Significance
The efficiency of production of genetically altered piglets for agricultural and biomedical purposes is very low, in large part because of the poor quality of the in vitro-matured oocytes used to initiate the process. Here, we describe a chemically defined culture medium that enhances porcine oocyte maturation and subsequent embryo development to the blastocyst stage, and increases the number of offspring born after embryo transfer, thereby effectively quadrupling the efficiency of piglet production. This research also provides some insights into the physiological events that lead to oocyte competence and how the efficacy of oocyte in vitro maturation might be improved in other species.
Keywords: cumulus cell, embryo development, genetic modification, in vitro fertilization, MAPK signaling
Abstract
Assisted reproductive technologies in all mammals are critically dependent on the quality of the oocytes used to produce embryos. For reasons not fully clear, oocytes matured in vitro tend to be much less competent to become fertilized, advance to the blastocyst stage, and give rise to live young than their in vivo-produced counterparts, particularly if they are derived from immature females. Here we show that a chemically defined maturation medium supplemented with three cytokines (FGF2, LIF, and IGF1) in combination, so-called “FLI medium,” improves nuclear maturation of oocytes in cumulus–oocyte complexes derived from immature pig ovaries and provides a twofold increase in the efficiency of blastocyst production after in vitro fertilization. Transfer of such blastocysts to recipient females doubles mean litter size to about nine piglets per litter. Maturation of oocytes in FLI medium, therefore, effectively provides a fourfold increase in piglets born per oocyte collected. As they progress in culture, the FLI-matured cumulus–oocyte complexes display distinctly different kinetics of MAPK activation in the cumulus cells, much increased cumulus cell expansion, and an accelerated severance of cytoplasmic projections between the cumulus cells outside the zona pellucida and the oocyte within. These events likely underpin the improvement in oocyte quality achieved by using the FLI medium.
In vitro maturation (IVM) of oocytes is a critical step in assisted reproductive technologies carried out in species, such as cattle and swine, for generating oocytes capable of being fertilized in vitro and providing healthy young useful for biomedical and agricultural purposes (1–3). IVM is also important for human in vitro fertilization (IVF) under conditions where exposure of the patient to high levels of superovulating regimens of hormones is contraindicated (4–6). Oocytes retrieved from small to medium-sized follicles, if cultured appropriately, can resume meiosis and mature to a state in which they can be fertilized. However, attaining full developmental competence in immature oocytes retrieved from unstimulated ovaries, particularly ones from prepubertal females, continues to be a challenge (2). In pigs, production of embryos from IVM oocytes has assumed increasing importance for biomedical applications, especially for introducing genetic changes into animals that might mimic human disease states (7). In creating such models, somatic cell nuclear transfer (SCNT) has allowed gene alterations previously engineered into cultured somatic cells by various gene-targeting tools to be relocated to enucleated oocytes (3), and newer CRISPR-Cas9 technologies can even introduce genetic changes directly into fertilized oocytes (zygotes) (8, 9). The reliance on slaughterhouse-derived ovaries from immature gilts as a source of oocytes markedly reduces the success of such procedures.
In vivo, the mammalian oocyte grows and matures within a somatic cell compartment consisting of cumulus cells, and gradually acquires meiotic and developmental competence at the antral follicle stage (10, 11). Oocytes and cumulus cells must interact harmoniously in terms of their metabolic activities for competence to be attained (12). Oocyte meiotic arrest is maintained by a high level of cAMP, which is largely produced by the oocytes (13, 14). Once cumulus–oocyte complexes (COCs) are removed from the follicular environment and placed into culture, a proportion of the oocytes usually resume meiosis spontaneously. This promiscuous progression to metaphase II most probably occurs as the result of a reduced influx of cGMP from the surrounding cumulus cells into the oocyte. cGMP maintains high intracellular cAMP concentrations by inhibiting the phosphodiesterase responsible for cAMP hydrolysis (15–17). An inappropriate drop in the concentrations of the cyclic nucleotides that control meiotic resumption causes unsynchronized nuclear and cytoplasmic maturation of the oocytes, thereby compromising their proper development (18).
The gonadotrophin-triggered activation of signaling networks during oocyte maturation is normally orchestrated by EGF and related factors, and the downstream MAPK1 and MAPK3 (19–21). The activation of MAPK1/3 in granulosa and cumulus cells plays a central role in triggering the essential steps of oocyte maturation and COC expansion (22, 23). Cumulus cells in antral follicles lack expression of luteinizing hormone (LH) receptors (24). Therefore, oocytes collected for IVM often respond poorly to LH (25), and that may result in dysregulated MAPK activation and compromised oocyte competence. As a result, medium used for IVM is usually supplemented with FSH and EGF to stimulate LH sensitivity and downstream signaling pathways (1, 25–28). However, even with these supplements, oocytes of many species still complete maturation quite poorly, suggesting that the necessary signal transduction pathways have been either understimulated or inappropriately stimulated. One explanation is that the follicular environment contains additional, locally produced factors that are needed to provide adequate support for the complex process of follicular growth and oocyte maturation. Accordingly, follicular fluid is sometimes added to the culture medium to enhance oocyte IVM, although outcomes have not been universally improved (2, 29). It seems counterintuitive to include follicular fluid to encourage oocyte maturation, as it contains oocyte maturation inhibitors, such as hypoxanthine, which arrest oocyte meiosis (30–32). Nevertheless, the beneficial effects of using follicular fluid during IVM in many studies (33–36) provided clues that its content of growth factors could be beneficial for oocyte maturation. Here, we provide evidence that three cytokines, fibroblast growth factor 2 (FGF2), leukemia inhibitory factor (LIF), and insulin-like growth factor 1 (IGF1), each known to be present in follicular fluid (37–40), when used in combination can provide much improved oocyte competence, most probably by influencing the timing of MAPK1/3 activation in the cumulus cells enveloping the oocyte.
Results
Effects of Individual Cytokines on Nuclear Maturation of Oocytes and Embryo Development to Blastocyst Stage.
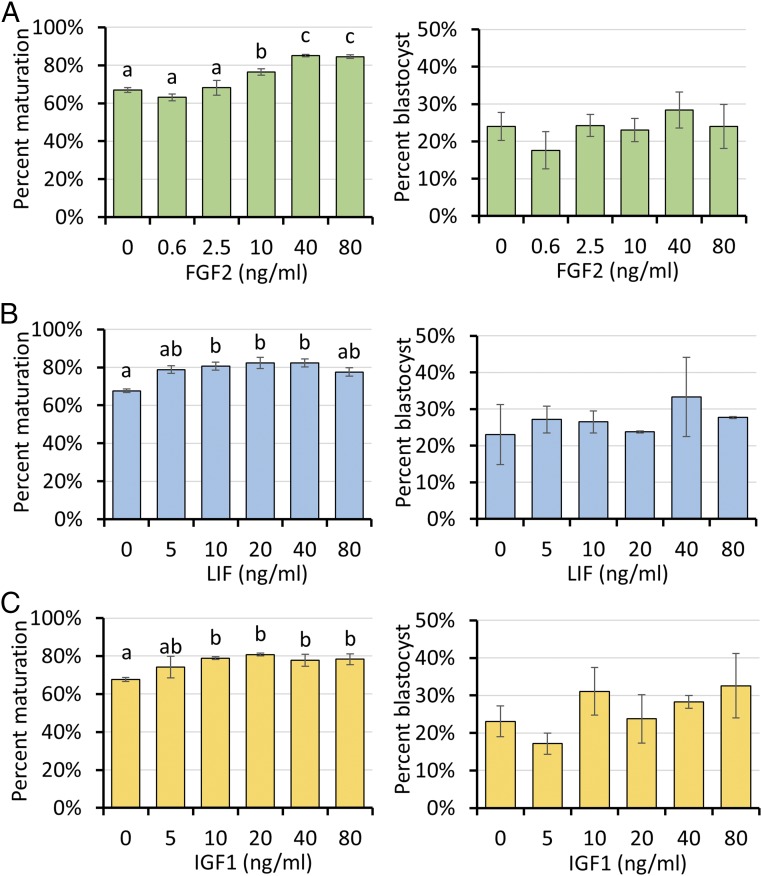
Initially, the individual effects of FGF2, LIF, and IGF1 (FLI) on maturation of oocytes derived from prepubertal gilts after commercial slaughter were examined after each had been added to an otherwise standard, chemically defined medium (41, 42). After 42 h, oocytes were in vitro fertilized and cultured for 6 d (41, 42). Whereas addition of FGF2 (40–80 ng/mL) (Fig. 1A), LIF (10–40 ng/mL) (Fig. 1B), and IGF1 (10–80 ng/mL) (Fig. 1C) each individually improved the efficiency of producing metaphase II oocytes, none of the cytokines improved oocyte developmental competence, as determined by the ability of the zygotes to form blastocysts after IVF.
Fig. 1.
Effects of various concentrations of FGF2 (A), LIF (B), and IGF1 (C) in porcine oocyte maturation medium on nuclear maturation and subsequent developmental competence. Data are reported as means ± SEM. Different superscripts (a and b) denote a significant difference from the control, P < 0.05. The experiments were replicated four times with a total of 3,554 oocytes.
Effects of FLI on Improving Oocyte Nuclear Maturation, IVF, and SCNT, Embryo Development to the Blastocyst Stage, and Litter Size After Embryo Transfer.
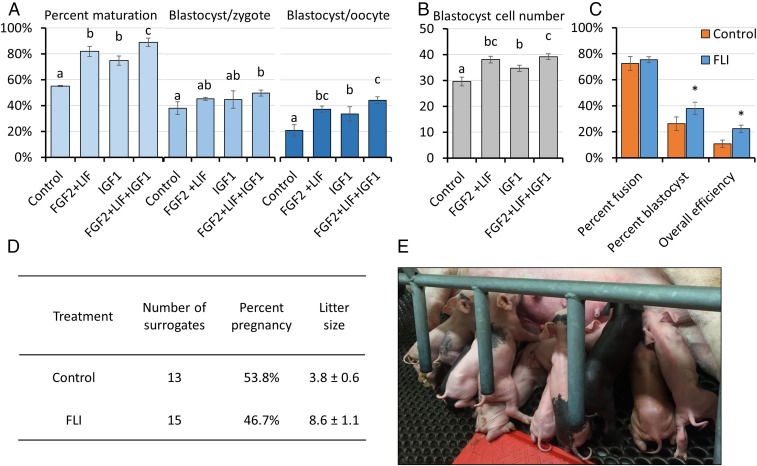
FGF2 and LIF at their optimal concentrations for nuclear maturation (40 ng/mL and 20 ng/mL, respectively) (Fig. 1 A and B) were added in combination to the IVM medium. This mix of two factors improved nuclear maturation beyond that achieved with the single factors but, more importantly, also increased the number of blastocyst-stage embryos (SI Appendix, Fig. S1). The effects of adding the third cytokine at its optimal concentration for promoting nuclear maturation (IGF1, 20 ng/mL) (Fig. 1C) along with the other two was then examined. This IVM medium, now supplemented with FGF2, LIF, and IGF1 in combination, increased oocyte nuclear maturation from 55% in controls to 89% in the experimental group (Fig. 2A). After IVF, the zygotes from FLI-treated oocytes advanced to the blastocyst stage more efficiently than controls (49.7% vs. 38%). Blastocyst cell number also increased, suggesting that embryo quality had likely improved, as well (Fig. 2B). Overall, the use of FLI medium during oocyte maturation, led to over a twofold increase in the number of blastocyst-stage embryos produced from the same number of retrieved oocytes (Fig. 2A). Oocytes matured in FLI medium also provided improved production of blastocysts following SCNT, a procedure that requires removal of nuclear material from the egg and subsequent fusion of the enucleated oocyte with a somatic donor cell (Fig. 2C). Finally, when blastocyst-stage embryos—in this case ones produced after injection of zygotes with CRISPR-Cas9 and guide RNAs targeted to a number of select genes, including CD163, CD1D, TMPRSS2, COL6A3, APC, and PAH—were transferred to surrogates, the number of piglets born was approximately doubled when the oocytes had first been matured in FLI medium (Fig. 2 D and E). Although no detailed measurements were made, there was no noticeable delay in blastocyst development after injection of these editing agents. The editing efficiency (method described in ref. 8) achieved in piglets born averaged 77% after oocyte maturation in control medium and 69% in FLI medium. Finally, none of these piglets showed obvious developmental abnormalities at birth. The combination of the twofold increase in blastocyst formation (assessed only in embryos that had not been gene-edited), and the doubling of litter size observed after zygotes had been injected with CRISPR-Cas9/guide RNA constructs suggests that the efficiency of producing piglets from oocytes matured in FLI medium can be effectively quadrupled relative to the more traditional form of oocyte maturation.
Fig. 2.
Supplementation of standard IVM medium with FLI improves porcine oocyte meiotic maturation and developmental competence. (A and B) Effects of FLI on nuclear maturation and subsequent blastocyst development following IVF. The experiments were replicated four times with a total of 796 oocytes. Different letters denote significant differences, P < 0.05. (C) Supplementation with FLI improves blastocyst development following SCNT. The experiments were replicated five times with a total of 488 oocytes. An asterisk (*) denotes a significant difference between control and treatment, P < 0.05. Percent fusion was calculated as fused embryos/enucleated oocytes; percent blastocyst was calculated as blastocysts/fused embryos; overall efficiency was calculated as blastocysts/enucleated oocytes. Embryo transfers were not performed in these experiments. (D) The in vivo developmental competence of the blastocysts produced from the FLI treated oocytes in terms of litter size after embryo transfer. Approximately 50 blastocysts that had been injected with CRISPR/Cas9 constructs at the zygote stage were transferred to surrogates on day 5 or 6. (E) Typical litter of 13 healthy piglets obtained after embryo transfer of blastocyst stage embryos derived from FLI-matured oocytes.
FLI-Matured COCs Display Distinctly Different Kinetics of MAPK Activation.
MAPK activation was measured in cumulus cells over the course of IVM. Cumulus cell samples from both control and FLI-treated COCs were collected at 0, 2, 6, 22, and 42 h after introduction of the COCs into the control and FLI IVM media. The concentrations of phosphorylated MAPK1/3 (pMAPK1/3) and total MAPK1/3 were measured by Western blotting to allow the fraction of pMAPK1/3 relative to total MAPK1/3 (pMAPK/MAPK) to be compared in the two groups at each time point. At 2 h after initiating IVM, the fraction of pMAPK was significantly reduced in the FLI-treated group compared with the control. Subsequently, relative pMAPK levels became elevated in the FLI-treated group, reaching a maximum at around 22 h. In contrast, the ratio of pMAPK to MAPK remained almost unchanged in the control over this period. However, by the end of the maturation period (42 h), the level of pMAPK in the FLI group had become very low, whereas it had continued to increase in the controls (Fig. 3 A and B). It is also important to note that the depletion of pMAPK at 42 h is only observed in the FLI medium and not when the factors are supplemented individually (Fig. 3C).
Fig. 3.
FLI regulation of MAPK1/3 activation in cumulus cells during IVM. (A) Representative images of Western blots showing the effects of FLI on MAPK1/3 activity in cumulus cells during IVM. (B) The relative expression of pMAPK/MAPK was quantified, and the ratios of the two forms compared between control and FLI treatments at the same time points. Each cumulus cell sample was pooled from 50 COCs. This experiment was replicated four times. Asterisks (*) denote a significant difference between control and treatment, P < 0.05. (C) Effects of FLI and each individual cytokine on MAPK1/3 activation in cumulus cells at 42 h after IVM as determined by Western blotting. (D) Effects of adding kinase inhibitors in FLI medium on oocyte meiotic maturation. The experiments were replicated four times with 1,208 oocytes. Different letters denote significant differences, P < 0.05.
Because the MAPK signaling pathway in the cumulus cells surrounding the oocyte is known to be essential for triggering oocyte maturation (22, 23), we hypothesized that the tightly regulated changes of MAPK1/3 activation in cumulus cells when all three cytokines were present together was the key as to why FLI promotes such a marked improvement in oocyte competence. Further evidence for this premise came from experiments in which oocytes were matured with different kinase inhibitors to block FGF2 (FGFR inhibitor, PD173074, 10 μM), LIF (JAK inhibitor I, 6 μM), and IGF1 (IGF1R inhibitor, OSI-906, 10 μM) signaling pathways individually and compared with data obtained when MAPK1/3 activity was directly inhibited (PD0325901, 10 μM). As anticipated, the MAPK inhibitor resulted in an almost complete block of meiotic maturation. However, more moderate declines were observed when the signaling pathways initiated by the individual cytokines were inhibited (Fig. 3D).
The Effect of FLI in IVM Medium on Cumulus Cell Expansion.
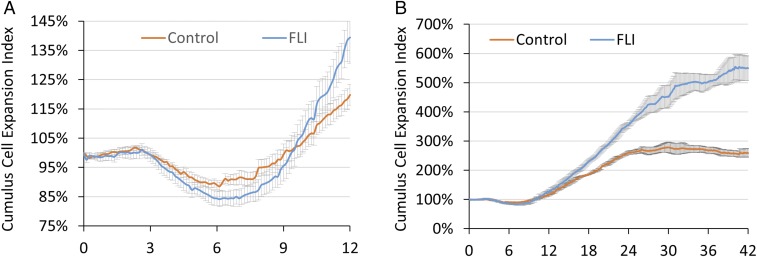
Cumulus cell expansion is generally considered to be an essential feature of oocyte maturation and is tightly regulated by signaling through the MAPK1/3 pathway (22). We adapted a live-imaging system (CytoSMART System; Lonza) to track COC expansion during IVM (Fig. 4 and Movies S1, control and S2, FLI). Surprisingly, COCs in both groups shrank in size between 3 and 6 h of culture (P < 0.05), a phenomenon that to our knowledge has never been previously reported (Fig. 4A). The decline during the initial stage of maturation, although quite small, was significantly greater in COCs cultured in FLI medium (Fig. 4A and SI Appendix, Table S1). After 6 h, this process of shrinkage stopped, and the COCs from both groups began an expansion phase. The expansion occurred more rapidly for COCs in FLI medium, such that by 22 h they had trebled in “field-of-view occupied” compared with the doubling observed in controls. After 22 h, the COCs in FLI medium continued their expansion and attained an over fivefold increase in apparent size by the end of IVM at 42 h IVM. In contrast, the median area by COCs in control medium reached its maximum by about 22 h and showed no increase thereafter (Fig. 4B).
Fig. 4.
Effects of FLI on cumulus cell expansion. (A) Cumulus cell expansion for the first 12 h of IVM. (B) Cumulus cell expansion occurring over the entire 42-h culture period of IVM. Images were taken every 7.5 min and recorded by the CytoSMART system. Size of COCs was estimated by dividing the total coverage of COCs provided by the system with the number of COCs within the images. Cumulus cell expansion index represents the relative size of COCs relative the median size of COCs at 0 h. The experiments were replicated six times for Fig. 3A, and three times for Fig. 3B.
The Effect of FLI in IVM Medium on Transzonal Projection Retraction.
Because MAPK activation is necessary to terminate gap-junctional communications between cumulus cells and oocytes through transzonal projection (TZPs) (43), the changes in MAPK activity shown in Fig. 3 prompted us to compare the relative numbers of intact TZPs in COCs matured in FLI vs. control medium. The COCs cultured in FLI medium possessed significantly more intact TZPs at 2 h than the controls (Fig. 5). Although numbers of TZPs in both groups subsequently declined, there were significantly more TZPs remaining intact in the control group at 22 and 42 h than in the FLI-cultured COCs (Fig. 5).
Fig. 5.
Effects of FLI on TZP integrity during IVM. (A) Quantification of TZPs during IVM. Number of intact TZPs was compared within each time point. These experiments were replicated three times, with a total of 142 COCs. Asterisks (*) denote a significant difference between control and treatment, P < 0.05. (B) Maximum-intensity projections of the equatorial cross-section of the oocytes stained for F-actin (rhodamine phalloidin) at different time points during IVM. (Scale bar, 20 μm.)
One consistent feature of the FLI-matured oocytes is a larger perivitelline space than observed in those matured in control medium. Such a gap between the zona pellucida and the exterior of the oocyte is nearly always observed in oocytes matured in vivo (SI Appendix, Fig. S2). We hypothesized that the expanded perivitelline space might be best explained by the more complete severance of TZPs in FLI-treated COCs relative to controls, thus allowing the oocyte to detach more completely from the zona pellucida, which encloses the oocyte and separates it from the innermost layer of cumulus cells. As shown in Fig. 5, the oocytes matured in FLI medium displayed slightly more intact TZPs at the time of maximal oocyte shrinkage than controls. At 6 h, no differences were observed, but by 22 and 42 h significantly more TZPs remained intact in the controls oocytes than in those undergoing maturation in FLI medium.
The Effect of FLI in IVM Medium on Cumulus Cell Gene Expression.
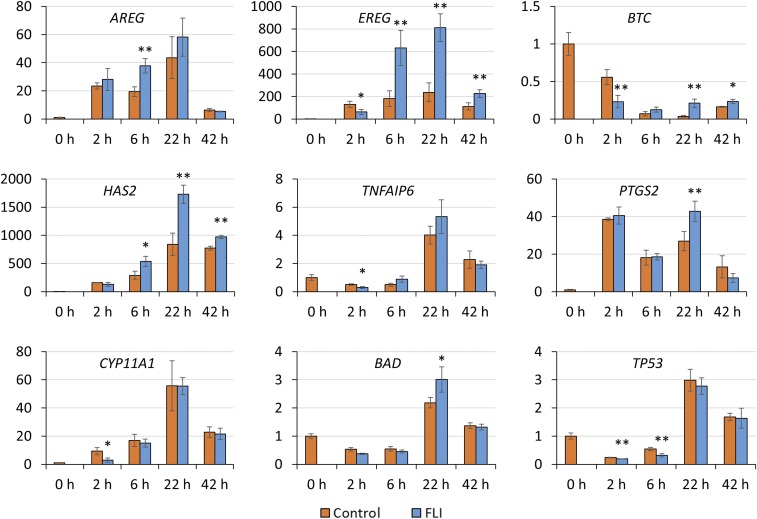
Finally, we analyzed whether the abundance of transcripts for certain genes implicated in oocyte maturation correlated with the changes in the pMAPK/MAPK ratio in cumulus cells. Genes for EGF-like factors (AREG, EREG, and BTC), so-called cumulus cell expansion factors (HAS2, TNFAIP6, and PTGS2), and stress-related genes (CYP11A1, BAD, and TP53) were analyzed throughout culture. At 2 h, transcripts for BTC, CYP11A1, and TP53 were down-regulated (P < 0.05) in cumulus cells from COCs cultured in FLI medium relative to controls. There was also a tendency for EREG and TNFAIP6 to be down-regulated (P < 0.1). These data may relate to the greater shrinkage of the FLI group in the initial phases of culture and that FLI might have provided improved protection against stress. The greater abundance of mRNA for AREG, EREG, and HAS2 at 6 h, and of EREG, BTC, HAS2, and PTGS2 at 22 h (P < 0.05) is consistent with the more rapid expansion of cumulus cells in FLI than in control medium (Fig. 6).
Fig. 6.
Effects of FLI on mRNA abundance of EGF-like factors (AREG, EREG, BTC), cumulus cell expansion factors (HAS2, TNFAIP6, PTGS2), and stress related genes (CYP11A1, BAD, TP53) during IVM. The relative abundance of mRNA was examined by quantitative PCR and compared between control and FLI cumulus cells at the same time point. The mRNA level for each gene was arbitrarily set to 1 for controls at 0 h. **P < 0.05, significant differences in mRNA abundance between control and treatment; *P < 0.1, a trend to be different. The experiments were replicated four times for the 0-, 2-, and 6-h time points, and three times for the 22- and 42-h time points.
Discussion
In this paper, we demonstrated that a combination of three cytokines, FGF2, LIF, and IGF1, enhances porcine oocyte maturation and embryo development to blastocyst stage, while effectively quadrupling the number of genetically engineered piglets born after embryo transfer.
The beneficial effects observed in this supplemented medium (Fig. 2) may be through a number of means. First, there is an early stage in the process, lasting no more than about 6 h following their introduction into maturation medium, when the COCs shrink rather than expand. It is unclear whether this phase in any way mimics occurrences in vivo. Instead, it may be a time when the COCs are adjusting to the stress of in vitro culture. Environmental factors can trigger premature MAPK1/3 activation (44, 45) and disrupt the normal time course of TZP disassembly, resulting in meiotic resumption and unsynchronized nuclear and cytoplasmic maturation (46–48). FLI may attenuate stress incurred at the early stage of IVM better than the control medium, as reflected in the lower level of pMAPK1/3 and the reduced transcriptional expression of genes encoding stress-related factors, “expansion factors,” and EGF-like factors in the cumulus cells of FLI-exposed cumulus cells. Possibly linked to these biochemical changes were more marked declines in the sizes of the COCs and a greater number of TZP connections. The better preservation of the TZPs during early-phase maturation would likely allow more trafficking of the metabolites and informational molecules essential to promote oocyte cytoplasmic maturation (49, 50).
The FLI medium provides a strikingly more rapid expansion of the COC over the subsequent 6–22 h of IVM than the control medium, and this is paralleled by an increase in transcripts for “expansion factors” and EGF-like factors. The kinetics of MAPK activation, specifically the rise in pMAPK1/3 during the 6- to 22-h period and its decline in the subsequent 20 h, phenomena not observed with COCs in the control medium, may also explain the success of FLI. The MAPK activation pattern is quite similar to that observed with the IVM of mouse COCs (51). The rapid rise in pMAPK in the cumulus cells during this period in FLI medium may reflect a more active metabolic state than in controls. This increase of pMAPK precedes a more complete breakdown of TZP connections after 22 h in the FLI-exposed COCs than in the controls. This severance of cytoplasmic connections between the cumulus cells and the oocyte would cut off the influx of inhibitory cyclic nucleotides and promote a permissive environment for meiosis to proceed. The fall in pMAPK during the final 22 h of culture on FLI medium was unexpected and difficult to explain. It could indicate a return of the cumulus cells to a more quiescent state as isolation of the oocyte from the enclosing somatic cells becomes more complete. Why a combination of the three cytokines is necessary to provide this pattern of MAPK activation is unclear, but it probably accounts for the dramatic improvement in oocyte maturation brought about by the FLI medium than by each factor alone. One difference, possibly critical, is that pMAPK1/3 in cumulus cells only becomes de-phosphorylated at 42 h when COCs are cultured with the three cytokines together (Fig. 3C). We speculate that COCs with higher concentrations of pMAPK1/3 at the end of IVM are less competent to be fertilized and to develop successfully than ones in which pMAPK1/3 remains quiescent. That a high concentration of pMAPK1/3 at the end of IVM is inhibitory to development is supported by the observation that the concentration of pMAPK1/3 in cumulus cells from bovine COCs after maturation in vitro is higher than in their fully competent in vivo counterparts (52).
As noted above, the beneficial effects of FLI relative to control medium may be achieved mainly through the timing of MAPK1/3 activation. Surprisingly, FLI medium did not cause an immediate and rapid increase of pMAPK1/3 in cumulus cells during the initial 6 h of IVM. Instead, there was an initial decline in relative pMAPK1/3 concentration, whereas maximal activation occurred between 6 and 22 h (Fig. 3). The increase during this intermediate stage of IVM may not, therefore, be so much a direct effect of FGF2, LIF, and IGF1, but instead be because of LH and its ability to up-regulate EGF-like growth factors, such as AREG, EREG, and BTC, which can also stimulate MAPK signaling (19, 53, 54) (Fig. 6). The decline in pMAPK1/3 observed after 22 h is also difficult to interpret, but may reflect desensitization following the earlier hyperstimulation of the pathway.
The effects of each individual cytokine on oocyte IVM have been relatively widely studied. Various FGFs have been reported to improve bovine and ovine oocyte IVM, but a functional linkage to events following fertilization is lacking (55–57). We speculate that FGF2 attenuates stress-induced cell damage, as observed for other cell types (58–60). LIF also may improve oocyte IVM in some species (61–63) through its ability to activate JAK-STAT3 and MAPK signaling pathways (61, 62). The former pathway is crucial for maintaining CD9 expression in mouse oocytes (61, 64). CD9 is a protein required for sperm binding to the oocyte (65, 66). The effects of IGF1 on IVM have been difficult to interpret because the media used have often been chemically undefined (e.g., containing follicular fluid or FBS) (67–70). We speculate that the beneficial effects we observed by using a chemically defined medium (Fig. 2) may be through activation of MAPK1/3 and PI3K-AKTsignaling pathways (71), plus a potential ability to increase expression of FGFR1, as has been observed in porcine granulosa cells (72). IGF1 may also cause increased production of the IL6 class of cytokines, such as LIF (73, 74). Therefore, each cytokine may exert different yet correlated roles in promoting oocyte maturation and development, but the precise basis of their synergistic action remains to be determined.
The significance of the FLI medium to the practical aspects of assisted reproductive technologies in swine should not be underestimated. FLI medium not only doubles the number of blastocyst-stage embryos that can be produced, it improves the success of somatic cell nuclear transfer and doubles the number of piglets born after genome modification by the CRISPR-Cas9 technology. Whether the three-cytokine combination will aid assisted reproductive technologies in species other than swine remains to be determined but is a topic that is being pursued.
Materials and Methods
Animal Care.
All experimental use of animals was approved by University of Missouri Animal Care and Use Committee under Protocol 7868.
Oocyte in Vitro Maturation, Fertilization, and Embryo Culture.
Ovaries from prepubertal gilts were collected from an abattoir (Farmland Foods Inc.). Prepubertal ovaries were confirmed by the absence of developed corpora lutea. Immature oocytes were aspirated from medium size (3–6 mm) follicles with an 18-gauge needle attached to a 10-mL syringe. Oocytes with several layers of unexpanded cumulus cells and evenly dark cytoplasm were selected for maturation. Around 50 COCs were placed in individual wells containing 500 μL of maturation medium (SI Appendix, Table S2) for 42–44 h at 38.5 °C in 5% CO2/humidified air. At the end of the maturation period, the cumulus cells adhering to the oocyte were removed by vortexing for 3 min in the presence of 0.1% (wt/vol) hyaluronidase. Matured oocytes were identified by the presence of a polar body and placed in 50-μL droplets of a Tris-buffered medium (75) in groups of 25–30 and coincubated with frozen-thawed sperm (0.25 × 106 cells/mL) for 5 h. After fertilization, the embryos were cultured in modified porcine zygote medium 3 (76), called MU1 (77), at 38.5 °C and 5% CO2, 5% O2, 90% N2. On day 6, blastocysts were stained with the nuclear stain Hoechst 33342 (0.01 mg/mL) to determine total cell number.
SCNT and Microinjection of CRISPR/Cas9 System into Zygotes and Embryo Transfer.
The SCNT and zygote microinjection were performed as previously described (8). Blastocyst-stage embryos generated by zygote injection were transferred into surrogates on day 5 or 6 after the first standing estrus. The embryos were surgically transferred into the ampullary–isthmic junction of the oviduct of the surrogate (8, 78).
Analysis of TZP.
Analysis of TZP was performed as previously described with minor modifications (49). COCs were fixed in 4% (vol/vol) paraformaldehyde in PBS for 30 min at room temperature. F-actin filaments were stained by rhodamine phalloidin (Cytoskeleton Inc.) to visualize the TZPs following the manufacturer’s instructions. COCs were then mounted with VECTASHIELD mounting medium with DAPI (Vector Laboratories) on coverslips, and images taken by using a Leica SP8 spectral confocal microscope. (research.missouri.edu/mcc//Leica%20SP8.html). Maximum-intensity projection of nine equatorial cross sections with the same depth (3 µm in total) of the COCs (as shown in Fig. 5B; scale bar, 20 μm) was used to measure the number of TZPs using ImageJ software.
COC Expansion Assessment.
CytoSMART live imaging system (Lonza) was used to track COC expansion during IVM. The instrument permitted the numbers of COCs and the overall area they occupied within a fixed field-of-view to be measured over time. Images were acquired automatically by the system every 7.5 min during the 42 h of IVM. A time-lapse video (Movies S1 and S2) and the overall coverage over time were obtained. The average size of the COCs at specific time points was estimated by dividing the overall coverage by the number of COCs in the field. A cumulus cell expansion index, representing the relative size of COCs at any time point relative the size of COCs at 0 h, was used to assess the degree of cumulus cell expansion.
Statistical Analyses.
Data were analyzed by using the software NCSS 2007. Percentage data were normalized by arcsin transformation. Differences were determined by either one-way ANOVA followed by Fisher’s least-significant difference multiple comparison test for experiments with multiple groups, or a Student t test for experiments with two treatment groups. For qPCR data, expression levels between treatments were determined by using the comparative threshold cycle (CT) method for each gene. The 2−ΔΔCT values were analyzed for normality. If not normally distributed, the data were log-transformed. The resulting values were then analyzed by using the Student t test. P < 0.05 values were considered significant. Data were reported as means ± SEM.
Supplementary Material
Acknowledgments
We thank Dr. Clifton Murphy and Josh Benne for their assistance with the embryo transfer surgeries; Jason Dowell and Melissa Samuel for all the animal care; Smithfield, Inc. of Milan, Missouri for providing the gilt ovaries for the experiments; and Dennis Reith for his editing and administrative assistance. This work was supported by NIH Grants R01HD069979 (to R.M.R.) and U42OD011140 (to R.S.P.) and the University of Missouri Food for the 21st Century Program (R.M.R. and R.S.P.).
Footnotes
Conflict of interest statement: Y.Y., L.D.S., R.S.P., and R.M.R. have a pending patent application “Medium Supplement to Increase the Efficiency of Oocyte maturation and Embryo Culture in vitro.”
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1703998114/-/DCSupplemental.
References
- 1.Yuan Y, Krisher RL. In vitro maturation (IVM) of porcine oocytes. Methods Mol Biol. 2012;825:183–198. doi: 10.1007/978-1-61779-436-0_14. [DOI] [PubMed] [Google Scholar]
- 2.Lonergan P, Fair T. Maturation of oocytes in vitro. Annu Rev Anim Biosci. 2016;4:255–268. doi: 10.1146/annurev-animal-022114-110822. [DOI] [PubMed] [Google Scholar]
- 3.Grupen CG. The evolution of porcine embryo in vitro production. Theriogenology. 2014;81:24–37. doi: 10.1016/j.theriogenology.2013.09.022. [DOI] [PubMed] [Google Scholar]
- 4.Seyhan A, et al. Severe early ovarian hyperstimulation syndrome following GnRH agonist trigger with the addition of 1500 IU hCG. Hum Reprod. 2013;28:2522–2528. doi: 10.1093/humrep/det124. [DOI] [PubMed] [Google Scholar]
- 5.Humaidan P, et al. Ovarian hyperstimulation syndrome: Review and new classification criteria for reporting in clinical trials. Hum Reprod. 2016;31:1997–2004. doi: 10.1093/humrep/dew149. [DOI] [PubMed] [Google Scholar]
- 6.Coticchio G, Dal-Canto M, Guglielmo MC, Mignini-Renzini M, Fadini R. Human oocyte maturation in vitro. Int J Dev Biol. 2012;56:909–918. doi: 10.1387/ijdb.120135gv. [DOI] [PubMed] [Google Scholar]
- 7.Prather RS. Pig genomics for biomedicine. Nat Biotechnol. 2013;31:122–124. doi: 10.1038/nbt.2490. [DOI] [PubMed] [Google Scholar]
- 8.Whitworth KM, et al. Use of the CRISPR/Cas9 system to produce genetically engineered pigs from in vitro-derived oocytes and embryos. Biol Reprod. 2014;91:78. doi: 10.1095/biolreprod.114.121723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang H, et al. One-step generation of mice carrying mutations in multiple genes by CRISPR/Cas-mediated genome engineering. Cell. 2013;153:910–918. doi: 10.1016/j.cell.2013.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gilchrist RB, Lane M, Thompson JG. Oocyte-secreted factors: Regulators of cumulus cell function and oocyte quality. Hum Reprod Update. 2008;14:159–177. doi: 10.1093/humupd/dmm040. [DOI] [PubMed] [Google Scholar]
- 11.Hyttel P, Fair T, Callesen H, Greve T. Oocyte growth, capacitation and final maturation in cattle. Theriogenology. 1997;47:23–32. [Google Scholar]
- 12.Sánchez F, Smitz J. Molecular control of oogenesis. Biochim Biophys Acta. 2012;1822:1896–1912. doi: 10.1016/j.bbadis.2012.05.013. [DOI] [PubMed] [Google Scholar]
- 13.Mehlmann LM, et al. The Gs-linked receptor GPR3 maintains meiotic arrest in mammalian oocytes. Science. 2004;306:1947–1950. doi: 10.1126/science.1103974. [DOI] [PubMed] [Google Scholar]
- 14.Horner K, et al. Rodent oocytes express an active adenylyl cyclase required for meiotic arrest. Dev Biol. 2003;258:385–396. doi: 10.1016/s0012-1606(03)00134-9. [DOI] [PubMed] [Google Scholar]
- 15.Norris RP, et al. Cyclic GMP from the surrounding somatic cells regulates cyclic AMP and meiosis in the mouse oocyte. Development. 2009;136:1869–1878. doi: 10.1242/dev.035238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Conti M, Hsieh M, Zamah AM, Oh JS. Novel signaling mechanisms in the ovary during oocyte maturation and ovulation. Mol Cell Endocrinol. 2012;356:65–73. doi: 10.1016/j.mce.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vaccari S, Weeks JL, 2nd, Hsieh M, Menniti FS, Conti M. Cyclic GMP signaling is involved in the luteinizing hormone-dependent meiotic maturation of mouse oocytes. Biol Reprod. 2009;81:595–604. doi: 10.1095/biolreprod.109.077768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sela-Abramovich S, Edry I, Galiani D, Nevo N, Dekel N. Disruption of gap junctional communication within the ovarian follicle induces oocyte maturation. Endocrinology. 2006;147:2280–2286. doi: 10.1210/en.2005-1011. [DOI] [PubMed] [Google Scholar]
- 19.Park JY, et al. EGF-like growth factors as mediators of LH action in the ovulatory follicle. Science. 2004;303:682–684. doi: 10.1126/science.1092463. [DOI] [PubMed] [Google Scholar]
- 20.Hsieh M, et al. Luteinizing hormone-dependent activation of the epidermal growth factor network is essential for ovulation. Mol Cell Biol. 2007;27:1914–1924. doi: 10.1128/MCB.01919-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shimada M, Hernandez-Gonzalez I, Gonzalez-Robayna I, Richards JS. Paracrine and autocrine regulation of epidermal growth factor-like factors in cumulus oocyte complexes and granulosa cells: Key roles for prostaglandin synthase 2 and progesterone receptor. Mol Endocrinol. 2006;20:1352–1365. doi: 10.1210/me.2005-0504. [DOI] [PubMed] [Google Scholar]
- 22.Su YQ, et al. Oocyte-dependent activation of mitogen-activated protein kinase (ERK1/2) in cumulus cells is required for the maturation of the mouse oocyte-cumulus cell complex. Dev Biol. 2003;263:126–138. doi: 10.1016/s0012-1606(03)00437-8. [DOI] [PubMed] [Google Scholar]
- 23.Fan HY, et al. MAPK3/1 (ERK1/2) in ovarian granulosa cells are essential for female fertility. Science. 2009;324:938–941. doi: 10.1126/science.1171396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eppig JJ, Wigglesworth K, Pendola F, Hirao Y. Murine oocytes suppress expression of luteinizing hormone receptor messenger ribonucleic acid by granulosa cells. Biol Reprod. 1997;56:976–984. doi: 10.1095/biolreprod56.4.976. [DOI] [PubMed] [Google Scholar]
- 25.Shimada M, Nishibori M, Isobe N, Kawano N, Terada T. Luteinizing hormone receptor formation in cumulus cells surrounding porcine oocytes and its role during meiotic maturation of porcine oocytes. Biol Reprod. 2003;68:1142–1149. doi: 10.1095/biolreprod.102.010082. [DOI] [PubMed] [Google Scholar]
- 26.Yamashita Y, et al. Positive feedback loop between prostaglandin E2 and EGF-like factors is essential for sustainable activation of MAPK3/1 in cumulus cells during in vitro maturation of porcine cumulus oocyte complexes. Biol Reprod. 2011;85:1073–1082. doi: 10.1095/biolreprod.110.090092. [DOI] [PubMed] [Google Scholar]
- 27.Prochazka R, Kalab P, Nagyova E. Epidermal growth factor-receptor tyrosine kinase activity regulates expansion of porcine oocyte-cumulus cell complexes in vitro. Biol Reprod. 2003;68:797–803. doi: 10.1095/biolreprod.102.005520. [DOI] [PubMed] [Google Scholar]
- 28.Kawashima I, et al. Sequential exposure of porcine cumulus cells to FSH and/or LH is critical for appropriate expression of steroidogenic and ovulation-related genes that impact oocyte maturation in vivo and in vitro. Reproduction. 2008;136:9–21. doi: 10.1530/REP-08-0074. [DOI] [PubMed] [Google Scholar]
- 29.Wrenzycki C, Stinshoff H. Maturation environment and impact on subsequent developmental competence of bovine oocytes. Reprod Domest Anim. 2013;48(Suppl 1):38–43. doi: 10.1111/rda.12204. [DOI] [PubMed] [Google Scholar]
- 30.Wigglesworth K, et al. Bidirectional communication between oocytes and ovarian follicular somatic cells is required for meiotic arrest of mammalian oocytes. Proc Natl Acad Sci USA. 2013;110:E3723–E3729. doi: 10.1073/pnas.1314829110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Downs SM, Coleman DL, Ward-Bailey PF, Eppig JJ. Hypoxanthine is the principal inhibitor of murine oocyte maturation in a low molecular weight fraction of porcine follicular fluid. Proc Natl Acad Sci USA. 1985;82:454–458. doi: 10.1073/pnas.82.2.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eppig JJ, Ward-Bailey PF, Coleman DL. Hypoxanthine and adenosine in murine ovarian follicular fluid: Concentrations and activity in maintaining oocyte meiotic arrest. Biol Reprod. 1985;33:1041–1049. doi: 10.1095/biolreprod33.5.1041. [DOI] [PubMed] [Google Scholar]
- 33.Vatzias G, Hagen DR. Effects of porcine follicular fluid and oviduct-conditioned media on maturation and fertilization of porcine oocytes in vitro. Biol Reprod. 1999;60:42–48. doi: 10.1095/biolreprod60.1.42. [DOI] [PubMed] [Google Scholar]
- 34.Ducolomb Y, et al. Effect of porcine follicular fluid proteins and peptides on oocyte maturation and their subsequent effect on in vitro fertilization. Theriogenology. 2013;79:896–904. doi: 10.1016/j.theriogenology.2013.01.024. [DOI] [PubMed] [Google Scholar]
- 35.Dell’Aquila ME, et al. Effects of follicular fluid supplementation of in-vitro maturation medium on the fertilization and development of equine oocytes after in-vitro fertilization or intracytoplasmic sperm injection. Hum Reprod. 1997;12:2766–2772. doi: 10.1093/humrep/12.12.2766. [DOI] [PubMed] [Google Scholar]
- 36.Kim K, Mitsumizo N, Fujita K, Utsumi K. The effects of follicular fluid on in vitro maturation, oocyte fertilization and the development of bovine embryos. Theriogenology. 1996;45:787–799. doi: 10.1016/0093-691x(96)00008-8. [DOI] [PubMed] [Google Scholar]
- 37.Malamitsi-Puchner A, et al. In vitro fertilization: Angiogenic, proliferative, and apoptotic factors in the follicular fluid. Ann N Y Acad Sci. 2003;997:124–128. doi: 10.1196/annals.1290.043. [DOI] [PubMed] [Google Scholar]
- 38.Arici A, et al. Leukaemia inhibitory factor expression in human follicular fluid and ovarian cells. Hum Reprod. 1997;12:1233–1239. doi: 10.1093/humrep/12.6.1233. [DOI] [PubMed] [Google Scholar]
- 39.Nilsson EE, Skinner MK. Kit ligand and basic fibroblast growth factor interactions in the induction of ovarian primordial to primary follicle transition. Mol Cell Endocrinol. 2004;214:19–25. doi: 10.1016/j.mce.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 40.Nilsson EE, Kezele P, Skinner MK. Leukemia inhibitory factor (LIF) promotes the primordial to primary follicle transition in rat ovaries. Mol Cell Endocrinol. 2002;188:65–73. doi: 10.1016/s0303-7207(01)00746-8. [DOI] [PubMed] [Google Scholar]
- 41.Zhang X, et al. Porcine oocytes denuded before maturation can develop to the blastocyst stage if provided a cumulous cell-derived coculture system. J Anim Sci. 2010;88:2604–2610. doi: 10.2527/jas.2009-2714. [DOI] [PubMed] [Google Scholar]
- 42.Spate LD, Brown A, Redel BK, Whitworth KM, Prather RS. PS48 can replace bovine serum albumin in pig embryo culture medium, and improve in vitro embryo development by phosphorylating AKT. Mol Reprod Dev. 2015;82:315–320. doi: 10.1002/mrd.22474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sela-Abramovich S, Chorev E, Galiani D, Dekel N. Mitogen-activated protein kinase mediates luteinizing hormone-induced breakdown of communication and oocyte maturation in rat ovarian follicles. Endocrinology. 2005;146:1236–1244. doi: 10.1210/en.2004-1006. [DOI] [PubMed] [Google Scholar]
- 44.Kyriakis JM, Avruch J. Mammalian mitogen-activated protein kinase signal transduction pathways activated by stress and inflammation. Physiol Rev. 2001;81:807–869. doi: 10.1152/physrev.2001.81.2.807. [DOI] [PubMed] [Google Scholar]
- 45.Kyriakis JM, Avruch J. Mammalian MAPK signal transduction pathways activated by stress and inflammation: A 10-year update. Physiol Rev. 2012;92:689–737. doi: 10.1152/physrev.00028.2011. [DOI] [PubMed] [Google Scholar]
- 46.Pandey AN, Chaube SK. A moderate increase of hydrogen peroxide level is beneficial for spontaneous resumption of meiosis from diplotene arrest in rat oocytes cultured in vitro. Biores Open Access. 2014;3:183–191. doi: 10.1089/biores.2014.0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.LaRosa C, Downs SM. Stress stimulates AMP-activated protein kinase and meiotic resumption in mouse oocytes. Biol Reprod. 2006;74:585–592. doi: 10.1095/biolreprod.105.046524. [DOI] [PubMed] [Google Scholar]
- 48.LaRosa C, Downs SM. Meiotic induction by heat stress in mouse oocytes: Involvement of AMP-activated protein kinase and MAPK family members. Biol Reprod. 2007;76:476–486. doi: 10.1095/biolreprod.106.057422. [DOI] [PubMed] [Google Scholar]
- 49.Macaulay AD, et al. Cumulus cell transcripts transit to the bovine oocyte in preparation for maturation. Biol Reprod. 2016;94:16. doi: 10.1095/biolreprod.114.127571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Suzuki H, Jeong BS, Yang X. Dynamic changes of cumulus-oocyte cell communication during in vitro maturation of porcine oocytes. Biol Reprod. 2000;63:723–729. doi: 10.1095/biolreprod63.3.723. [DOI] [PubMed] [Google Scholar]
- 51.Su YQ, Wigglesworth K, Pendola FL, O’Brien MJ, Eppig JJ. Mitogen-activated protein kinase activity in cumulus cells is essential for gonadotropin-induced oocyte meiotic resumption and cumulus expansion in the mouse. Endocrinology. 2002;143:2221–2232. doi: 10.1210/endo.143.6.8845. [DOI] [PubMed] [Google Scholar]
- 52.Salhab M, et al. In vitro maturation of oocytes alters gene expression and signaling pathways in bovine cumulus cells. Mol Reprod Dev. 2013;80:166–182. doi: 10.1002/mrd.22148. [DOI] [PubMed] [Google Scholar]
- 53.Ashkenazi H, et al. Epidermal growth factor family members: Endogenous mediators of the ovulatory response. Endocrinology. 2005;146:77–84. doi: 10.1210/en.2004-0588. [DOI] [PubMed] [Google Scholar]
- 54.Sekiguchi T, et al. Expression of epiregulin and amphiregulin in the rat ovary. J Mol Endocrinol. 2004;33:281–291. doi: 10.1677/jme.0.0330281. [DOI] [PubMed] [Google Scholar]
- 55.Zhang K, Hansen PJ, Ealy AD. Fibroblast growth factor 10 enhances bovine oocyte maturation and developmental competence in vitro. Reproduction. 2010;140:815–826. doi: 10.1530/REP-10-0190. [DOI] [PubMed] [Google Scholar]
- 56.Zhang K, Ealy AD. Supplementing fibroblast growth factor 2 during bovine oocyte in vitro maturation promotes subsequent embryonic development. Open J Anim Sci. 2012;2:119–126. doi: 10.1016/j.domaniend.2011.12.006. [DOI] [PubMed] [Google Scholar]
- 57.Mondal S, Mor A, Reddy IJ, Nandi S, Parameswaragupta PS. Effect of fibroblast growth factor 2 (FGF2) and insulin transferrin selenium (ITS) on in vitro maturation, fertilization and embryo development in sheep. Braz Arch Biol Technol. 2015;58:521–525. [Google Scholar]
- 58.Upadhyay D, Bundesmann M, Panduri V, Correa-Meyer E, Kamp DW. Fibroblast growth factor-10 attenuates H2O2-induced alveolar epithelial cell DNA damage: Role of MAPK activation and DNA repair. Am J Respir Cell Mol Biol. 2004;31:107–113. doi: 10.1165/rcmb.2003-0064OC. [DOI] [PubMed] [Google Scholar]
- 59.Planavila A, et al. Fibroblast growth factor 21 protects the heart from oxidative stress. Cardiovasc Res. 2015;106:19–31. doi: 10.1093/cvr/cvu263. [DOI] [PubMed] [Google Scholar]
- 60.Mark RJ, Keller JN, Kruman I, Mattson MP. Basic FGF attenuates amyloid beta-peptide-induced oxidative stress, mitochondrial dysfunction, and impairment of Na+/K+-ATPase activity in hippocampal neurons. Brain Res. 1997;756:205–214. doi: 10.1016/s0006-8993(97)00196-0. [DOI] [PubMed] [Google Scholar]
- 61.Mo X, et al. Leukemia inhibitory factor enhances bovine oocyte maturation and early embryo development. Mol Reprod Dev. 2014;81:608–618. doi: 10.1002/mrd.22327. [DOI] [PubMed] [Google Scholar]
- 62.Dang-Nguyen TQ, et al. Leukemia inhibitory factor promotes porcine oocyte maturation and is accompanied by activation of signal transducer and activator of transcription 3. Mol Reprod Dev. 2014;81:230–239. doi: 10.1002/mrd.22289. [DOI] [PubMed] [Google Scholar]
- 63.De Matos DG, et al. Leukemia inhibitory factor induces cumulus expansion in immature human and mouse oocytes and improves mouse two-cell rate and delivery rates when it is present during mouse in vitro oocyte maturation. Fertil Steril. 2008;90:2367–2375. doi: 10.1016/j.fertnstert.2007.10.061. [DOI] [PubMed] [Google Scholar]
- 64.Oka M, et al. CD9 is associated with leukemia inhibitory factor-mediated maintenance of embryonic stem cells. Mol Biol Cell. 2002;13:1274–1281. doi: 10.1091/mbc.02-01-0600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Le Naour F, Rubinstein E, Jasmin C, Prenant M, Boucheix C. Severely reduced female fertility in CD9-deficient mice. Science. 2000;287:319–321. doi: 10.1126/science.287.5451.319. [DOI] [PubMed] [Google Scholar]
- 66.Miyado K, et al. Requirement of CD9 on the egg plasma membrane for fertilization. Science. 2000;287:321–324. doi: 10.1126/science.287.5451.321. [DOI] [PubMed] [Google Scholar]
- 67.Grupen CG, Nagashima H, Nottle MB. Role of epidermal growth factor and insulin-like growth factor-I on porcine oocyte maturation and embryonic development in vitro. Reprod Fertil Dev. 1997;9:571–575. doi: 10.1071/r96115. [DOI] [PubMed] [Google Scholar]
- 68.Kiapekou E, et al. Effects of GH and IGF-I on the in vitro maturation of mouse oocytes. Hormones (Athens) 2005;4:155–160. doi: 10.14310/horm.2002.11153. [DOI] [PubMed] [Google Scholar]
- 69.Quetglas MD, Coelho LA, Garcia JM, Oliveira Filho EB, Esper CR. Effect of insulin-like growth factor-1 during in vitro oocyte maturation and in vitro culture of bovine embryos. Arq Bras Med Vet Zootec. 2001;53:207–211. [Google Scholar]
- 70.Feng P, Catt KJ, Knecht M. Transforming growth factor-beta stimulates meiotic maturation of the rat oocyte. Endocrinology. 1988;122:181–186. doi: 10.1210/endo-122-1-181. [DOI] [PubMed] [Google Scholar]
- 71.Siddle K. Signalling by insulin and IGF receptors: Supporting acts and new players. J Mol Endocrinol. 2011;47:R1–R10. doi: 10.1530/JME-11-0022. [DOI] [PubMed] [Google Scholar]
- 72.Evans JR, Schreiber NB, Williams JA, Spicer LJ. Effects of fibroblast growth factor 9 on steroidogenesis and control of FGFR2IIIc mRNA in porcine granulosa cells. J Anim Sci. 2014;92:511–519. doi: 10.2527/jas.2013-6989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kooijman R, Coppens A, Hooghe-Peters E. IGF-I stimulates IL-8 production in the promyelocytic cell line HL-60 through activation of extracellular signal-regulated protein kinase. Cell Signal. 2003;15:1091–1098. doi: 10.1016/s0898-6568(03)00069-x. [DOI] [PubMed] [Google Scholar]
- 74.Tu W, Cheung PT, Lau YL. IGF-I increases interferon-gamma and IL-6 mRNA expression and protein production in neonatal mononuclear cells. Pediatr Res. 1999;46:748–754. doi: 10.1203/00006450-199912000-00019. [DOI] [PubMed] [Google Scholar]
- 75.Abeydeera LR, Day BN. Fertilization and subsequent development in vitro of pig oocytes inseminated in a modified tris-buffered medium with frozen-thawed ejaculated spermatozoa. Biol Reprod. 1997;57:729–734. doi: 10.1095/biolreprod57.4.729. [DOI] [PubMed] [Google Scholar]
- 76.Yoshioka K, Suzuki C, Tanaka A, Anas IM, Iwamura S. Birth of piglets derived from porcine zygotes cultured in a chemically defined medium. Biol Reprod. 2002;66:112–119. doi: 10.1095/biolreprod66.1.112. [DOI] [PubMed] [Google Scholar]
- 77.Redel BK, Tessanne KJ, Spate LD, Murphy CN, Prather RS. Arginine increases development of in vitro-produced porcine embryos and affects the protein arginine methyltransferase-dimethylarginine dimethylaminohydrolase-nitric oxide axis. Reprod Fertil Dev. 2015;27:655–666. doi: 10.1071/RD14293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lee K, et al. Piglets produced from cloned blastocysts cultured in vitro with GM-CSF. Mol Reprod Dev. 2013;80:145–154. doi: 10.1002/mrd.22143. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.