampling and dilution system designed to mimic real-world nanoparticle formation. The shadowed areas represent the error limits for the PSM measurements formed by the SDs.
Fig. S2.
Exhaust particle size distributions measured after the thermal treatment of diluted exhaust samples using the PSM (solid lines) and SMPS (dashed lines).
Because CO2 is an inert gas and a product of combustion, it can be used as a tracer for combustion-originated products in the atmosphere, i.e., to determine fuel-specific emission factors for other byproducts of combustion (30, 31). Such emission factors enable a comparison of emissions from different combustion sources. Fig. 4 shows the dependence of the NCA concentration on CO2 for each experiment conducted in this study (two roadside experiments, the on-road study, and three experiments in the engine laboratory). For the roadside and on-road experiments, the figure shows the measurement data averaged over discrete CO2 levels (at intervals of 5 or 10 ppm), and linear fits for the averaged data points above the background CO2 level (SI Text, Background CO2 Concentrations of Ambient Air). For the engine laboratory experiments, the figure shows lines measured at one phase of exhaust dilution and calculated into the larger dilution scene.
Fig. 4.
NCA number concentration as a function of simultaneously measured CO2. Both roadside measurements, high-speed motorway driving during the long-range on-road study through Europe, and engine laboratory experiments are shown. The dots represent the experimental data averaged over discrete CO2 levels. For the roadside and on-road studies, the solid lines represent fits for the experimental data, whereas for engine laboratory studies, the dashed lines represent results generated based on the NCA concentration measured at one well-defined dilution stage.
For the roadside experiment conducted in the street canyon and for the on-road experiment, the NCA concentration increased linearly as a function of CO2 concentration when the CO2 values were above the background value. In the semiurban roadside experiment, the linear dependence was not as clear-cut. For all three cases, slopes of the fits were similar: 1,475 ± 918 (cm3ppm)-−1 for the semiurban roadside environment, 1,625 ± 1,062 (cm3ppm)−1 for the street canyon, and 1,784 ± 309 (cm3ppm)−1 for high-speed motorway driving during the trans-European on-road study. Assuming a CO2 emission of 3,140 g/kg of fuel (an average of conversion factors for diesel and gasoline fuels) and normal temperature and pressure (NTP) (30), we computed fuel-specific emission factors for NCA: 2.4·1015 ± 1.5·1015 (kgfuel)−1, 2.6·1015 ± 1.7·1015 (kgfuel)−1, and 2.9·1015 ± 0.5·1015 (kgfuel)−1 for the three environments, respectively. The error limits were formed from the 95% confidence limits obtained for the slopes of the fits (Fig. 4). For the engine laboratory experiments, the fuel-specific emission factors for NCA were 1.6·1012 (kgfuel)−1 at low load, 2.2·1014 (kgfuel)−1 at medium load, and 4.3·1015 (kgfuel)−1 at high load (for diesel fuel, assuming a CO2 emission of 3,160 g/kg of fuel and NTP) (30). Thus, the traffic NCA emissions determined in the three field experiments were between the minimum and maximum emission factors observed in the laboratory study. Compared with other studies (30, 31), our findings show that the NCA emission factors of traffic are at same levels as the emission factors of larger particles. From a global perspective, the annual NCA emissions of road traffic are likely to exceed 4.2·1027 per year (SI Text), representing a significant increase over the existing estimate of 17·1027 a−1 for global anthropogenic particle sources (32).
Recent studies (33) have strongly suggested that a large fraction of secondary organic aerosol formation is due to the condensation of extremely low-volatility vapors on available aerosol surfaces. We show here that traffic-emitted NCA increases the aerosol particle number concentrations in environments near traffic. Previous research has shown that particle concentrations decrease when moving away from the roadway; this effect is generally attributed to coagulation and dilution (2, 26, 34), although evaporation has been suggested as well (35). The scavenging time scale of diluted NCA is of the order of 20 min to reach the levels observed for urban background aerosol; thus, NCA can be transported for several kilometers before removal by loss processes, meaning that in urban areas, the traffic-originated NCA is likely ubiquitous and contributes significantly to the background levels. Compared with other NCA sources, in urban environments traffic-emitted NCA is likely to exceed the number of particles formed by photochemical nucleation. High anthropogenic aerosol concentrations typically have been considered an inhibiting factor in terrestrial boundary layer nucleation owing to their capability of scavenging both condensing vapors and fresh nanoparticles from the air. Regional photochemical formation is considered the major source of NCA-sized aerosol in the atmosphere. Our findings update this view, showing that human activity also directly produces nanosized aerosol, which may allow even a majority of the condensational growth events of atmospheric aerosol particles to begin in urban areas (36), by acting as nanosized condensation nuclei for both secondary anthropogenic and biogenic low-volatility organic compounds. Thus, NCA has the potential to enhance tropospheric aerosol formation and, by that route, modify terrestrial cloud cover. Therefore, our findings help to understand atmospheric processes affecting climate, in addition to presenting an anthropogenic NCA source that may affect urban air quality and thus public health as well.
SI Text
Background CO2 Concentrations of Ambient Air.
The average atmospheric CO2 level is currently considered to be ∼400 ppm; however, atmospheric CO2 concentration exhibits significant spatial and temporal variation, depending on, e.g., nearby vegetation (46), and, as in the present study, nearby combustion emission sources. The background CO2 concentrations display temporal variation as well, typically seen as relatively strong diurnal and seasonal cycles (47). The variation in local background concentrations of atmospheric CO2 are seen as plateaus in measurements obtained in street canyons (long measurement times) and the on-road experiment (large spatial variations). In addition, in the on-road experiment, the motorway driving caused slight mechanical shaking and tremors to the instrument, which introduced random normally distributed noise component to the data, likely by the electronic components of the instrument. Because this random component can be negative as well as positive, values below the background value also were recorded by the instrument. In the on-road experiment, the greatest number of CO2 observations were in the concentration ranges of 390–400 ppm (28.5%; no elevated NCA concentration) and 400–410 ppm (21.5%; slightly elevated NCA concentration), and a minor fraction (3.5%) were <380 ppm.
The sideways wind on the bridge crossing the Danish Straits (Fig. 2C) enabled us to avoid the effects of nearby CO2 sources and sinks to the CO2 measurement and thus to quantify the local background CO2 concentration and the aforementioned driving-caused noise. The mean CO2 concentration during bridge driving was 388.9 ± 12.5 ppm (median, 389.3 ppm). Importantly, the variation in CO2 concentration during this local background measurement was random and followed a normal distribution. The aforementioned variation in background CO2 concentrations did not affect the NCA emission factors described in the main text, because those were determined from slopes of increasing NCA concentration.
Computing Total Global NCA Emissions.
In 2013, road transport produced 5.5 Gt of CO2 worldwide (48). Using the same conversion factors as earlier (3.14 gCO2/gfuel), we can now convert the emission factors to total global road transport emissions.
For the roadside minimum NCA emission factor of 2.4 × 1015 kgfuel−1, we get as the total emission [(5.5 × 1012 kg/3.14)/a] × 2.4 × 1015 kgfuel−1 = 4.2 × 1027 a−1 from road transport alone. This can be compared with the estimated total anthropogenic particle number emissions (32), which estimates worldwide particle number emissions at 17 × 1027; from this, we see that the annual NCA emissions resulting from road traffic is a considerable percentage (25%) of total emissions.
Methods
Atmospheric Measurements.
The studies consisted of three atmospheric measurements covering a wide range of environments: stationary measurements at the roadside of a main road in a semiurban area, stationary measurements in a street canyon in an urban area, and a long-distance on-road study using a mobile aerosol laboratory. The NCA measurements were performed using a PSM capable of detecting nanoparticles with diameters as small as ∼1.3 nm (27). NCA measurements were reinforced by measurements with other instruments.
The experiment that produced the first observation of NCA in an urban traffic environment was conducted in a roadside environment on October 19–30, 2012. The measurement station was located 5 m from the pavement of Ring 1, one of the main roads in Helsinki (Fig. S3). Daily traffic rate on that road during workdays is ∼70,000 vehicles, including both heavy-duty vehicles (8%) and passenger cars. Ambient temperature and relative humidity varied between −5 and +14 °C and 43% and 93%, respectively, and were continuously measured at a weather station located in Pasila (Fig. S3). Weather parameters were temporarily measured in a mobile laboratory (37) located next to the measurement station, with good correlation with the continuous measurements. The measurement station data were classified based on the wind direction data into two relevant sectors and the sectors between them (Fig. S3B). The road-influenced sector was defined as wind direction 110–270° and the clean sector was defined as wind direction 325–55° (with 0° as north).
Fig. S3.
(A) Locations of the roadside measurement station, the street canyon measurement station, and the weather station on a map of the Helsinki metropolitan area. (B and C) Exact locations of the measurement station and the road influenced (red) and clean (blue) sectors with respect to the wind direction measured at the weather station for the roadside (B) and street canyon (C) stations.
The aerosol sample was drawn into the measurement station from the roof of the station (height, 3 m) and then to the online aerosol instruments. The combination of a PSM (A09; Airmodus Oy) and a CPC (model 3775; TSI), referred to as a PSM hereinafter, measured the concentration of particles larger than 1.3 nm. It was used in parallel with another CPC (model 3776; TSI) that measured the number concentration of particles larger than 3 nm in diameter. The particle size distribution was measured with a differential mobility particle sizer (DMPS) equipped with a Vienna-type differential mobility analyzer (DMA) and a CPC (model 5.401; Grimm). The DMPS covered a size range from 6 nm to 1 µm. Gaseous pollutants NOx (APNA-360 ambient NOx monitor; HORIBA) and CO2 (SIDOR gas analyzer; SICK) were monitored as well. CO2 concentrations were used in the evaluation of the NCA emission factors.
The time series of the number concentrations measured by the PSM and CPC is shown in Fig. S4A. For further analysis and visualization, the data were averaged over a 5-min time window. The concentration of the NCA (i.e., particles of 1.3–3 nm) was defined as the difference in the concentrations measured by these two devices. The relative number concentration, normalized by the total number concentration, Ntot, measured by the PSM and classified according to the road-influenced and clean sectors, is shown in Fig. S4B. As shown in Fig. S4A, both the PSM and the CPC measured rather high concentrations, ∼106/cm3 at maximum. The actual counting instruments, CPC models 3775 and 3776, were concentration-calibrated in the laboratory after the field campaign based on the single charged aerosol reference (38). The calibration results were applied in the data analysis. Owing to the material dependency of the lower detection limits of a diethylene glycol based PSM and of a butanol-based CPC (39), this study may have slightly underestimated the NCA concentrations.
Fig. S4.
Time series of the particle number concentrations measured by the PSM and the CPC (A), the relative amount of NCA (B), and the wind direction (C). Red and blue represent the road-influenced and background sectors, respectively, and black indicates the sector between them.
In a street canyon environment in an urban area, NCA concentrations were measured for 3 mo. The measurement station was located on the pavement along a relatively busy road that leads toward Helsinki’s city center (Fig. S3; address, Mäkelänkatu 50). The site can be classified as an avenue canyon with an aspect ratio (height/width) of 0.4 (Fig. S5). The road (Mäkelänkatu, total width, 42 m) has six lanes with two tramlines and rows of trees in the middle, and is flanked by four- and five-story buildings. The traffic rate is ∼28,000 vehicles/workday, of which heavy-duty vehicles account for 9%. Measurements were conducted between April 7 and June 26, 2015. The temperature and relative humidity measured at the station varied between 2.1 °C and 21 °C (average, 8.6 °C) and between 18.9% and 92.9% (average, 65.1%), respectively. Wind direction measurements were performed at the weather station located in Pasila at 53 m above ground level and 78 m above sea level (Fig. S3), representing the rooftop wind at the measurement site. The measurement station data were divided into two sectors according to the wind direction and the sectors between these (Fig. S3C). In the event of perpendicular flow, the local wind within the cavity of the canyon was assumed to be contrary to the rooftop wind. Consequently, the road-influenced sector was between the angles 165° and 285°, and the clean sector was between the angles 0 and 105° and 345° and 360°.
Fig. S5.
Profile of the measurement site in the street canyon environment. The sampling location is marked with red dots.
The particle sample was drawn from the roof of the station (height, 4 m) and then conducted to the online aerosol instruments in the measurement station. The setup consisted of a PSM (model A10; Airmodus Oy) and a CPC (model A20; Airmodus Oy), referred to simply as a PSM hereinafter. A bridge diluter (dilution ratio, 7.55) was inserted in the sampling line before the PSM. The PSM was set to measure the number concentrations in a step mode with four different cutoff sizes: 1.2, 1.5, 1.8, and 2.7 nm. The measurement duration for one step was 60 s, and thus one cycle took a total of 4 min. The diurnal hourly averages were calculated separately for each step, and the NCA concentrations were determined from the concentration difference between the first and last steps. Sub–3-nm measurement points for the particle number size distributions were determined similarly from the step data. Particle size distributions for larger particles were measured with a DMPS equipped with a Vienna-type DMA and a CPC (model A20; Airmodus Oy). The DMPS covered a size range of 6–800 nm.
The on-road NCA emissions were studied with a mobile laboratory unit while driving in traffic. The mobile laboratory consisted of a large van equipped with sampling systems for both gaseous and particulate compounds, a compartment in which the instruments were installed, a desktop space for the operator to control the measurement and a power source to supply the instruments. Fig. S6A shows the mobile unit, and Fig. S6B shows an on-road measurement scenario. The measurement route (Fig. 2B) started eastward from the Atlantic coast in northern Spain. It then turned toward north form the Mediterranean coast in southern France and continued through France, Luxembourg, Belgium, The Netherlands, Germany, and Denmark to the large bridges over the Great Belt and the Sound. From there, the route continued through southern Sweden toward a ferry connection to Finland. The total distance covered over 6 d was ∼3,600 km. Although the route consisted mainly of larger motorways, smaller highways and city traffic were also included when the schedule allowed.
Fig. S6.
The mobile unit shown from the side (A) and on the road (B) conducting the long-range experiment through Europe.
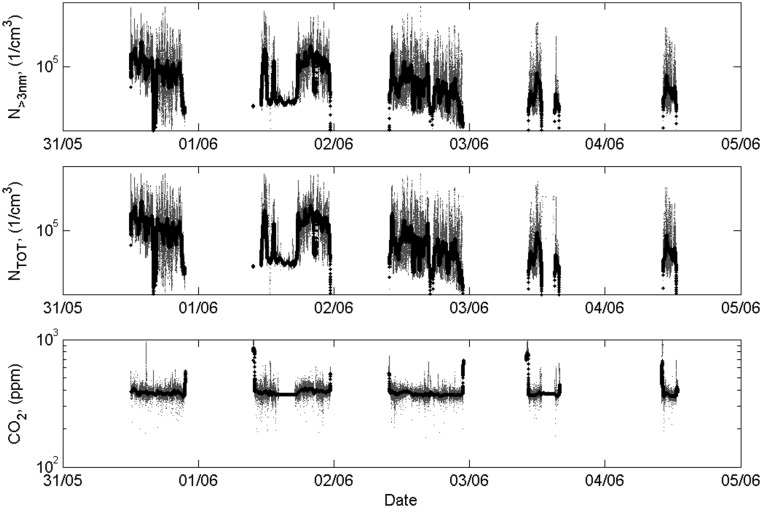
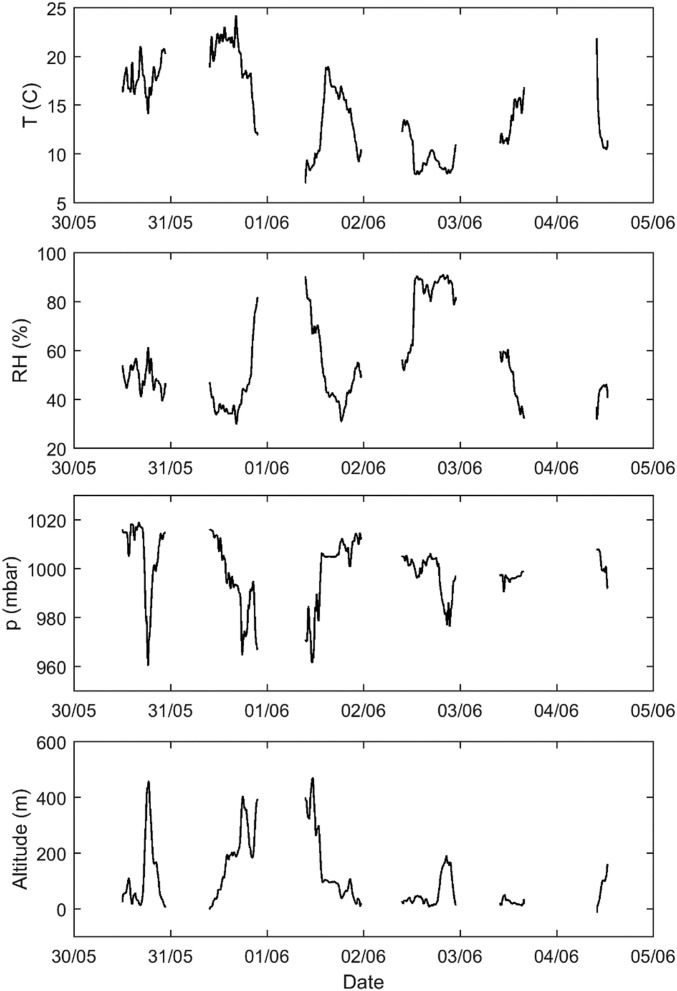
The aerosol samples were obtained in front of the van from the centerline, just above the windscreen. The sampling probe was bent downstream of the flow to keep insects and rain droplets from entering the sample. The sample line continued to the instrument compartment with a residence time of <1 s, where the sample was divided via a bridge diluter (dilution ratio, 42) to the CPC (model 3776; TSI) and the PSM consisting of a PSM (model A10; Airmodus Oy) and a CPC (model 3775; TSI). The CPC model 3776 measured the total particle concentration for particles larger than 3 nm, and the PSM measured the total concentration of particles larger than ∼1.3 nm. CO2 concentration was measured using an IR photometric analyzer (SIDOR; SICK). The measured data from all these instruments (shown in Fig. S7) were saved at a 1-s time resolution. A weather station (WeatherStation 200WX; Airmar Technology) provided information on wind speed and direction, air temperature, barometric pressure, relative humidity, GPS location, vehicle speed, and vehicle direction in the sampling point. Time series of the measured air temperature, relative humidity, barometric pressure, and altitude are shown in Fig. S8.
Fig. S7.
Measured number concentrations for particles larger than 3 nm in diameter (Upper), total particle number concentrations (Middle), and CO2 concentrations (Bottom) obtained from the on-road measurements.
Fig. S8.
Weather data obtained during the on-road measurements. From top to bottom, air temperature (T), relative humidity (RH), barometric pressure (p), and altitude.
Engine Laboratory Experiments.
Laboratory experiments with a modern four-cylinder turbocharged common rail heavy-duty diesel engine (displacement, 4.4 dm3) equipped with an intermediary cooling system for the intake air, a diesel oxidation catalyst, a diesel particle filter, and a selective catalytic reduction system were conducted to verify the traffic environment observations. The ultra-low-sulfur diesel fuel [fuel sulfur content (FSC) <10 ppm] was used. Thus, the technology level of the test engine corresponded to the typical modern (and even future) heavy-duty diesel buses, trucks, and working machines. Three experiments were conducted at an engine test bench, each at a different engine loading. The driving parameters and regulated emissions of each experiment are shown in Table S1.
Table S1.
Driving parameters (engine load and torque) and regulated emissions of the heavy-duty test engine
| Parameter | Load at 1,500 rpm | ||
| 100% | 75% | 50% | |
| Torque, Nm | 560 | 422 | 282 |
| FSN, mg/kWh | 0.91 | 0.44 | 0.43 |
| NOx, g/kWh | 0.92 | 1.04 | 1.03 |
| HC, g/kWh | 0.00 | 0.00 | 0.01 |
| CO, g/kWh | 0.01 | 0.01 | 0.01 |
FSN, filter smoke number, indicating the particulate mass emission of the engine; HC, hydrocarbons, kWh, kilowatt hours.
The measurement setup is shown in Fig. S9. Real-world nanoparticle formation was mimicked using an exhaust sampling system (40) that has been reported to mimic exhaust nucleation mode particle formation relatively well (29, 41). With this sampling system, a portion of exhaust was sampled directly from the tailpipe and further diluted immediately using a porous tube-type primary diluter (42), led through a residence time chamber with a residence time of 2.6 s, and finally diluted by an ejector secondary dilution unit. In the primary diluter, the dilution air temperature was kept at 30 °C and the dilution ratio, calculated from CO2 data for undiluted and diluted exhaust, was maintained at 12. The relative humidity for the pressurized and filtered dilution air was close to zero. After the secondary dilution unit, the exhaust aerosol was led into the aerosol instruments. Because of the high stability of the particle concentrations, the PSM was used in the scanning mode, thereby providing information on both the number concentration and size distribution of particles smaller than 3 nm in diameter. The cumulative number concentrations measured by the PSM were converted to particle size distributions using calibrations for different saturator flows of the PSM (25). In addition to the PSM, exhaust particle number size distributions were measured using a scanning mobility particle sizer (SMPS) (43) equipped with a DMA 3085 (TSI) and a CPC 3025 (TSI), called here a nano-SMPS; an engine exhaust particle sizer (EEPS; TSI) (44); and an electrical low-pressure impactor (ELPI; Dekati) (45). The EEPS and the ELPI were used to monitor the stability of the larger particle emissions during the experiment. Particle volatility was studied using a thermodenuder (5), in which the aerosol sample was first heated to 265 °C and then led through the denuder to collect the evaporated compounds on active charcoal.
Fig. S9.
Measurement setup for the engine laboratory experiments. The after-treatment system consisted of a diesel oxidation catalyst (DOC), a diesel particulate filter (DPF), and a selective catalytic reduction (SCR) system. The sample was diluted with clean pressurized air (PA) in a two-stage dilution system consisting of a porous tube diluter (PTD) and an ejector diluter. Instruments used for the measurements included an ELPI, a CPC, a PSM, an SMPS, and an EEPS.
Acknowledgments
We thank Anders Svens and Harri Portin (Helsinki Region Environmental Services Authority) for their technical support with the roadside measurements. Financial support was provided by Tekes–the Finnish Funding Agency for Innovation; Academy of Finland Grants 283455, 259016, and 293437; Cleen Ltd. (MMEA project); Dinex Ecocat Oy; Neste Oil Oyj; AGCO Power; and Ab Nanol Technologies Oy.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The metadata have been uploaded to the Etsin research data service (https://etsin.avointiede.fi/dataset/urn-nbn-fi-csc-kata20170615095020292611) with a permanent download link and also added to the Tampere University of Technology’s research data system, TUTCRIS (https://tutcris.tut.fi/portal/en/datasets/search.html).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1700830114/-/DCSupplemental.
References
- 1.Pey J, et al. Source apportionment of urban fine and ultra-fine particle number concentration in a western Mediterranean city. Atmos Environ. 2009;43:4407–4415. [Google Scholar]
- 2.Hu S, et al. A wide area of air pollutant impact downwind of a freeway during pre-sunrise hours. Atmos Environ (1994) 2009;43:2541–2549. doi: 10.1016/j.atmosenv.2009.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kittelson D. Engines and nanoparticles: A review. J Aerosol Sci. 1998;29:575–588. [Google Scholar]
- 4.Sgro LA, et al. Measurements of nanoparticles of organic carbon and soot in flames and vehicle exhausts. Environ Sci Technol. 2008;42:859–863. doi: 10.1021/es070485s. [DOI] [PubMed] [Google Scholar]
- 5.Rönkkö T, et al. Effects of gaseous sulphuric acid on diesel exhaust nanoparticle formation and characteristics. Environ Sci Technol. 2013;47:11882–11889. doi: 10.1021/es402354y. [DOI] [PubMed] [Google Scholar]
- 6.Lähde T, et al. Heavy-duty diesel engine exhaust aerosol particle and ion measurements. Environ Sci Technol. 2009;43:163–168. [Google Scholar]
- 7.Maher BA, et al. Magnetite pollution nanoparticles in the human brain. Proc Natl Acad Sci USA. 2016;113:10797–10801. doi: 10.1073/pnas.1605941113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hoek G, Brunekreef B, Goldbohm S, Fischer P, van den Brandt PA. Association between mortality and indicators of traffic-related air pollution in The Netherlands: A cohort study. Lancet. 2002;360:1203–1209. doi: 10.1016/S0140-6736(02)11280-3. [DOI] [PubMed] [Google Scholar]
- 9.Oberdörster G, Oberdörster E, Oberdörster J. Nanotoxicology: An emerging discipline evolving from studies of ultrafine particles. Environ Health Perspect. 2005;113:823–839. doi: 10.1289/ehp.7339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alföldy B, Gieschaskiel B, Hofmann W, Drossinos Y. Size distribution-dependent lung deposition of diesel exhaust particles. J Aerosol Sci. 2009;40:652–663. [Google Scholar]
- 11.Lelieveld J, Evans JS, Fnais M, Giannadaki D, Pozzer A. The contribution of outdoor air pollution sources to premature mortality on a global scale. Nature. 2015;525:367–371. doi: 10.1038/nature15371. [DOI] [PubMed] [Google Scholar]
- 12.McConnell R, et al. Traffic, susceptibility, and childhood asthma. Environ Health Perspect. 2006;114:766–772. doi: 10.1289/ehp.8594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haywood J, Boucher O. Estimates of the direct and indirect radiative forcing due to tropospheric aerosols: A review. Rev Geophys. 2000;38:513–543. [Google Scholar]
- 14.Bond T, et al. Bounding the role of black carbon in the climate system: A scientific assessment. J Geophys Res, D, Atmospheres. 2013;118:5384–5388. [Google Scholar]
- 15.Maricq MM, Chase RE, Xu N, Laing PM. The effects of the catalytic converter and fuel sulfur level on motor vehicle particulate matter emissions: Light-duty diesel vehicles. Environ Sci Technol. 2002;36:283–289. doi: 10.1021/es010962l. [DOI] [PubMed] [Google Scholar]
- 16.Vaaraslahti K, et al. Effect of lubricant on the formation of heavy-duty diesel exhaust nanoparticles. Environ Sci Technol. 2005;39:8497–8504. doi: 10.1021/es0505503. [DOI] [PubMed] [Google Scholar]
- 17.Rönkkö T, et al. Vehicle engines produce exhaust nanoparticles even when not fueled. Environ Sci Technol. 2014;48:2043–2050. doi: 10.1021/es405687m. [DOI] [PubMed] [Google Scholar]
- 18.Shi J, Harrison R. Investigation of ultrafine particle formation during diesel exhaust dilution. Environ Sci Technol. 1999;33:3730–3736. [Google Scholar]
- 19.Arnold F, et al. First online measurements of sulfuric acid gas in modern heavy-duty diesel engine exhaust: Implications for nanoparticle formation. Environ Sci Technol. 2012;46:11227–11234. doi: 10.1021/es302432s. [DOI] [PubMed] [Google Scholar]
- 20.Robinson AL, et al. Rethinking organic aerosols: Semivolatile emissions and photochemical aging. Science. 2007;315:1259–1262. doi: 10.1126/science.1133061. [DOI] [PubMed] [Google Scholar]
- 21.Platt SM, et al. Two-stroke scooters are a dominant source of air pollution in many cities. Nat Commun. 2014;5:3749. doi: 10.1038/ncomms4749. [DOI] [PubMed] [Google Scholar]
- 22.Gentner D, et al. Elucidating secondary organic aerosol from diesel and gasoline vehicles through detailed characterization of organic carbon emissions. Proc Natl Acad Sci USA. 2012;109:18318–18323. doi: 10.1073/pnas.1212272109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kulmala M, et al. Toward direct measurement of atmospheric nucleation. Science. 2007;318:89–92. doi: 10.1126/science.1144124. [DOI] [PubMed] [Google Scholar]
- 24.Kirkby J, et al. Ion-induced nucleation of pure biogenic particles. Nature. 2016;533:521–526. doi: 10.1038/nature17953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alanen J, et al. The formation and physical properties of the particle emissions from a natural gas engine. Fuel. 2015;162:155–161. [Google Scholar]
- 26.Zhu Y, Hinds W, Kim S, Shen S, Sioutas C. Study of ultrafine particles near a major highway with heavy-duty diesel traffic. Atmos Environ. 2002;36:4323–4335. [Google Scholar]
- 27.Vanhanen J, et al. Particle size magnifier for nano-CN detection. Aerosol Sci Technol. 2011;45:533–542. [Google Scholar]
- 28.Iida K, Stolzenburg M, McMurry P. Effect of working fluid on sub–2-nm particle detection with a laminar flow ultrafine condensation particle counter. Aerosol Sci Technol. 2009;43:81–96. [Google Scholar]
- 29.Keskinen J, Rönkkö T. Can real-world diesel exhaust particle size distribution be reproduced in the laboratory? A critical review. J Air Waste Manag Assoc. 2010;60:1245–1255. [PubMed] [Google Scholar]
- 30.Yli-Tuomi T, et al. Emissions of the particles, NOx, and CO from on-road vehicles in Finland. Atmos Environ. 2005;39:6696–6706. [Google Scholar]
- 31.Pirjola L, et al. Physical and chemical characterization of real-world particle number and mass emissions from city buses in Finland. Environ Sci Technol. 2016;50:294–304. doi: 10.1021/acs.est.5b04105. [DOI] [PubMed] [Google Scholar]
- 32.Paasonen P, et al. Continental anthropogenic primary particle number emissions. Atmos Chem Phys. 2016;16:6823–6840. [Google Scholar]
- 33.Ehn M, et al. A large source of low-volatility secondary organic aerosol. Nature. 2014;506:476–479. doi: 10.1038/nature13032. [DOI] [PubMed] [Google Scholar]
- 34.Jacobson M, Seinfeld J. Evolution of nanoparticle size and mixing state near the point of emission. Atmos Environ. 2004;38:1839–1850. [Google Scholar]
- 35.Zhang K, Wexler A, Zhu Y, Hinds W, Sioutas C. Evolution of particle number distribution near roadways, II: The “road-to-ambient” process. Atmos Environ. 2004;38:6655–6665. [Google Scholar]
- 36.Ahlm L, et al. Formation and growth of ultrafine particles from secondary sources in Bakersfield, California. J Geophys Res, D, Atmospheres. 2012;117:D00V08. [Google Scholar]
- 37.Pirjola L, et al. “Sniffer”—a novel tool for chasing vehicles and measuring traffic pollutants. Atmos Environ. 2004;38:3625–3635. [Google Scholar]
- 38.Yli-Ojanperä J, Mäkelä JM, Marjamäki M, Rostedt A, Keskinen J. Towards traceable particle number concentration standard: Single charged aerosol reference (SCAR) J Aerosol Sci. 2010;41:719–728. [Google Scholar]
- 39.Kangasluoma J, et al. Sub–3-nm particle size and composition dependent response of a nano-CPC battery. Atmos Meas Tech. 2014;7:689–700. [Google Scholar]
- 40.Ntziachristos L, et al. 2004 Performance evaluation of a novel sampling and measurement system for exhaust particle characterization. SAE Technical Paper Series 2004-01-1439. Available at: papers.sae.org/2004-01-1439/. Accessed June 15, 2017.
- 41.Rönkkö T, et al. Effect of dilution conditions and driving parameters on nucleation mode particles in diesel exhaust: Laboratory and on-road study. Atmos Environ. 2006;40:2893–2901. [Google Scholar]
- 42.Mikkanen P, Moisio M, Keskinen J, Ristimäki J, Marjamäki M. 2001 Sampling method for particle measurements of vehicle exhaust. SAE Technical Paper Series 2001-01-0219. Available at: papers.sae.org/2001-01-0219/. Accessed June 15, 2017.
- 43.Wang S, Flagan R. Scanning electrical mobility spectrometer. Aerosol Sci Technol. 1990;13:230–240. [Google Scholar]
- 44.Johnson T, Caldow R, Pöcher A, Mirme A, Kittelson D. 2004 A new electrical mobility particle sizer spectrometer for engine exhaust particle measurements. SAE Technical Paper Series 2004-01-1341. Available at: papers.sae.org/2004-01-1341/. Accessed June 15, 2017.
- 45.Keskinen J, Pietarinen K, Lehtimäki M. Electrical low-pressure impactor. J Aerosol Sci. 1992;23:353–360. [Google Scholar]
- 46.Fernández-Duque B, Pérez IA, Sánchez ML, García MÁ, Pardo N. Temporal patterns of CO2 and CH4 in a rural area in northern Spain described by a harmonic equation over 2010-2016. Sci Total Environ. 2017;593-594:1–9. doi: 10.1016/j.scitotenv.2017.03.132. [DOI] [PubMed] [Google Scholar]
- 47.Wu J, et al. Evolution of atmospheric carbon dioxide concentration at different temporal scales recorded in a tall forest. Atmos Environ. 2012;61:9–14. [Google Scholar]
- 48.International Energy Agency 2016 CO2 emissions from fuel combustion highlights 2015. Available at: https://www.iea.org/publications/freepublications/. Accessed September 14, 2016.