Significance
Starch granules of Solanum jamesii extracted from ground stone tools establish wild potato use as early as 10,900–10,100 calendar years B.P. in southern Utah. This discovery is the earliest documented use of potatoes in North America, an important energy source that has been largely undervalued or even ignored when diet breadth analyses and optimal foraging theory have been applied in archaeological studies. Younger deposits also contained tools with S. jamesii granules, indicating at least 4,000 years of intermittent use. Ethnographic and historical accounts from the region extend the period of use to more than 10,000 years. Given this long prehistory and history, the question arises as to whether some S. jamesii populations could have undergone transport, cultivation, and eventual domestication over such a long period of time.
Keywords: potato, Solanum jamesii, starch granule analysis, tuber use, Colorado Plateau
Abstract
The prehistory of wild potato use, leading to its domestication and diversification, has been well-documented in, and confined to, South America. At least 20 tuber-bearing, wild species of Solanum are known from North and Central America, yet their importance in ancient diets has never been assessed from the archaeological record. Here, we report the earliest evidence of wild potato use in North America at 10,900–10,100 calendar years (cal) B.P. in the form of well-preserved starch granules extracted from ground stone tools at North Creek Shelter, southern Utah. These granules have been identified as those of Solanum jamesii Torr. (Four Corners potato), a tuber-bearing species native to the American Southwest. Identification was based on applying five strictly defined diagnostic characteristics (eccentric hilum, longitudinal fissure, lack of fissure branching, fissure ratio, and maximum granule size) to each of 323 archaeological granules. Of those, nine were definitively assigned to S. jamesii based on possession of all characteristics, and another 61 were either likely or possibly S. jamesii depending on the number of characteristics they possessed. The oldest granules were found in substratum 4k (10,900–10,100 cal B.P.). Younger deposits, dating to ∼6,900 cal B.P., also contained tools with S. jamesii granules, indicating at least 4,000 y of intermittent use. Ethnographic and historical accounts extend the period of use to more than 10,000 y. The question then arises as to whether some S. jamesii populations could have undergone transport, cultivation, and eventual domestication over such a long period of time.
The discovery of starch granules persisting on ground stone artifacts has established early dates for the dietary use of many wild plant species, including 32,600 calendar years (cal) B.P. for oat (Avena) in southern Italy (1); 30,000 cal B.P. for cattail (Typha) in central Italy and the Czech Republic (2); 23,000 cal B.P. for barley (Hordeum) and wheat (Triticum) in Israel (3); and 23,000–19,500 cal B.P. for grasses (Triticeae and Paniceae), beans (Vigna), yams (Dioscorea), and snakegourd roots (Trichosanthes) in China (4). These dates are significant because they are often used to establish the ancestry of domesticated plants that eventually diverged from wild relatives (5–8).
In the case of tuber-forming species of Solanum, archaeological tubers found in Casma Valley, Peru, have been directly dated to 7,800 cal B.P. (9, 10). The tubers contained starch granules resembling those of the domesticated Solanum tuberosum but may have still represented a wild species (10). Intensive exploitation, perhaps of domesticated strains, took place between 3,400 and 1,600 cal B.P. in the western Titicaca Basin (11).
Herein, we report on starch granules from the wild Four Corners potato, Solanum jamesii (Fig. 1), found on ground stone tools in deposits dating between 10,900 and 10,100 cal B.P. at North Creek Shelter (NCS) near Escalante, Utah. This discovery is the earliest documented use of potatoes in North America, an important energy source that has been largely undervalued or even ignored when diet breadth analyses and optimal foraging theory are applied in archaeological studies (12, 13).
Fig. 1.
S. jamesii (Left), tubers produced by a single individual after one growing season (Center), and a tuber at the end of a stolon (Right).
Study Site: Archaeological Context
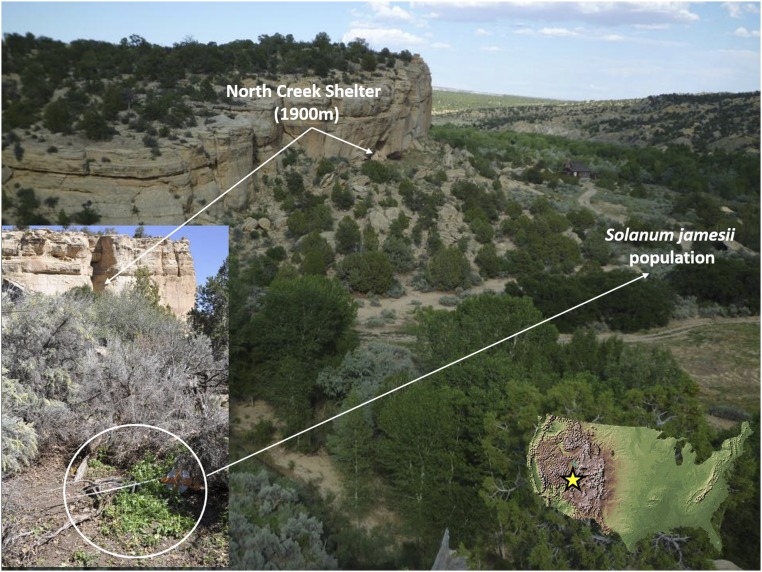
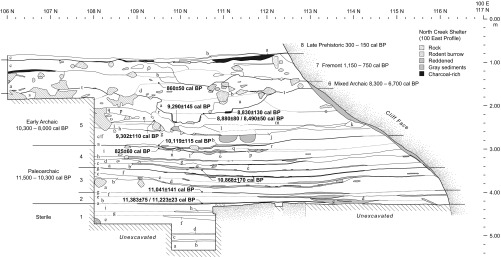
NCS is a deeply stratified site at the base of a south-facing sandstone cliff at an elevation of 1,900 m (37°46.200′ N, 111°40.812′ W) (Fig. 2). Excavations of NCS occurred between 2004 and 2008, uncovering numerous hearths and pits and abundant stone tools, faunal assemblages, and botanical remains (14). The excavations revealed seven cultural strata, dating from 11,500 to 150 cal B.P. Thirty-five radiocarbon dates were obtained on various materials (bone/collagen, dentin, wood/charcoal, fruits/seeds; Table S1), allowing strata to be further subdivided into 68 substrata, some interpreted as short-term living/use surfaces (14) (Fig. S1 and Table S1).
Fig. 2.
Location of NCS in southern Utah and nearby population of S. jamesii (Inset).
Table S1.
Radiocarbon dates for North Creek Shelter (after ref. 14)
| Sample no. | Substratum | Material | Conventional age 14C yrs B.P. | Calibrated yrs B.P. (± 2σ) |
| Beta-197358 | 7a | Zea mays | 940 ± 40 | 760–940 |
| AA78631 | 7c | Dentin | 902 ± 40 | 736–916 |
| Beta 195226 | 4i | Atriplex | 890 ± 40 | 710–920 |
| AA89634 | 7c | Collagen | 196 ± 38 | 136–225 |
| Beta-221411 | 7b | Zea mays | 1,050 ± 40 | 920–1,050 |
| Beta 261678 | 6c | Zea mays | 1,030 ± 40 | 910–1,050 |
| Beta-26176 | 7a | Zea mays | 1,130 ± 40 | 950–1,160 |
| Beta-261677 | 6d | Zea mays | 1,130 ± 40 | 950–1,160 |
| Beta-261678 | 6c | Zea mays | 1,030 ± 40 | 910–1,050 |
| Beta-221414 | 6d | Juniperus | 6,020 ± 60 | 6,710–7,000 |
| AA89632 | 6d | Dentin | 7,526 ± 70 | 8,183–8,429 |
| Beta-221412 | 5u | Acer, Pseudotsuga, Pinus | 7,670 ± 80 | 8,350–8,600 |
| Beta-239024 | 5u | Juniperus | 7,700 ± 50 | 8,400–8,590 |
| PRI-07–102-4364 | 5u | Juniperus | 7,990 ± 30 | 8,720–9,000 |
| Beta 207167 | 5t | Juniperus | 7,970 ± 80 | 8,590–9,030 |
| Beta 210253 | 5t | Juniperus/Pinus | 8,320 ± 120 | 9,010–9,530 |
| Beta 197359 | 5q | Pinus | 8,310 ± 70 | 9,100–9,490 |
| Beta 239023 | 5h | Juniperus | 8,310 ± 40 | 9,140–9,170 |
| AA89637 | 5a | bone collagen | 8,816 ± 78 | 9,612–10,171 |
| PRI-07–102-4029 | 5h | Juniperus | 8,860 ± 25 | 9,860–10,250 |
| Beta 194030 | 5c | Pinus | 9,020 ± 70 | 10,120–10,250 |
| AA89640 | 4a | bone collagen | 9,490 ± 92 | 10,546–11,132 |
| AA89642 | 3f | O. hemionus | 9,642 ± 84 | 10,741–11,208 |
| AA89641 | 3e | bone collagen | 9,237 ± 83 | 10,234–10,589 |
| AA89637 | 3e | Dentin | 9,406 ± 96 | 10,372–11,081 |
| AA89638 | 3e | coll/dent | 9,384 ± 91 | 10,290–10,807 |
| AA89635 | 1i | coll/dent | 9,410 ± 82 | 10,405–10,872 |
| AA89633 | 3b | Collagen | 9,556 ± 84 | 10,657–11,173 |
| Beta 207168 | 4a | Pinus | 9,510 ± 80 | 10,560–11,140 |
| Beta 221415 | 3a | Juniperus/Pinus | 9,690 ± 60 | 11,060–11,200 |
| AA89643 | 2g | O. hemionus bone collagen | 9,733 ± 84 | 10,774–11,272 |
| AA89639 | 1i | Dentin | 9,736 ± 95 | 10,760–11,316 |
| AA89645 | 2e | O. hemionus bone collagen | 9,739 ± 81 | 10,782–11,288 |
| Beta 239022 | 2a | Salicaceae | 9,800 ± 50 | 11,170–11,260 |
| PRI-07–102-3716 | 2a | Salicaceae | 9,960 ± 30 | 11,260–11,420 |
Fig. S1.
Stratigraphic profile of NCS. The strata of interest in this study are 4, 5, and 6, dating between 10,900 and 6,700 cal BP. Adapted from ref. 14.
Among the archaeological materials were 196 ground stone tools (manos and metates, hand and milling stones, respectively) varying in abundance from Paleoarchaic to Late Prehistoric times (10,900–150 cal B.P.) (14). The majority had been manufactured from sandstone (88%) and most (85%) were fragmented (Table S2). Manos were bun-shaped, one- or two-sided, and tended to be complete. The seven complete metates were slabs up to 46 cm in length with one or two smoothly ground basin surfaces (Fig. 3). The ages of these artifacts were established by radiocarbon dating of associated materials from the same substratum. The greatest number of tools occurred in substrata 5t and 5u (9,300–8,500 cal B.P.), suggesting a technological and dietary shift toward intensive use of plant resources that has been observed at about this time in many other locations across arid western North America (15–18).
Table S2.
Description and measurements of the 24 GS tools that yielded starch granules with eccentric hila
| Substratum | GS sample (FS no.) | GS type | Raw material | Complete/ fragmented | Weight, g | Thick, cm | Length, cm | Width, cm | Description |
| 6d | 1316 | Metate | S | F | 1,389.2 | 5.65 | 17 | 13.26 | Two-sided slab metate |
| 1719.1 | Metate | S | F | 258.5 | 1.75 | 13.57 | 10.45 | One-sided slab metate | |
| 1719.2 | Metate | S | F | 254.2 | 2.68 | 12.72 | 8.18 | One-sided slab metate | |
| 1759 | Mano | S | C | 765.5 | 6.16 | 10.15 | 8.85 | One-sided bun-shaped mano | |
| 6a | 505 | Mano | S | C | 221.7 | 4.03 | 7.39 | 6.12 | Two-sided bun-shaped mano |
| 548 | Mano | S | C | 878.9 | 6.12 | 10.21 | 9.81 | One-sided bun-shaped mano | |
| 603 | Metate | S | F | 1,275.8 | 4.42 | 17.4 | 13.53 | One-sided slab metate | |
| 3489 | Mano | S | F | 371.9 | 4.46 | 10.81 | 6.41 | Two-sided bun-shaped mano | |
| 5u | 2346 | Metate | S | F | 4,536 | 4.02 | 33 | 20.2 | Two-sided slab with basin metate |
| 2347 | Mano | S | C | 793.8 | 5.12 | 10.34 | 9.42 | One-sided bun-shaped mano | |
| 2667.1 | Metate | S | F | 1,938 | 1.87 | 34 | 33.2 | One-sided slab metate | |
| 2672 | Metate | S | F | 1,105.7 | 4.45 | 19.5 | 11.72 | One-sided slab metate | |
| 2673 | Metate | S | F | 1,786.1 | 4.01 | 22.5 | 14.5 | Two-sided slab metate | |
| 2674 | Metate | S | F | 1,332.5 | 5.28 | 15.56 | 10.22 | One-sided slab metate | |
| 2676 | Metate | S | F | — | — | — | — | Two-sided slab metate | |
| 3070 | Mano | S | C | 737.1 | 5.84 | 9.54 | 8.57 | Two-sided bun-shaped mano | |
| 3316 | Metate | S | F | 141.2 | 3.17 | 7.69 | 4.4 | One-sided slab metate | |
| 3446 | Metate | S | C | 2,013 | 10.07 | 21 | 10.69 | One-sided slab metate | |
| 5t | 3474.1 | Mano | S | F | 40 | 2.25 | 3.94 | 4.64 | Two-sided bun-shaped mano |
| 3474.2 | Mano | S | C | 708 | 4.21 | 11.08 | 9.26 | Two-sided bun-shaped mano | |
| 3564 | Metate | S | F | 878 | 3.72 | 10.42 | 9.81 | Two-sided slab metate | |
| 3573 | Metate | S | F | 114.8 | 0.75 | 10.59 | 10.12 | Two-sided slab metate | |
| 3607.2 | Mano | Q | F | 174.8 | 4.59 | 7.28 | 5.72 | Two-sided bun-shaped mano | |
| 4k | 4856 | Metate | S | F | 14.9 | 0.55 | 4.68 | 3.7 | One-sided slab metate |
All GS tools were sandstone (S), except 3607.2, which is quartzite (Q). Measurements and descriptions provided by Mark Bodily.
Fig. 3.
Slab metate (2004.8.2346.1) from substratum 5u and two-sided mano (2004.8.3474.1 and 2004.8.3474.2) from substratum 5t. Both yielded starch granules of S. jamesii and dated between 9,300 and 8,500 cal B.P. (NCS collections held at BYU Museum of Peoples and Cultures).
Results
Extractions of surface and interstitial residues were performed on 101 ground stone tools from strata 4, 5, and 6 and on seven sediment samples for purposes of establishing background starch concentrations (19). Although 90 of these tools yielded starch granules, 24 manos and metates bore granules with eccentric hila and were from substrata 4k, 5t, 5u, 6a, and 6d (Table 1 and Table S3). Recovered granules were well-preserved, tested positive with potassium iodide, and retained most, if not all, of their diagnostic characteristics, unlike whole tubers that apparently degrade in archaeological contexts. The levels of background starch granules from surrounding sediments were negligible (19) (Materials and Methods). The normalized yields of starch granules (mean number per gram) from the surfaces of ground stone tools were 100 times more than all sediment samples combined. Residue samples had an average of 129 ± 51 (95% CI) starch granules per gram of residue, whereas sediment samples yielded an average of 1.3 ± 2.7 (95% CI) starch granules per gram of sediment (19).
Table 1.
Summary of starch granules extracted from ground stone tools excavated at North Creek Shelter
| Granules assigned to S. jamesii | ||||||||
| Substratum | Dates (cal B.P.) | Ground stone tools | Total starch granules | Total granules w/ eccentric hila | Eccentric hila granules w/ longitudinal fissure | Total no. | Mean fissure ratio,wf/wg | Mean length, µm |
| 6d | 6,900 | 4 | 46 | 5 | 1 | 1 | 0.28* | 41.78* |
| 6a | 6,900–8,300 | 4 | 30 | 7 | 3 | 0 | — | — |
| 5u | 8,500–8,800 | 10 | 101 | 55 | 35 | 5 | 0.25 | 49.00 |
| 5t | 9,300 | 5 | 101 | 35 | 18 | 1 | 0.25* | 48.36* |
| 4k | 10,100–10,900 | 1 | 45 | 20 | 13 | 2 | 0.26 | 43.65 |
| Totals | 24 | 323 | 122 | 70 | 9 | 0.25 | 42.43 | |
Measurements based on a single grain.
Definitive assignment of nine granules to S. jamesii based upon the possession of five diagnostic characteristics. Another 61 granules were likely or possibly S. jamesii based upon possession of fewer characteristics (Figs. S3–S5 and Table S3). Fissure ratio calculated from the width of the longitudinal fissure (wf) and width of the grain (wg). Mean lengths are from log-transformed data.
Table S3.
Inventory of starch granules from ground stone tools within substrata 4k, 5t, 5u, 6a, and 6d at NCS
| Substratum | Ground stone sample (FS no.) | Ground stone type | Total starch granules | Granules w/ eccentric hilum | Granule length, µm | Fissure characteristics | Taxonomic assignment | ||
| Longitudinal fissure? | Branched? | Fissure ratio, wf/wg | |||||||
| 6d | 1316 | Metate | 8 | 1 | 35.36 | No | — | — | — |
| 1719.1 | Metate | 13 | 1 | 24.66 | No | — | — | — | |
| 1719.2 | Metate | 17 | 1 | 41.78 | Yes | No | 0.28 | S. jamesii | |
| 1759 | Mano | 8 | 2 | 46.21 | No | — | — | — | |
| 35.70 | No | — | — | — | |||||
| 6a | 505 | Mano | 11 | 3 | 33.83 | Yes | No | 0.21 | Likely S. jamesii |
| 35.66 | No | — | — | — | |||||
| 64.38 | No | — | — | — | |||||
| 548 | Mano | 2 | 1 | 39.70 | No | — | — | — | |
| 603 | Metate | 8 | 1 | 32.21 | Yes | No | 0.16 | Possibly S. jamesii | |
| 3489 | Mano | 9 | 2 | 32.85 | Yes | No | 0.24 | Likely S. jamesii | |
| 60.10 | No | — | — | — | |||||
| 5u | 2346 | Metate | 11 | 8 | 17.46 | No | — | — | — |
| 30.65 | No | — | — | — | |||||
| 44.84 | No | — | — | — | |||||
| 45.29 | No | — | — | — | |||||
| 51.45 | No | — | — | — | |||||
| 52.95 | Yes | No | 0.34 | Likely S. jamesii | |||||
| 55.17 | Yes | No | 0.20 | Likely S. jamesii | |||||
| 74.67 | No | — | — | — | |||||
| 2347 | Mano | 8 | 7 | 22.66 | No | — | — | — | |
| 32.23 | Yes | No | 0.29 | Possibly S. jamesii | |||||
| 38.25 | Yes | No | 0.23 | S. jamesii | |||||
| 44.64 | Yes | No | 0.09 | Likely S. jamesii | |||||
| 49.92 | Yes | No | 0.04 | Likely S. jamesii | |||||
| 51.27 | Yes | No | 0.31 | Likely S. jamesii | |||||
| 64.30 | Yes | No | 0.68 | Likely S. jamesii | |||||
| 2667.1 | Metate | 9 | 6 | 29.11 | No | — | — | — | |
| 36.94 | No | — | — | — | |||||
| 44.15 | Yes | No | 0.30 | Likely S. jamesii | |||||
| 50.99 | Yes | No | 0.27 | S. jamesii | |||||
| 51.16 | Yes | No | 0.15 | Likely S. jamesii | |||||
| 56.19 | Yes | No | 0.10 | Likely S. jamesii | |||||
| 2672 | Metate | 24 | 21 | 24.88 | Yes | No | 0.56 | Possibly S. jamesii | |
| 25.13 | Yes | No | 0.22 | Likely S. jamesii | |||||
| 27.66 | No | — | — | — | |||||
| 29.19 | No | — | — | — | |||||
| 29.72 | No | — | — | — | |||||
| 37.49 | Yes | No | 0.12 | Likely S. jamesii | |||||
| 39.50 | Yes | No | 0.18 | Likely S. jamesii | |||||
| 40.06 | No | — | — | — | |||||
| 41.44 | Yes | No | 0.05 | Likely S. jamesii | |||||
| 42.75 | Yes | No | 0.27 | S. jamesii | |||||
| 44.26 | No | — | — | — | |||||
| 44.59 | Yes | No | 0.12 | Likely S. jamesii | |||||
| 44.78 | Yes | No | 0.23 | S. jamesii | |||||
| 47.92 | No | — | — | — | |||||
| 51.16 | Yes | Yes | 0.45 | Possibly S. jamesii | |||||
| 59.69 | Yes | No | 0.13 | Likely S. jamesii | |||||
| 62.85 | Yes | Yes | 0.54 | Possibly S. jamesii | |||||
| 65.83 | Yes | Yes | 0.74 | Possibly S. jamesii | |||||
| 66.83 | Yes | Yes | 0.43 | Possibly S. jamesii | |||||
| 68.23 | Yes | No | 0.24 | S. jamesii | |||||
| 74.00 | Yes | Yes | 0.52 | Possibly S. jamesii | |||||
| 2673 | Metate | 3 | 3 | 28.18 | No | — | — | — | |
| 60.68 | Yes | No | 0.32 | Likely S. jamesii | |||||
| 88.57 | Yes | Yes | 0.62 | Possibly S. jamesii | |||||
| 2674 | Metate | 8 | 4 | 43.29 | No | — | — | — | |
| 49.67 | Yes | No | 0.20 | Likely S. jamesii | |||||
| 57.28 | Yes | Yes | 0.48 | Possibly S. jamesii | |||||
| 63.58 | Yes | No | 0.17 | Likely S. jamesii | |||||
| 2676 | Metate | 4 | 1 | 75.06 | Yes | Yes | 0.59 | Possibly S. jamesii | |
| 3070 | Mano | 7 | 1 | 23.86 | Yes | No | 0.15 | Possibly S. jamesii | |
| 3316 | Metate | 19 | 1 | 19.62 | No | — | — | — | |
| 3446 | Metate | 8 | 3 | 22.06 | No | — | — | — | |
| 43.03 | No | — | — | — | |||||
| 63.88 | Yes | Yes | 0.79 | Possibly S. jamesii | |||||
| 5t | 3474.1 | Mano | 13 | 6 | 19.26 | No | — | — | — |
| 35.18 | No | — | — | — | |||||
| 42.95 | Yes | No | 0.35 | Likely S. jamesii | |||||
| 53.31 | Yes | No | 0.05 | Likely S. jamesii | |||||
| 65.68 | Yes | No | 0.20 | Likely S. jamesii | |||||
| 78.93 | Yes | No | 0.06 | Likely S. jamesii | |||||
| 3474.2 | Mano | 7 | 4 | 18.15 | No | — | — | ||
| 22.39 | No | — | — | ||||||
| 39.29 | Yes | Yes | 0.64 | Possibly S. jamesii | |||||
| 57.30 | Yes | No | 0.05 | Likely S. jamesii | |||||
| 3564 | Metate | 6 | 1 | 15.54 | No | — | — | — | |
| 3573 | Metate | 19 | 1 | 20.52 | No | — | — | — | |
| 3607.2 | Mano | 56 | 23 | 20.74 | Yes | No | 0.33 | Possibly S. jamesii | |
| 21.26 | Yes | No | 0.49 | Possibly S. jamesii | |||||
| 22.07 | No | — | — | — | |||||
| 22.98 | No | — | — | — | |||||
| 24.33 | Yes | No | 0.19 | Possibly S. jamesii | |||||
| 29.78 | No | — | — | — | |||||
| 32.91 | No | — | — | — | |||||
| 33.32 | Yes | No | 0.12 | Possibly S. jamesii | |||||
| 33.58 | No | — | — | — | |||||
| 33.68 | No | — | — | — | |||||
| 35.72 | Yes | No | 0.12 | Likely S. jamesii | |||||
| 37.6 | No | — | — | — | |||||
| 40.82 | Yes | No | 0.31 | Likely S. jamesii | |||||
| 42.7 | No | — | — | — | |||||
| 44.73 | No | — | — | — | |||||
| 44.83 | Yes | No | 0.13 | Likely S. jamesii | |||||
| 48.36 | Yes | No | 0.25 | S. jamesii | |||||
| 52.82 | Yes | No | 0.18 | Likely S. jamesii | |||||
| 53.60 | Yes | No | 0.51 | Likely S. jamesii | |||||
| 56.28 | No | — | — | — | |||||
| 58.12 | Yes | No | 0.29 | Likely S. jamesii | |||||
| 62.14 | Yes | No | 0.45 | Likely S. jamesii | |||||
| 72.78 | No | — | — | — | |||||
| 4k | 4856 | Metate | 45 | 20 | 29.71 | No | — | — | — |
| 33.65 | No | — | — | — | |||||
| 35.15 | Yes | Yes | 0.39 | Possibly S. jamesii | |||||
| 35.29 | No | — | — | — | |||||
| 35.96 | Yes | No | 0.46 | Likely S. jamesii | |||||
| 36.16 | No | — | — | — | |||||
| 37.19 | Yes | No | 0.07 | Likely S. jamesii | |||||
| 39.56 | Yes | No | 0.14 | Likely S. jamesii | |||||
| 39.83 | Yes | Yes | 0.35 | Possibly S. jamesii | |||||
| 39.89 | No | — | — | — | |||||
| 40.59 | Yes | No | 0.08 | Likely S. jamesii | |||||
| 40.62 | Yes | No | 0.13 | Likely S. jamesii | |||||
| 42.28 | Yes | No | 0.25 | S. jamesii | |||||
| 42.46 | Yes | No | 0.05 | Likely S. jamesii | |||||
| 44.43 | No | — | — | — | |||||
| 44.49 | Yes | No | 0.13 | Likely S. jamesii | |||||
| 45.01 | Yes | No | 0.27 | S. jamesii | |||||
| 45.56 | Yes | Yes | 0.69 | Possibly S. jamesii | |||||
| 45.81 | No | — | — | — | |||||
| 78.27 | Yes | Yes | 0.37 | Possibly S. jamesii | |||||
Definitive taxonomic assignment of granules to S. jamesii is based on the possession of five diagnostic characteristics. Granules that possessed four (likely S. jamesii) or three (possibly S. jamesii) characteristics were also assigned. Characteristics of S. jamesii possessed by each granule are bolded. Damaged granules extracted from ground stone tools are not included.
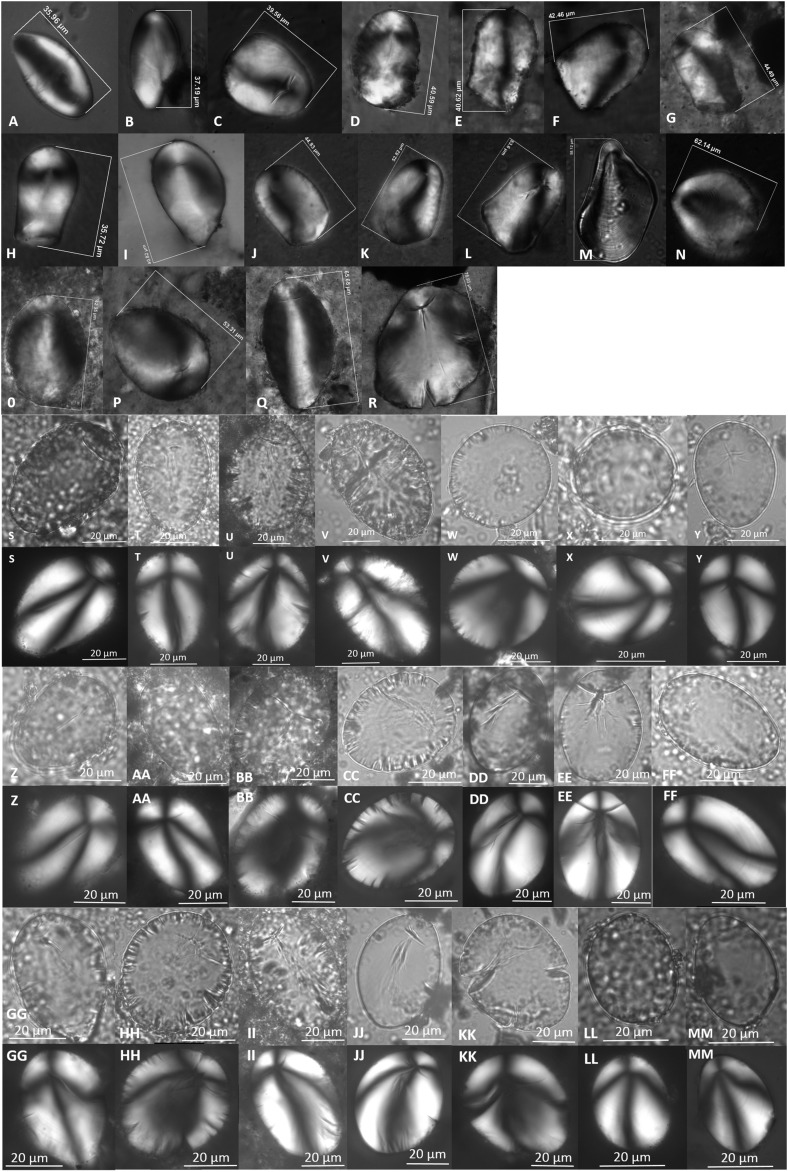
A total of 323 starch granules were recovered from the 24 tools, of which 122 granules had pronounced eccentric hila (Table 1). Those with eccentric hila are almost exclusively produced by underground storage organs (USOs) (20), reducing the number of possible sources to two plant families (Solanaceae and Liliaceae) and only six species within the greater Colorado Plateau region (21). Using morphometric measurements pertaining to another four diagnostic characteristics (Materials and Methods), nine granules were definitively assigned to S. jamesii and 61 (for a total of 70) were likely or possibly S. jamesii based on possession of three or two characteristics (Table S3 and Figs. S2–S4).
Fig. S2.
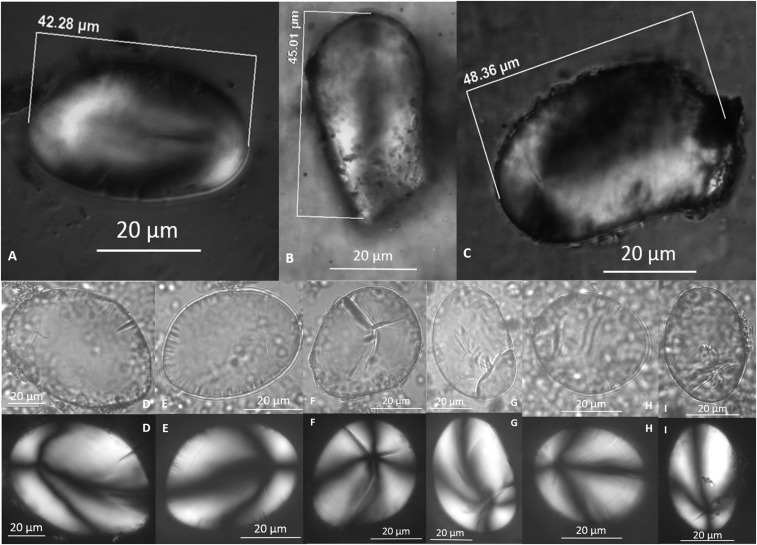
Starch granules possessing all five diagnostic characteristics, assigned to S. jamesii. Nomarski optics (Top): Str. 4k (10,900–10,100 cal BP, GS tool 4856) (A and B) and Str. 5t (9,300 cal BP, GS tool 3607.2) (C). Brightfield and polarized (Middle and Bottom): Str. 5u (8,800–8,500 cal BP, GS tools 2672, 2667.1, 2347) (D–H) and Str. 6d (6,900 cal BP, GS tool 1719.2) (I). Panels with the same letter are images of the same granule in brightfield (Middle) and polarized (Bottom).
Fig. S4.
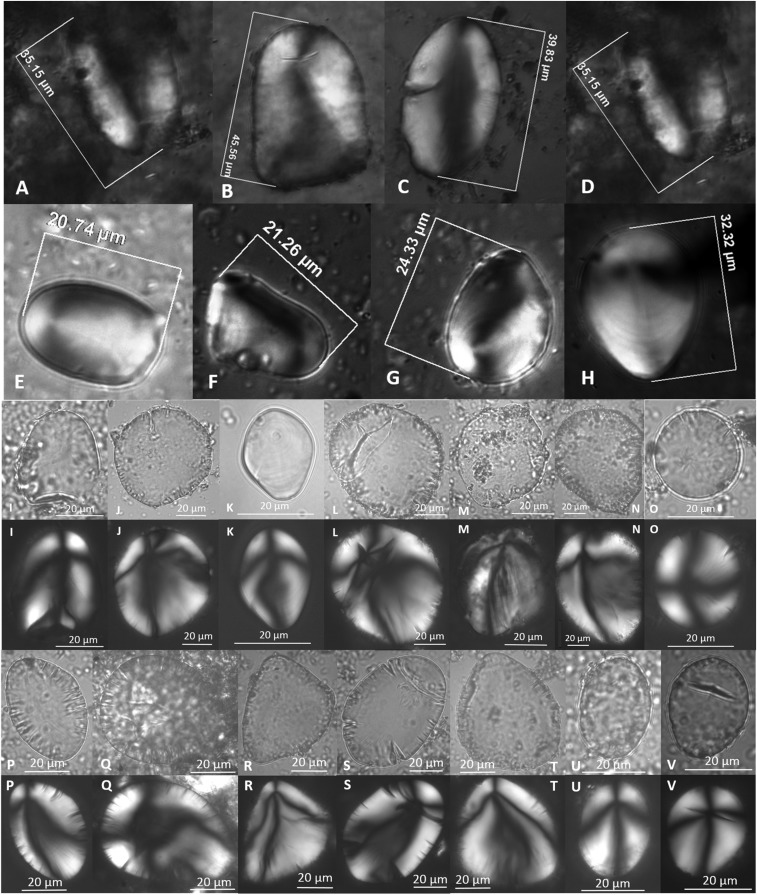
Starch granules possessing three diagnostic characteristics, possibly S. jamesii. Nomarski optics (top two rows) Str. 4k (GS tool 4856) (A–D), Str. 5t (GS tool 3607.2) (E–H). Brightfield and polarized (bottom four rows): Str. 5t (GS tool 3474.2) (I), Str. 5t (GS tools 3446, 3070, 2676, 2674, 2673, 2672, 2347) (J–U), Str. 6a (GS tool 603) (V). Panels with the same letter are images of the same granule in brightfield (Top) and polarized (Bottom).
Fig. S3.
Starch granules possessing four diagnostic characteristics, likely S. jamesii. Nomarski optics (top three rows): Str. 4k (GS tool 4856) (A–G), Str. 5t (GS tool 3607.2) (H–N), Str. 5t (GS tool 3474.1) (O–R). Brightfield and polarized (bottom six rows): Str. 5t (GS tool 3474.1) (S), Str. 5u (GS tools 2674, 2673, 2674, 2667.1, 2347, 2346) (T–KK), Str. 6a (GS tools 3489, 505) (LL and MM). Panels with the same letter are images of the same granule in brightfield (Top) and polarized (Bottom).
Reduced confidence in identification was most often because the fissure ratio (width of longitudinal fissure/width of granule; wf/wg) was not within the strictly defined range (0.21–0.28). Nevertheless, these starch residues place the earliest use of S. jamesii tubers at 10,900 cal B.P. The overlying substratum 5t also contained tools with at least one granule definitively assigned to S. jamesii and another 17 likely or possibly S. jamesii (Fig. 4). This layer is described as an extensive living surface with at least 10 roasting pits in addition to 20 ground stone tools (14) (Fig. S5). The pits span contiguous surfaces and could have been available for use over several hundred years.
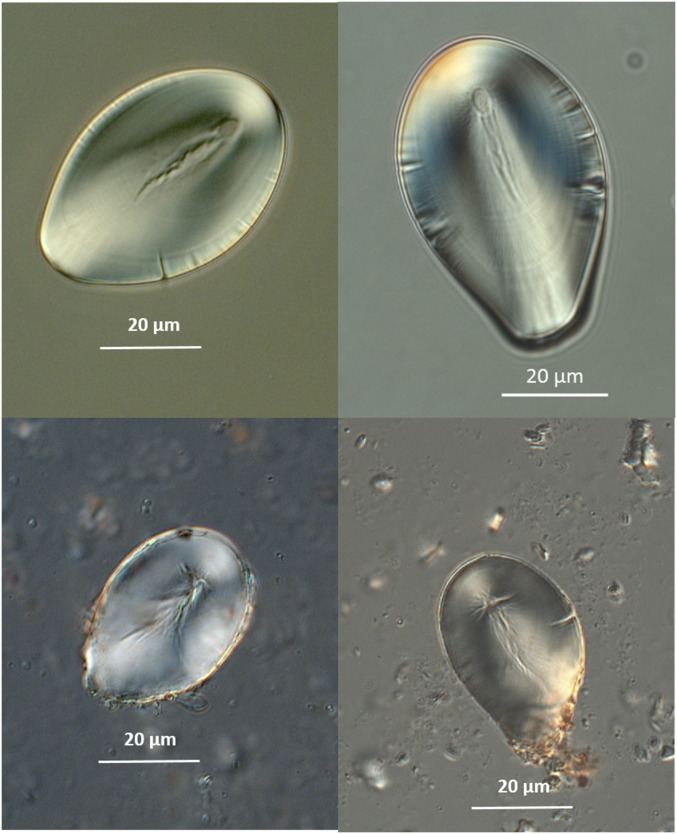
Fig. 4.
Starch granules of S. jamesii. (Upper) Modern reference material from Escalante, Utah. (Lower) Archaeological granules from a ground stone tool (2004.8.3607.2) in substratum 5t (9,300 cal B.P.).
Fig. S5.
Uncovering multiple roasting pits on living surface 5t (9,300 cal B.P.) at NCS. (Photo courtesy of Joel Janetski).
Younger deposits, such as 6a and 6d (∼6,900 cal B.P.), also contained tools with S. jamesii granules, indicating at least 4,000 y of intermittent use. Although tools from the late Holocene strata (1,150–150 cal B.P.) have yet to be examined, ethnographic and historic records indicate the importance of this species in the local diet during recent times (22–27).
Discussion
Ethnographic accounts of S. jamesii use come from Apache, Hopi, Kawaik, Navajo, Southern Paiute, Tewa, Zia, and Zuni groups (22–27). There were various cooking and processing techniques associated with the species. For example, tubers were collected by Apache during late summer, then “boiled, unpeeled and thus eaten. The S. jamesii product was sometimes dried, stored, and later ground into flour for making bread” (22, p. 42). S. jamesii tubers were also eaten with a pinch of clay to reduce bitterness (24–26).
Tissues of S. jamesii are reported to contain the glycoalkaloids solanine, tomatine, and chaconine, which produce the bitterness. These compounds are steroid-based with various sugar moieties attached to hydroxyl groups on the genin portion (28). One report on the glycoalkaloid content of S. jamesii found ∼100 mg tomatine/g of dried tuber from samples collected at Zuni Pueblo (29). However, collections of tubers from other archaeological sites in New Mexico and Arizona were found to have significantly lower total glycoalkaloid content (30).
Calvary soldiers on early expeditions into Escalante also consumed wild tubers of S. jamesii and remarked that the place had already been named “Potato Valley” by pioneers: “We have found wild potatoes growing from which the valley takes its name” (Captain James Andrus, August 1866, among the first Europeans to enter this region). A soldier’s diary (John S. Adams, Cavalryman, 1866) recorded, “…we gathered some wild potatoes which we cooked and ate them; they were somewhat like the cultivated potato, but smaller” (31). Settlers and their descendants also consumed this potato from the 1870s through the Great Depression of the 1930s and are even now grown in a few backyard gardens.
S. jamesii is known to be highly nutritious, having twice the protein, zinc, and manganese and three times the calcium and iron content as S. tuberosum (32). Under optimal growing conditions in a greenhouse, a single “mother” tuber produced 125 progeny tubers during a 6-mo growing season. In the field, tubers do not produce shoots until the onset of summer rain, usually in July, but carbon allocation to forming progeny tubers begins as soon as leaves are aboveground. Summer drought and autumn frost cause immediate senescence of shoots. Tubers, however, can persist underground for at least 8 y (33), achieving high densities and yielding at least 1.7 kg of starchy food per 10 m2 of habitat area (32). Thus, a summer-active and highly productive herbaceous perennial would have provided a reliable, yearlong source of carbohydrate and minerals that significantly improved dietary quality.
Evidence for the processing of starch-rich tubers has implications with respect to the ecology of human diets and optimal foraging theory. Calculations of dietary species richness and evenness, used to define the energetics of food choices, have traditionally been based on faunal evidence. When macro- and microbotanical evidence are added to the calculations, we are able to achieve a fuller picture of dietary change through time (34). Tubers and other USOs rank just below small- and large-bodied animals (35, 36), therefore energetic gains would be greater than those obtained from small seeds alone. Adding S. jamesii to the tally of dietary species richness at NCS would accentuate a shift toward broader diets, especially during the middle Holocene (34). Similar to pine nuts and perhaps acorns, S. jamesii would disproportionally mitigate for the loss of higher ranked resources. However, unlike pine nuts and acorns, these perennial tubers would not be susceptible to reproductive failure during the increasing aridity and changing ecosystem composition of the Holocene.
Five small and isolated populations of S. jamesii are known from the Escalante Valley, including one only 150 m from NCS (34, 37) (Fig. 2). The next closest population is 150 km to the east in the vicinity of Newspaper Rock, another well-known archeological site in southern Utah. At both locations, the species occupies woodlands of pinyon, oak, and cottonwood that did not become established until the mid-Holocene (38). It is likely, therefore, that during the late Pleistocene and early Holocene, when climatic conditions were cooler and the vegetation surrounding NCS was dominated by spruce-fir forest (34), S. jamesii was confined to warmer, lower elevation regions of the southwest where monsoonal climates persisted (39). Two other tuber-producing species (S. fendleri A. Gray ex Torr. and S. cardiophyllum Lindl.) are also restricted to warm, subtropical climates much farther south (40, 41) that would not have been present in the Escalante/Colorado Plateau region during the late Pleistocene and early Holocene. The confinement of tuber-bearing Solanum species in North America to warm, monsoonal climates suggests a physiological constraint on distribution that might only be overcome by human transport into favorable microsites at higher latitudes during ancient times. The long prehistory of S. jamesii in North America raises the question of whether its populations in the Four Corners region could have undergone transport, cultivation, and eventual domestication.
Materials and Methods
Starch Granule Extraction.
Starch granules were extracted from ground stone tools by using three different treatment methods (19). Each residue sample was sieved by using a 125-μm mesh Endecott sieve (to remove material >125 μm). Water plus sieved sample (<125 μm) was transferred to a 50-mL Falcon tube. Samples were centrifuged for 3 min at 2,720 × g. The supernatant was discarded, and the resuspended sample was transferred to a 15-mL centrifuge tube.
A heavy liquid separation technique was used to isolate any starch in the archaeological sample. Sodium polytungstate (Specific Gravity 2.35) was added to the sample pellet in each tube, resuspended with a vortex mixer, and centrifuged for 15 min at 300 × g. The suspended fraction (with starch if present) was decanted into another set of labeled centrifuge tubes. Two more rinses (addition of DH2O and centrifugation, 3 min at 2,720 × g) were undertaken to remove any residual heavy liquid. Acetone was added to each, spun, and allowed to dry overnight.
The pellets were resuspended in a few drops of a 50% glycerol and 50% DH2O solution, and the sample was mounted on a glass slide. Total slide scans were completed by using a Zeiss Axioskop II transmitting, brightfield microscope fitted with polarizing filters and Nomarski optics. A Zeiss HRc digital camera and Zeiss Axiovision and Zen software were used for image capture and archiving (all manufactured by Zeiss International).
Starch Granule Identification.
Our approach to identifying archaeological starch granules with eccentric hila began with an ethnographic inventory of all native food plants significant to Utah’s southern Paiute people, inhabitants of the Basin-Plateau region (42) (Table S4). Of these 65 plant taxa, those belonging to only two plant families (Solanaceae and Liliaceae) have starch granules with pronounced eccentric hila similar to those of S. jamesii (21). We were then able to develop a set of strict, statistically defined diagnostic characteristics so that archaeological granules from many species could be sorted for purposes of identification. Using reference materials from three populations of S. jamesii (21), those characteristics included (i) possession of an eccentric hilum, (ii) the presence of a longitudinal fissure, (iii) the absence of fissure branching, (iv) a ratio of fissure width to granule width in the range of 0.21–0.28 (mean ratio ± 99% CI), and (v) mean maximum granule length (top 20% of granules possessing characteristics 1–3) greater than 34.88 µm (mean minus the 99% CI).
Table S4.
Inventory of plants of ethnobotanical significance and associated food materials (e.g., seeds, fruits, USOs) used by the Utah Southern Paiute
| Family | Taxon | Plant parts | Hilum position | ||
| Seeds | Fruits | USOs | |||
| Agavaceae | Yucca baccata Torr. | x | x | Centric | |
| Yucca baileyi Wooton and Standl. | x | x | Centric | ||
| Alismataceae | Sagittaria latifolia Willd. | x | Slightly eccentric | ||
| Amaranthaceae | Amaranthus sp. L. | x | Centric | ||
| Anacardiaceae | Rhus aromatica Aiton var. trilobata (Nutt.) A. Gray ex S. Watson | x | No starch | ||
| Apiaceae | Cymopterus bulbosus A. Nelson | x | Centric | ||
| Lomatium cous (S. Watson) J.M. Coult. and Rose | x | Centric | |||
| Lomatium macrocarpum (Nutt. ex Torr. and A. Gray) J.M. Coult. and Rose | x | Centric | |||
| Lomatium roseanum Cronquist | x | Centric | |||
| Orogenia linearifolia S. Watson | x | Centric | |||
| Perideridia sp. Rchb. | x | Centric | |||
| Asteraceae | Balsamorhiza sagittata (Pursh) Nutt. | x | x | Centric | |
| Helianthus annuus L. | x | Centric | |||
| Helianthus nuttallii Torr. and A. Gray | x | x | Centric | ||
| Helianthus tuberosus L. | x | x | Centric | ||
| Berberidaceae | Mahonia fremontii (Torr.) Fedde | x | Centric | ||
| Brassicaceae | Arabis holboellii Hornem. | x | Centric | ||
| Lepidium fremontii S. Watson | x | Centric | |||
| Lepidium lasiocarpum Nutt. | x | Centric | |||
| Cactaceae | Cylindropuntia sp. (Engelm.) Kreuzinger | x | Centric | ||
| Cylindropuntia whipplei (Engelm. and J.M. Bigelow) F.M. Knuth | x | Centric | |||
| Chenopodiaceae | Allenrolfea occidentalis (S. Watson) Kuntz | x | Centric | ||
| Atriplex canescens (Pursh) Nutt. | x | Centric | |||
| Atriplex confertifolia (Torr. and Frém.) S. Watson | x | Centric | |||
| Chenopodium berlandieri Moq. | x | Centric | |||
| Chenopodium fremontii S. Watson | x | Centric | |||
| Sarcocornia utahensis (Tidestr.) A.J. Scott | x | Centric | |||
| Cucurbitaceae | Cucurbita foetidissima (Kunth) | x | Centric | ||
| Cupressaceae | Juniperus osteosperma (Torr.) Little | x | Centric | ||
| Cyperaceae | Schoenoplectus acutus (Muhl. ex Bigelow) A. Löve and D. Löve | x | Centric | ||
| Schoenplectus americanus (Pers.) Volkart ex Schinz and R. Keller | Centric | ||||
| Bolboschoenus maritamus (L.) Palla | x | Centric | |||
| Elaeagnaceae | Shepherdia argentea (Pursh) Nutt. | x | x | Centric | |
| Shepherdia rotundifolia Parry | x | x | Centric | ||
| Fabaceae | Pediomelum aromaticum (Payson) W.A. Weber | x | Centric | ||
| Pediomelum cuspidatum (Pursh) Rydb. | x | Centric | |||
| Pediomelum megalanthum (Wooton and Standl.) Rydb. | x | Centric | |||
| Pediomelum mephiticum (S. Watson) Rydb. | x | Centric | |||
| Fagaceae | Quercus gambelii Nutt. | x | Centric | ||
| Grossulaceae | Ribes aureum Pursh | x | Centric | ||
| Liliaceae | Allium brevistylum S. Watson | x | No starch | ||
| Allium cernuum Roth. | x | No starch | |||
| Calochortus leichtlinii Hook. f. | x | Eccentric | |||
| Calochortus nuttallii Torr. and A. Gray | x | Eccentric | |||
| Camassia quamash (Pursh) Greene | x | Slightly eccentric | |||
| Erythronium grandiflorum Pursh | x | Eccentric | |||
| Fritillaria atropurpurea Nutt. | x | Eccentric | |||
| Fritillaria pudica (Pursh) Spreng. | x | Eccentric | |||
| Malvaceae | Sphaeralcea parvifolia A. Nelson | x | Centric | ||
| Pinaceae | Pinus edulis Engelm. | x | Centric | ||
| Pinus monophylla Torr. and Frém. | x | Centric | |||
| Poaceae | Achnatherum hymenoides (Roem. and Schult.) Barkworth | x | Centric | ||
| Leymus cinereus (Scrib. and Merr.) A. Löve | x | Centric | |||
| Muhlenbergia sp. Schreb. | x | Centric | |||
| Sporobolus sp. R. Br. | x | Centric | |||
| Sporobolus airiodes (Torr.) Torr. | x | Centric | |||
| Sporobolus cryptandrus (Torr.) A. Gray | x | Centric | |||
| Zea mays L. | x | Centric | |||
| Polygonaceae | Eriogonum inflatum Torr. and Frém. | x | Centric | ||
| Polygonaceae (cont.) | Eriogonum heracleoides Nutt. | x | Centric | ||
| Eriogonum racemosum Nutt. | x | Centric | |||
| Eriogonum umbellatum Torr. | x | Centric | |||
| Polygonum bistortoides Pursh. | x | Centric | |||
| Portulaceae | Lewisia sp. Pursh | x | Centric | ||
| Solanaceae | S. jamesii Torr. | x | Eccentric | ||
| Typhaceae | Typha sp. L. | x | x | Centric | |
Based on a master list published by Fowler (42) and the authors’ seed reference collection at Natural History Museum of Utah Archaeobotany Lab. Excluded are nonnative and cultivated plants that would not have been available before European contact. Nomenclature follows US Department of Agriculture Germplasm Resources Information Network (www.ars-grin.gov/).
The utility of starch granules in archaeobotanical studies depends heavily on achieving high levels of confidence during the process of identification (43, 44). Assignment of archaeological granules to any taxonomic level is facilitated by larger sample sizes, a greater number of diagnostic characteristics, the use of metrics for establishing objective criteria, and comparisons with modern reference material (21). Statistical analyses can then be used to characterize interpopulation and interspecific variation and the effects of selection under domestication. Confidence in our identifications has been elevated by (i) the use of substantial archaeological starch assemblages, (ii) statistically robust reference sample sizes systematically examined for variation within and between populations and (iii) the derivation of at least five measurable or strictly defined diagnostic characteristics. Having a smaller subset of potential food plants, in this case possessing eccentric hila, limited the number of comparisons between archaeological and reference materials.
Background Starch Concentrations from Surrounding Sediments.
Sediment samples were collected from the east and west walls of the NCS excavations during 2006 and 2007 (14) and included substrata 5a, 5f, 5q, 5s, 5t, 6a, and 6d. No sediment samples were collected from substrata 4k, 5r, or 5u. Sediment samples were processed for starch to establish background concentrations. All were weighed and transferred to 50-mL Falcon tubes. Deflocculation of clays was facilitated by the addition of Calgon (sodium hexametaphosphate) and gentle agitation for several hours.
To directly compare starch granule counts, sediment samples and a subsample of residue samples (19) were normalized by weight. One gram of material from seven sediment samples was processed according to standard protocols (45). Weights were determined for residue samples (n = 34) by recording the dry weight of stones before and after sonication. All residue weights were less than 0.1 g, about one-10th of the sediment weights. Because there was 10 times more sediment than residue in any one sample, the probability of detecting background levels of starch granules was increased.
Contamination Sources.
Starch contamination can originate in soils, air currents, and on laboratory and human surfaces (46–49), so precautions were taken to minimize contamination of the samples. In this study, sources of contamination were linked to slide preparation rather than laboratory processing. For example, new sheets of Whatman’s lens papers and human fingertips could be sources of starch contaminants. Fresh pairs of powder-free laboratory gloves, new microscope slides and coverslips, DH2O, and bottled glycerol did not, however, contribute detectable amounts of starch granules. We also tested the plastic and paper bags that held the ground stone tools in collections, and the paper labels with accession numbers, none of which were detectable sources of contamination.
Acknowledgments
Processing for starch residues was conducted in Dr. Judith Field’s laboratory at University of New South Wales. Nomarski images (in supporting figures) were taken with Dr. Field’s Zeiss microscope. All other imaging was conducted either at University of Washington or the Archaeobotany Lab at the Natural History Museum of Utah. Joel Janetski generously provided data on archaeological material and chronology from North Creek Shelter. Paul Stavast [Brigham Young University (BYU) Museum of Peoples and Cultures] gave us access to the collections. We thank our friends and colleagues, John Bamberg, Alfonso del Rio, Nicole Herzog, David Kinder, and David Rhode, for thoughtful conversations on starch granule analysis and potato biology; Don Grayson, Joel Janetski, and Benjamin Pavlik for reading an earlier version of the manuscript and whose comments and suggestions improved our paper; DeLane Griffin, Joette-Marie Rex, David Delthony, and Drew Parkin of Escalante, Utah, for their hospitality and enthusiastic support; and to the native peoples of the greater Four Corners region, the original innovators and stewards of S. jamesii. Funding for this research was provided by National Science Foundation Doctoral Dissertation Improvement Grant 1262835 and a Funding Incentive Seed Grant from the University of Utah.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1705540114/-/DCSupplemental.
References
- 1.Mariotti Lippi M, Foggi B, Aranguren B, Ronchitelli A, Revedin A. Multistep food plant processing at Grotta Paglicci (Southern Italy) around 32,600 cal B.P. Proc Natl Acad Sci USA. 2015;112:12075–12080. doi: 10.1073/pnas.1505213112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Revedin A, et al. Thirty thousand-year-old evidence of plant food processing. Proc Natl Acad Sci USA. 2010;107:18815–18819. doi: 10.1073/pnas.1006993107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Piperno DR, Weiss E, Holst I, Nadel D. Processing of wild cereal grains in the Upper Palaeolithic revealed by starch grain analysis. Nature. 2004;430:670–673. doi: 10.1038/nature02734. [DOI] [PubMed] [Google Scholar]
- 4.Liu L, Bestel S, Shi J, Song Y, Chen X. Paleolithic human exploitation of plant foods during the last glacial maximum in North China. Proc Natl Acad Sci USA. 2013;110:5380–5385. doi: 10.1073/pnas.1217864110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fuller DQ. Agricultural origins and frontiers in South Asia: A working synthesis. J World Prehist. 2006;20:1–86. [Google Scholar]
- 6.Piperno DR, Dillehay TD. Starch grains on human teeth reveal early broad crop diet in northern Peru. Proc Natl Acad Sci USA. 2008;105:19622–19627. doi: 10.1073/pnas.0808752105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Perry L, et al. Starch fossils and the domestication and dispersal of chili peppers (Capsicum spp. L.) in the Americas. Science. 2007;315:986–988. doi: 10.1126/science.1136914. [DOI] [PubMed] [Google Scholar]
- 8.Willcox G, Nesbitt M, Bittmann F. From collecting to cultivation: Transitions to a production economy in the Near East. Veg Hist Archaeobot. 2012;21:81–83. [Google Scholar]
- 9.Hawkes JG. The Potato: Evolution, Biodiversity and Genetic Resources. Smithsonian Institution; Washington, DC: 1990. [Google Scholar]
- 10.Ugent D, Pozorski S, Pozorski T. Archaeological potato tuber remains from the Casma Valley of Peru. Econ Bot. 1982;36:182–192. [Google Scholar]
- 11.Rumold CU, Aldenderfer MS. Late Archaic-Early Formative period microbotanical evidence for potato at Jiskairumoko in the Titicaca Basin of southern Peru. Proc Natl Acad Sci USA. 2016;113:13672–13677. doi: 10.1073/pnas.1604265113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bird DW, O’Connell JF. Behavioral ecology and archaeology. J Archaeol Res. 2006;14:143–188. [Google Scholar]
- 13.Henry AG, Brooks AS, Piperno DR. Plant foods and the dietary ecology of Neanderthals and early modern humans. J Hum Evol. 2014;69:44–54. doi: 10.1016/j.jhevol.2013.12.014. [DOI] [PubMed] [Google Scholar]
- 14.Janetski JC, Bodily ML, Newbold BA, Yoder DT. The Paleoarchaic to early Archaic transition on the Colorado Plateau: The archaeology of North Creek Shelter. Am Antiq. 2012;77:125–159. [Google Scholar]
- 15.Geib PR, Jolie EA. The role of basketry in early Holocene small seed exploitation: Implications of a ca. 9,000 year-old basket from Cowboy Cave, Utah. Am Antiq. 2008;73:83–102. [Google Scholar]
- 16.Grayson DK. The Great Basin: A Natural Prehistory. Univ of California Press; Berkeley, CA: 2011. [Google Scholar]
- 17.Rhode D, Madsen DB, Jones KT. Antiquity of early Holocene small seed consumption and processing at Danger Cave, Utah, USA. Antiquity. 2006;80:328–339. [Google Scholar]
- 18.Herzog NM, Lawlor AT. Reevaluating diet and technology in the Archaic Great Basin using Hogup Cave starch assemblages. Am Antiq. 2016;83:664–681. [Google Scholar]
- 19.Louderback LA, Field J, Janetski JC. Curation practices and extraction methods in relation to starch grain yields from ground stone artifacts. J Archaeol Sci Rep. 2015;4:535–540. [Google Scholar]
- 20.Reichert ET. The Differentiation and Specificity of Starches in Relation to Genera, Species, etc. Chapman; London: 1913. [Google Scholar]
- 21.Louderback LA, Herzog NM, Pavlik BM. A new approach for identifying starch granules of wild food plants from arid western North America. Starke. 2016;68:1–7. [Google Scholar]
- 22.Castetter EF, Opler ME. Ethnobiology Studies in the American Southwest. Vol III. Univ of New Mexico Bulletin; Albuquerque: 1936. The ethnobiology of the Chiricahua and Mescalero Apache. A. The uses of plants for food, beverages, and narcotics; pp. 1–39. [Google Scholar]
- 23.Elmore FH. Monographs of the School of American Research. Univ of New Mexico, School of Amer Res; Santa Fe, NM: 1944. Ethnobotany of the Navajo; pp. 9–15. [Google Scholar]
- 24.Vestal PA. Notes on a collection of plants from the Hopi Indian region of Arizona made by J. G. Owens in 1891. Botanical Museum Leaflets. 1940;8:153–168. [Google Scholar]
- 25.Nequatewa E. Some Hopi recipes for the preparation of wild plant foods. Plateau. 1943;18:18–20. [Google Scholar]
- 26.White LA. The Pueblo of Sia, New Mexico, Bureau of American Ethnology Bulletin. Smithsonian Inst; Washington, DC: 1962. [Google Scholar]
- 27.Yarnell RA. Implications of distinctive flora on Pueblo ruins. Am Anthropol. 1965;67:662–674. [Google Scholar]
- 28.Savarese S, et al. Glycoalkaloids as biomarkers for recognition of cultivated, wild, and somatic hybrids of potato. Chem Biodivers. 2009;6:437–446. doi: 10.1002/cbdv.200800247. [DOI] [PubMed] [Google Scholar]
- 29.Johns T, Alonso JG. Glycoalkoloid change during the domestication of the potato, Solanum Section Petota. Euphytica. 1990;50:203–210. [Google Scholar]
- 30.Nzaramba MN, Reddivari L, Bamberg JB, Miller JC., Jr Antiproliferative activity and cytotoxicity of Solanum jamesii tuber extracts on human colon and prostate cancer cells in vitro. J Agric Food Chem. 2009;57:8308–8315. doi: 10.1021/jf901567k. [DOI] [PubMed] [Google Scholar]
- 31.Roundy JC. Advised Them to Call the Place Escalante. Art City Publishing; Springville, UT: 2000. [Google Scholar]
- 32.Kinder DH, Adams KR, Wilson HR. Solanum jamesii Torr: Evidence for cultivation of wild potato tubers by ancestral Pueblo groups. J Ethnobiol. in press. [Google Scholar]
- 33.Bamberg JB. Tuber dormancy lasting eight years in the wild potato Solanum jamesii. Am J Potato Res. 2010;87:226–228. [Google Scholar]
- 34.Louderback LA. 2014 The ecology of human diets during the Holocene at North Creek Shelter, Utah. PhD dissertation (University of Washington, Seattle, WA). Available at https://digital.lib.washington.edu/researchworks/handle/1773/25980.
- 35.Kelly RL. The Foraging Spectrum: Diversity in Hunter-Gatherer Lifeways. Smithsonian Inst Press; Washington, DC: 1995. [Google Scholar]
- 36.Simms SR. British Archaeological Reports, International Series 381. British Archaeological Reports; Oxford: 1987. Behavioral ecology and hunter-gatherer foraging: An example from the Great Basin. [Google Scholar]
- 37.Bamberg JB, et al. Core collections of potato (Solanum) species native to the USA. Am J Potato Res. 2016;93:564–571. [Google Scholar]
- 38.Cole KL, Fisher JF, Ironside K, Mead JI, Koehler P. The biogeographic histories of Pinus edulis and Pinus monophylla over the last 50,000 years. Quat Int. 2013;310:96–110. [Google Scholar]
- 39.Betancourt JL. Late quaternary plant zonation and climate in Southwestern Utah. Great Basin Nat. 1984;44:1–35. [Google Scholar]
- 40.Hijmans RJ, Spooner DM, Salas AR, Guarino L, de la Cruz J. Atlas of Wild Potatoes, Systematic and Ecogeographic Studies on Crop Genepools, 10. Intl Plant Genet Resour Inst; Rome: 2002. [Google Scholar]
- 41.Correll DS. The Potato and its Wild Relatives: Section Tuberarium of the Genus Solanum. Texas Res Found; Renner, TX: 1962. [Google Scholar]
- 42.Fowler CS. In: Subsistence. Handbook of North American Indians. D’Azevedo WL, editor. Vol 11. Smithsonian Inst; Washington, DC: 1986. pp. 64–97. [Google Scholar]
- 43.Liu L, Ma S, Cui J. Identification of starch granules using a two-step identification method. J Archaeol Sci. 2014;52:421–427. [Google Scholar]
- 44.Barton H, Torrence R. Cooking up recipes for ancient starch: Assessing current methodologies and looking to the future. J Archaeol Sci. 2015;56:194–201. [Google Scholar]
- 45.Fullagar R, Field J, Kealhofer L. Grinding stones and seeds of change: Starch and phytoliths as evidence of plant food processing. In: Rowan YM, Ebeling JR, editors. New Approaches to Old Stones: Recent Studies of Ground Stone Artifacts. Equinox; London: 2008. pp. 159–172. [Google Scholar]
- 46.Crowther A, Haslam M, Oakden N, Walde D, Mercader J. Documenting contamination in ancient starch laboratories. J Archaeol Sci. 2014;49:90–104. [Google Scholar]
- 47.Laurence AR, Thoms AV, Bryant VM, McDonough C. Airborne starch granules as a potential contamination source at archaeological sites. J Ethnobiol. 2011;31:213–232. [Google Scholar]
- 48.Dozier CA. Airborne starch dispersal from stone grinding: Experimental results and implications. J Archaeol Sci Rep. 2016;8:112–115. [Google Scholar]
- 49.Mercader J, et al. Starch contamination landscapes in field archaeology: Olduvai Gorge, Tanzania. Boreas. March 20, 2017 doi: 10.1111/bor.12241. [DOI] [Google Scholar]