Significance
Tuberculosis, caused by Mycobacterium tuberculosis (Mtb) bacteria, is the most prevalent infectious disease, affecting one-third of the global population, especially in developing countries. First-line therapies to treat this disease are losing efficacy due to the emergence of drug resistance. Accordingly, new therapeutic agents of novel mechanisms of action remain an urgent medical need. The isocitrate lyases (ICL1 and ICL2) comprise metabolically essential enzymes of Mtb, are absent in mammals, and thereby provide therapeutically important drug targets for tuberculosis. Here, we describe the first example of a mechanism-based inactivator of ICL1 and ICL2 that could provide a starting point for the development of new drugs to treat tuberculosis.
Keywords: mechanism-based inactivation, isocitrate lyase, tuberculosis, 2-vinyl isocitrate, covalent adduct
Abstract
Isocitrate lyase (ICL, types 1 and 2) is the first enzyme of the glyoxylate shunt, an essential pathway for Mycobacterium tuberculosis (Mtb) during the persistent phase of human TB infection. Here, we report 2-vinyl-d-isocitrate (2-VIC) as a mechanism-based inactivator of Mtb ICL1 and ICL2. The enzyme-catalyzed retro-aldol cleavage of 2-VIC unmasks a Michael substrate, 2-vinylglyoxylate, which then forms a slowly reversible, covalent adduct with the thiolate form of active-site Cys191. 2-VIC displayed kinetic properties consistent with covalent, mechanism-based inactivation of ICL1 and ICL2 with high efficiency (partition ratio, <1). Analysis of a complex of ICL1:2-VIC by electrospray ionization mass spectrometry and X-ray crystallography confirmed the formation of the predicted covalent S-homopyruvoyl adduct of the active-site Cys191.
Tuberculosis (TB) is the leading cause of death from an infectious disease. The World Health Organization reported that, in 2014, an estimated 9.6 million people became infected with TB, with 1.5 million deaths [World Health Organization report: Global Tuberculosis Report 2015 (1)]. Infection by Mycobacterium tuberculosis (Mtb), the causative agent of TB, may assume a latent state when harbored within macrophage phagosomes during extended, “persistent” stages (2). In this hypoxic environment, fatty acids provide the primary carbon source, and mycobacteria activate the glyoxylate shunt, a functional abridgement of the tricarboxylic acid cycle (3). Here, d-isocitrate is converted to glyoxylate and succinate by the action of two isocitrate lyases (ICLs) of 27% sequence identity, ICL1 (428 aa) and ICL2 (766 aa), encoded by the genes icl1 and aceA, respectively (4–7). Glyoxylate is subsequently converted to l-malate by malate synthase, encoded by the gene glcB (8). Enzymes of the glyoxylate shunt are found in prokaryotes, lower eukaryotes, and plants, but are absent in mammals (6). Deletion of both ICL genes leads to growth impairment of Mtb in infected mice and rapid elimination of bacteria from the lungs (4, 5). The ICL inhibitor, 3-nitropropionate (3NP), inhibited Mtb in cell cultures (4, 9, 10). Collectively, these results demonstrated the essentiality of the ICLs in persistent-stage Mtb. Despite an absence of drug quality inhibitors of Mtb ICLs, they remain validated targets for the development of new drugs to treat TB. Accordingly, the exploitation of the chemical mechanism to discover covalent inactivators could reinvigorate drug discovery for the ICLs, particularly because their active sites contain conserved, catalytic cysteines. Compounds that form covalent bonds, especially reversible ones, with cysteine residues in or near the enzymatic active sites have received renewed attention as a strategy for the development of enzyme inactivators (11, 12).
The catalytic mechanism of Mtb ICL derived from both structural data (10) and kinetic analysis (13–15) is depicted in Fig. 1. (2R,3S)-Isocitrate (1) [d-isocitrate (IC)] coordinates an active-site magnesium ion and undergoes a base-catalyzed retro-aldol reaction (a) to form glyoxylate (2) and the aci-anion (3) of succinate. Cys191 then protonates C-2 of 3 to afford succinate (4). Ko and McFadden (16) demonstrated that the affinity label, 3-bromopyruvate (3BP) (Fig. 1), exerted time-dependent inactivation of Escherichia coli ICL. A crystal structure of Mtb ICL1 treated with 3BP revealed that Cys191 had been S-pyruvoylated (10). One could envision synthetic analogs of d-isocitrate that bear nascent electrophilic substituents at C-2, which are unmasked only following ICL-catalyzed retro-aldol cleavage. We speculated that 2-C-vinyl-d-isocitrate (2-VIC) (numbering in Fig. 1 based on d-isocitrate) would provide such an inactivator. ICL-catalyzed cleavage of the C2–C3 bond would produce succinate as well as an enzyme-bound Michael acceptor, 2-vinylglyoxylate (2VG), which is poised to react with the thiolate form of the proximal Cys191. Mechanism-based inactivation of target enzymes to produce drug candidates has a history of proven success (17–19). In this work, we describe the preliminary kinetic and structural analysis of the mechanism-based inactivation of Mtb isocitrate lyases 1 and 2 by 2-VIC.
Fig. 1.
Chemical mechanism of isocitrate lyase and structures of inactivators. Proposed two-step chemical mechanism of Mtb ICL1 based on structural (10) and kinetic (13–15) data. Structures of 3-bromopyruvate, 2-vinyl d-isocitrate, and 2-vinylglyoxylate.
Results
Time-Dependent Inactivation of Mtb ICL1 and ICL2 with 2-VIC.
Preincubation of ICL1 (800 nM) with 0–40 µM 2-VIC (5a) (chemistry described in SI Appendix) over a time course of 0–70 min, followed by dilution into reaction mixtures containing high concentrations of the substrates glyoxylate and succinate, resulted in a time-dependent loss of ICL1 activity conforming to first-order kinetics (Fig. 2A). More than 95% of ICL1 was inactivated after a 65-min preincubation with 2-VIC at concentrations of ≥20 µM. Data were fitted globally to Eq. 1, resulting in a maximal rate constant of inactivation, kinact = 0.080 ± 0.006 min−1, and a concentration of 2-VIC leading to half-maximal inactivation, Kinact = 22 ± 3 μM. Using the method of Kitz and Wilson (20), the apparent first-order rate constant of inactivation, kobs, obtained from Eq. 2 demonstrated a hyperbolic dependence on the concentration of the inactivator upon fitting to Eq. 3 (Fig. 2C), indicating saturation behavior of inactivation. The (2R,3R)-diastereomer of 2-VIC (5b) displayed no inactivation of ICL1 when preincubated with enzyme at 1–80 μM concentrations for as long as 1,200 min. 2-VIC effected no appreciable time-dependent inhibition of the coupling enzymes isocitrate dehydrogenase or lactate dehydrogenase. A sample of 2-VIC–inactivated ICL1 that retained 7% residual ICL1 activity [(vi/v0) = 0.07 ± 0.02] was then subjected to spin filtration (Amicon Ultra) comprising a 100,000-fold dilution of 2-VIC. The fraction of remaining activity, compared with a control sample, was vi/v0 = 0.08 ± 0.01, invariant from the input sample, suggesting that the inactivation was apparently irreversible. However, 50% activity was restored following 24-h dialysis (room temperature) of replicate samples of 2-VIC–inactivated ICL1 against 50 mM Hepes (pH 7.5) and 10 mM MgCl2; dialysis for 24 and 48 h in the same buffer containing 10 mM DTT resulted in activity recoveries of 50% and nearly 100%, respectively. Accordingly, the formation of an apparently covalent adduct of 2-VIC and ICL1 was reversible at room temperature, albeit at a slow rate of reactivation (0.0002 min−1).
Fig. 2.
Inactivation of ICL1 and ICL2 by 2-VIC. Residual activity (vi/v0) of (A) ICL1 (800 nM) following preincubation with 0–40 μM 2-VIC and (B) ICL2 (15.0 µM) with 0–3 mM 2-VIC. C and D are replots of rates of inactivation, (kobs) vs. [2-VIC], from A and B, respectively. (E) Residual ICL1 activity (vi/v0) after preincubation of 1.0 μM ICL1 with 0–5.0 μM 2-VIC. The line drawn through the experimental data points results from data fitting to Eq. 4, resulting in a value of p, the partition coefficient, of 0.24 ± 0.04. The curve drawn through the data results from fitting of the data to Eq. 5. (Inset) Concentrations of 2-VIC–inactivated ICL1 versus product succinate formed during inactivation (slope = 1.24).
2-VIC also demonstrated saturable, time-dependent inactivation of ICL2, from which we obtained kinetic parameters of kinact = 0.019 ± 0.001 min−1 and Kinact = 420 ± 70 µM, upon global fitting of the data to Eq. 1 (Fig. 2B). As with ICL1, replotting values of kobs versus [2-VIC] indicated a hyperbolic dependence of enzyme inactivation by 2-VIC (Fig. 2D). Kinetic parameters for catalysis and mechanism-based inactivation are summarized in SI Appendix, Table S1. Briefly, initial velocity data of the forward reaction (isocitrate cleavage) resulted in kinetic parameters of kcat = 1,000 ± 120 min−1, KIC = 37 ± 8 μM, and kcat/KIC = 28 ± 5 μM−1⋅min−1 for ICL1, and kcat = 102 ± 3 min−1, KIC = 100 ± 12 μM, and kcat/KIC = 1.0 ± 0.2 μM−1⋅min−1 for ICL2, demonstrating that, although the affinity of isocitrate binding to either isozyme differs by threefold, the turnover of ICL2 is only 10% that of ICL1. The kinetic parameter kinact/Kinact is analogous to the specificity constant kcat/KIC (that is, the higher the value, the more efficient the inactivator) (19). Values of kinact/Kinact for 2-VIC of (3.5 ± 0.7) × 10−3 μM−1⋅min−1 (ICL1) and (4.6 ± 0.7) × 10−5 μM−1⋅min−1 (ICL2) are 7,800- and 22,400-fold lower, respectively, than kcat/KIC, despite the fact that comparable values of KIC and Kinact were observed. Ratios of kcat/kinact for ICL1 and ICL2 were 13,000 and 5,000, respectively, indicating that reaction steps of inactivation are considerably slower than for turnover of isocitrate. 2-VIC was an inhibitor of human isocitrate dehydrogenase (IC50 of 10 ± 1.3 µM, similar to its binding to Mtb ICL1), but 2-VIC displayed no cytotoxicity in human dermal fibroblasts upon treatment for 72 h at ≤400 μM.
Protection of ICL from 2-VIC Inactivation by d-Malate, Glyoxylate, Succinate, and Added Thiols.
Concentrations of 0.7 mM succinate or 0.1 mM glyoxylate afforded protection from inactivation by 30 μM 2-VIC. d-Malate, a competitive inhibitor of isocitrate (Ki = 310 ± 30 μM; SI Appendix, Fig. S1), served as an isocitrate surrogate to demonstrate that the inactivation effected by 2-VIC occurs at the active site. In preincubation studies of ICL1 with 50 μM 2-VIC, the presence of 0.1–3.0 mM d-malate exhibited a concentration-dependent ablation of ICL1 inactivation by 2-VIC (SI Appendix, Fig. S2). The inactivation was almost entirely eliminated at 3 mM d-malate, which is 10-fold higher than its inhibition constant. The concentration dependence of protection from inactivation by d-malate was evaluated by fitting data (SI Appendix, Eq. S7), resulting in an apparent dissociation constant of Kd = 360 ± 60 μM, nearly equal to its value of Ki, and a Hill coefficient n = 0.56 (SI Appendix, Fig. S2, Inset).
Addition of either DTT or glutathione to preincubation mixtures diminished inactivation of ICL1 by 2-VIC, presumably by interception of an enzyme-generated electrophilic species by reaction with the added thiols. Addition of micromolar concentrations of either DTT or glutathione to preincubation mixtures of ICL1 and 2-VIC demonstrated concentration-dependent protection from 2-VIC inactivation of ICL1. As shown in SI Appendix, Fig. S3A, increasing fixed concentrations of 10–1,000 μM DTT in preincubation mixtures afforded increasing protection from inactivation, and indicated apparent, saturable binding of DTT to ICL1. Fitting of the fractional protection versus [DTT] (SI Appendix, Fig. S3A, Inset) as described provided an apparent binding constant of DTT of Kd = 8.4 ± 0.1 μM. Similar results were found from protection studies using glutathione (Kd = 3.8 ± 0.2 μM; SI Appendix, Fig. S3B). DTT has been shown to reduce the ability of 3BP to inactivate ICL from E. coli, as a result of its reaction with 3BP during preincubation with the enzyme (16). To determine whether DTT reacts with intact 2-VIC in solution in the absence of enzyme, we combined 0.5–1.0 mM 2-VIC with 1 mM DTT for 60 min, which was then diluted 10-fold into reaction mixtures containing ICL1, yielding final concentrations of 100 μM DTT and 100 μM 2-VIC. Both DTT-treated 2-VIC and untreated 2-VIC in the presence of 100 μM DTT effected complete inactivation of ICL1 at identical rates of 0.009 min−1. These results indicated that 2-VIC does not react with DTT in the time frame of the inactivation studies. Accordingly, either the thiol compounds form covalent adducts with an electrophilic species outside the active site of ICL (which would not demonstrate saturation behavior), thereby preventing its rebinding and reaction with ICL, or both thiol compounds are capable of gaining access to an enzyme-bound electrophile generated from 2-VIC or its putative covalent enzyme-bound adduct. The latter is more likely due to the small partition ratios observed (see below), indicating that very little of the enzyme-generated electrophilic species escapes from the active site before the formation of a covalent adduct with ICL1.
Partition Ratio.
To determine the efficiency of 2-VIC inactivation, we measured its partition ratio: the fraction of bound 2-VIC that dissociates versus that which forms a covalent adduct with ICL1 (19). The residual catalytic activity (vi/v0) of 1.0 μM ICL1 was determined after a 20-h preincubation with 0–5.0 μM 2-VIC (Fig. 2E). The value of (vi/v0) failed to reach zero when [2-VIC] exceeded [ICL1], such that a constant level of ∼10% ICL1 activity remained at [2-VIC] ≤ 5.0. Because inactivated ICL1 recovered 50% activity at room temperature after 24 h, conditions similar to this study, we attribute this loss of linearity to the slow reversibility of the covalent adduct. Fitting of the data plotted as activity (vi/v0) versus [2-VIC] (Eq. 4; [2-VIC] = 0–5.0 μM, [ICL1] = 1.0 μM) resulted in an extrapolated x intercept of 1.24 ± 0.04, from which we obtained a partition ratio (P = k9/k11, Fig. 3) of 0.24 ± 0.04. This indicates that 1.2 mol of 2-VIC was required to inactivate 1 mol of ICL1. A similar analysis of ICL2 resulted in P = 0.6 ± 0.1 (SI Appendix, Fig. S4), wherein the titration curve significantly deviated from linearity.
Fig. 3.
Proposed inactivation mechanism of ICL1 by 2-vinyl isocitrate. Crystal structure of (Upper Left) unliganded Mtb ICL1 (Eopen; blue) showing closure (Eclosed; orange) of the active-site loop upon substrate binding (k1). 2-VIC docked into ICL1 (Eclosed–2VIC), for which the Inset shows the base-catalyzed, retro-aldol cleavage of 2-vinyl isocitrate (k3) forming aci-succinate (E–2VG–aci-Succ; green) and 2VG (6) (magenta). Protonation of aci-succinate by Cys191 forms succinate (k5) and Cys191 thiolate. Desorption of succinate (k7) provides steric freedom for Cys191-S− in Eclosed–2VG to proceed to either reaction with enzyme-bound 2VG (k11) (8, Eclosed–HP) or desorption (k9) of 2VG. In the k8 step, either succinate (succ) or DTT (thiols) may enter the active site.
Correction for the reversibility of the covalent adduct is provided by plotting of data in Fig. 2E and SI Appendix, Fig. S4 to Eq. 5, for which Kd is the dissociation constant for the covalent adduct formed from 2-VIC and ICL1/2. Fitting yielded values of P = k9/k11 = 0.11 ± 0.04 (ICL1) and 0.13 ± 0.08 (ICL2) and Kd = k9k12/k10k11 = 0.04 ± 0.02 μM (ICL1) and 4 ± 2 μM (ICL2) (rate constants as in Fig. 3). Note that when none of the covalent adduct decomposes to form free enzyme, k12 = Kd = 0, and Eq. 5 is identical to Eq. 4. Values of Kd, although poorly determined from fitting, suggested that, for ICL1 and ICL2, k12/k10 ∼ 10−7 M and ∼ 10−5 M, respectively, indicating a more rapid recovery of enzyme activity from the ICL2-adduct than that of ICL1.
The partition ratio was also evaluated by a second approach. The ICL-catalyzed generation of succinate from 2-VIC compared with the amount of inactivated enzyme provides a measure of the stoichiometry for inactivation. We ascertained the concentration of succinate produced from 2-VIC during the mechanism-based inactivation of ICL1 with saturating concentrations of 2-VIC. Here, concentration of free enzyme is negligible for rebinding of 2VG (k10 step, Fig. 3) and the preincubation time is sufficiently short (90 min) that the reversal of covalent adduct formation (k12 step) was insignificant. The stoichiometric ratio of quantifiable succinate versus inactivated ICL1 (Fig. 2E, Inset) indicated that 1.24 molecules of succinate were released per inactivation event, thereby providing an independent assessment of the partition ratio (k9/k11 = 0.24). A similar analysis of ICL2 demonstrated that 1.4 ± 0.1 molecules of succinate were released per inactivation event of ICL2 (P = 0.4 ± 0.1; SI Appendix, Fig. S4, Inset), in good agreement with results from fitting to Eqs. 4 and 5. These results indicate that 2-VIC is a highly efficient inactivator of both ICL1 and ICL2, where the partition ratios, averaged from all three evaluations above, are, respectively, P = 0.2 ± 0.08 and 0.4 ± 0.2. The rate of succinate formation (ksucc) from 2-VIC (0.5 mM) catalyzed by ICL1 (20 µM) was also measured and compared with its corresponding and apparent rate of enzyme inactivation for this concentration of 2-VIC; ksucc = 0.06 min−1 and kinact = 0.04 min−1. The rate of succinate cleavage from 2-VIC is much slower than full turnover of isocitrate (kcat = 1,000 min−1), indicating that carbon–carbon bond cleavage is very slow for 2-VIC and that the reaction of 2-vinylglyoxylate with a residue on ICL1 cannot be the sole rate-limiting step of inactivation.
Mass Spectrometry of Mtb ICL1 Treated with 2-VIC.
Electrospray ionization time-of-flight mass spectrometry was performed to affirm the existence of an enzyme–inactivator covalent adduct. An untreated sample of ICL1 enzyme demonstrated a peak of an average mass of 48,788 ± 1 Da, consistent with the theoretical molecular weight of ICL1 monomers (48,787 Da) (SI Appendix, Fig. S5A). A 2-VIC–treated ICL1 sample displayed none of this peak, but instead a major peak at 48,887 ± 1 Da was observed (SI Appendix, Fig. S5B). The apparent increased mass of the enzyme by 99 ± 1 Da is consistent with the addition of C4H3O3 to an ICL1 monomer resulting from formation of a covalent adduct between ICL1 and 2-vinylglyoxylate (2VG). Treatment of the 2-VIC–inactivated ICL1 with 10 mM DTT (90 min; 37 °C) demonstrated an approximate 60:40 ratio of the 48,887-Da and 48,788-Da (2-VIC–ICL1 and ICL1) species, indicating that treatment with DTT had removed nearly 40% of the covalent adduct from the inactivated enzyme (SI Appendix, Fig. S5C). In concert with these results, kinetic analysis demonstrated that, upon addition of 10 mM DTT (37 °C), 2-VIC–treated ICL1 was reactivated to 30% or more of its initial activity before treatment with 2-VIC (SI Appendix, Fig. S6).
Crystallographic Analysis of 2-VIC–Treated Isocitrate Lyase.
As kinetic and mass-spectrometric characterization of the inactivation of ICL1 by 2-VIC is consistent with alkylation of the enzyme, we sought a crystallographic assessment of the mechanism of inactivation. The active site of the ICL1–Mg2+ enzyme assumes an open, solvent-accessible conformation (10). The ICL1–glyoxylate–3NP complex of the C191S mutant of ICL1 was characterized by movement of the K189KCGH193 loop by about 15 Å (measured as the Cα of His193), to yield a closed, solvent-inaccessible active site (10). A crystal structure of 2-VIC–treated ICL1 at 1.8-Å resolution displayed the electron density of a covalent, thioether-linked, homopyruvoyl (HP) moiety attached to the active-site Cys191 in all four subunits of the asymmetric unit (Fig. 4A). Occupancy of the covalent adduct was different in the four monomers, which likely reflects the observed reversibility of the homopyruvoyl adduct during crystallization. Chain A exhibited the highest occupancy for the electron density of all atoms, and all of the interactions described below refer to those of chain A. Although the α-hydroxy-carboxylate substituent of 2-VIC would be expected to bind initially to the Mg2+ ion to undergo retro-aldol cleavage to form 2VG, the observed homopyruvoyl adduct is not coordinated to this Mg2+ ion.
Fig. 4.
X-ray structural characterization of 2-VIC–treated ICL1. (A) Density map structure of ICL1 displaying the S-homopyruvolyated Cys191 residue following treatment with 2-VIC. (B) Cys191 modified with 2-VIC (purple). Potential hydrogen bonds (dashed lines) and interatomic distances are shown. (C) Superposition of crystal structures of ICL1 bound to glyoxylate and 3-NP (gold) or after treatment with 2-VIC (purple). (D) Superposition of active-site structures of ICL1 including Cys191 (yellow) treated with 3-bromopyruvate (S-pyruvoylated enzyme, gray) (10) and 2-VIC (S-homopyruvoylated, purple). The labeling scheme is as follows: nitrogen, blue; oxygen, red; sulfur, yellow; water, red spheres; Mg2+ ion, green sphere; and hydrogen bonds, dashed lines.
Instead, the covalently tethered keto acid (electron density was modeled as an α-keto acid) has turned away from the Mg2+ ion toward the succinate binding site, as in the case of 3BP (10). The closed active site provides adequate sequestration of the reactive 2VG for its eventual migration to and reaction with Cys191. One of its carboxylate oxygens forms apparent hydrogen bonds with His193 and Ser315 (both at distances of 2.5 Å), whereas the other carboxylate oxygen is within hydrogen-bonding distance to Asn313, Ser317, and Thr347 (3.3-, 2.6-, and 2.8-Å distances, respectively; Fig. 4B). The α-keto oxygen forms no apparent hydrogen bonds with the protein, and it is oriented toward the indole ring of Trp93; Trp93 possibly prevents the recoordination of the cysteine-tethered keto acid to the Mg2+ ion. In the crystal structure of the C191S ICL1 mutant complexed with glyoxylate and 3NP, the bound 3NP is structurally indistinguishable from succinate, and this structure provides a model for the ICL1–glyoxylate–succinate complex (10). When the 3NP-bound structure is overlaid with the HP-ICL structure, the HP moiety appears to bind preferentially in the succinate binding pocket (Fig. 4C). In order for the homopyruvoyl group to adopt these interactions, the succinate formed from 2-VIC would need to dissociate from enzyme. Additionally, the homopyruvoyl group attached to C191 in ICL1 assumes a binding mode that is very similar to that of the S-pyruvoylated form of 3BP-treated ICL1 (10). Fig. 4D shows the overlay of the structures of 3BR-treated ICL1 (gray; PDB ID code 1F8M) and 2-VIC–treated ICL1 (purple). All of the residues in the active site are nearly superimposable. In both covalently modified structures, the binding site in which glyoxylate is chelated to the Mg2+ ion is vacant, as both S-pyruvoyl and S-homopyruvoyl groups bind in the succinate binding pocket, and interact with the same five residues via apparent hydrogen bonds. Unlike the S-homopyruvoyl structure, only one of the carboxylate oxygens from the S-pyruvoyl group is in adequate proximity to Thr347, Ser315, and Asn313 for hydrogen bonding. The α-keto group of the S-pyruvoyl complex is within hydrogen-bonding distance to Ser317 and His193.
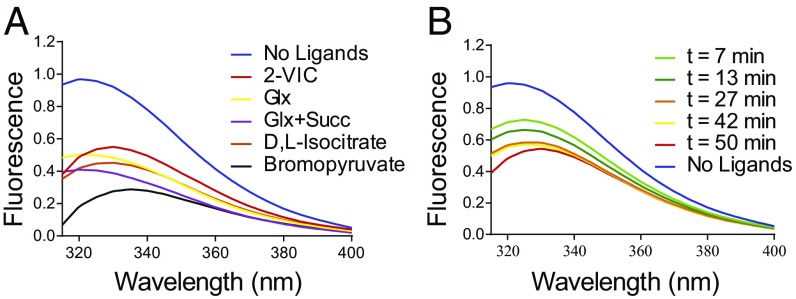
Effects of Ligand Binding on the Intrinsic Protein Fluorescence of ICL1.
The intrinsic protein fluorescence of Mtb ICL1 (λex = 290 nm, λem = 320 nm) is diminished by ligand binding, and by covalent reaction with 3BP and 2-VIC (Fig. 5A). Tyrosine and tryptophan residues proximal to the active site of ICL1 [e.g., Tyr89 and Trp93 (Figs. 3 and 4)] likely contribute to the observed changes in fluorescence upon ligand binding in the active site. In comparison with the unliganded enzyme, 3BP-inactivated ICL1 exhibits the largest decrease in protein fluorescence (−60%) followed by ICL1 plus Glx (−50%), ICL1 plus isocitrate (−55%), and 2-VIC-inactivated enzyme (−40%). Because an equilibrium between isocitrate and the products of enzymatic turnover is established within minutes of mixing ICL1 and isocitrate at our experimental concentrations, the nearly identical fluorescence emission spectra of ICL1 plus isocitrate and ICL1 plus Glx suggests that the binary ICL1–Glx complex, and not ICL1–isocitrate, would be the predominant species in the ICL1 plus isocitrate sample. The decrease in intrinsic protein fluorescence accompanies a significant change in ICL1 conformation, as observed in crystallographic analysis of ICL1, arising in part from the large (15-Å) movement of the aforementioned active-site loop (containing Cys191) upon ligand binding (10). Notably, the apparent covalent inactivation arising from added 0.1 mM 2-VIC (red) or 0.1 mM 3BP (black) led to a diminution of ICL1 fluorescence, which is consistent with the closed conformation of the active site for the covalently modified ICL1 structure. We also observed a time-dependent shift and decrease of the relative fluorescence of ICL1, from 1.0 to an invariant value of 0.6, during a 20-min incubation of ICL1 with 100 μM 2-VIC (Fig. 5B). An apparent, first-order rate constant of change in fluorescence (kobs = 0.01 min−1) was comparable to kinact obtained from kinetic studies. To evaluate the structural changes occurring during 2-VIC inactivation, 2-VIC and its reaction products 2VG and succinate were docked into the closed conformation of the ICL1 crystal structures using AutoDock Vina (21) based on these previous crystallographic structures, and the results are discussed below.
Fig. 5.
Intrinsic protein fluorescence changes arising from ligand binding to ICL1. (A) Free 1 µM ICL1 (blue line) displayed fluorescence maxima at λmax = 325 nm, which is diminished upon binding of 1 mM d,l-isocitrate (orange), 1 mM glyoxylate (yellow), 0.1 mM 2-VIC (red), 0.1 mM 3BP (black), and 10 mM glyoxylate and succinate (purple). (B) Time-dependent fluorescence change upon incubation of 1 µM ICL1 with 0.1 mM 2-VIC.
Discussion
A proposed chemical mechanism, accompanied with docked structures of the enzyme species and ligands formed during ICL1 inactivation by 2-VIC, is depicted in Fig. 3. The unliganded form of ICL1 preferentially maintains the open conformation (Fig. 3, Eopen), in which the active-site loop (residues 189–193) is distal to the bound Mg2+ ion. Molecular docking of 2-VIC into the active site of C191S ICL1, for which the 1-carboxylate and 2-hydroxyl groups are coordinated to Mg2+ (E–2-VIC), suggests that 2-VIC binds to ICL1 (step k1) in the same manner as isocitrate (Eclosed–2VIC). Closing of the active site upon ligand binding (Eclosed) results in movement of this loop as well as slight movement of the Mg2+ ion. This brings Cys191 into position for catalysis. Base-catalyzed cleavage of the C2–C3 bond (k3) produces Mg2+-bound 2VG (green) and the aci-anionic form of succinate (E–2VG–aci-Succ). Protonation of aci-succinate by Cys191 (k5) then produces succinate and the thiolate form of Cys191 (E–2VG–Succ). Modeling of the ICL1–2VG–succinate complex is consistent with the coordination of 2VG to the Mg2+ ion, and its interaction with active-site residues is similar to that of unsubstituted glyoxylate. It is noteworthy that succinate blocks access of Mg2+-bound 2VG to the thiolate ion of Cys191, which is consistent with the observed protection from 2-VIC inactivation afforded by added succinate (k8). We propose that the expulsion of succinate from the partially or fully opened active site of E–2VG–Succ (k7) allows 2VG to rotate and migrate to the succinate binding site, forming the covalent complex ICL–HP (8) via Michael addition of the Cys191 thiolate to 2VG (k11). Desorption of succinate from a partially opened active site also allows 2VG either to escape from the active site (k9), or to access added thiols for preemptive reaction with enzyme-bound 2VG (k8).
The release of 2VG from ICL1–2VG occurs at a rate that is 20% that of its reaction with Cys191 to form E-HP, as indicated by the partition analysis from which k9/k11 = 0.20. Because little 2VG desorbs from the active site before formation of ICL-HP (k9/k11 = 0.2), it is unlikely that k11 is the rate-limiting step in 2-VIC inactivation, as is supported by the measured rate of succinate formation from 2-VIC equal to 1.5kinact. ICL1-catalyzed cleavage of 2-methyl-isocitrate has a value of kcat (60 min−1), which is 5% that of isocitrate (7), suggesting that the substitution of a methyl or vinyl group on carbon-2 of isocitrate may further reduce the ability of ICL to cleave the C2–C3 bond of 2-VIC, and render k3 a slow step in inactivation (kinact). We propose that the E–2VG complex (vacant succinate subsite) is the complex at which covalent reaction with enzyme is interdicted by added thiols (k8). As shown in the dialysis studies that the ICL1-HP adduct was slowly reversible by a presumed retro-Michael reaction, DTT likely intercepts and reacts with reformed 2-VG upon its release from Cys191. Although we observed that extended incubation of DTT with ICL1-HP resulted in reactivation of enzyme, both kinetically and by mass spectrometric analysis, incubation of ICL1-3BP with DTT does not restore ICL1 activity (SI Appendix, Fig. S6). This suggests that the apparent restoration of free Cys191 from S-homopyruvoylated ICL1, possibly mediated by DTT, likely occurs by a retro-Michael reaction, a reaction not available to the S-pyruvoylated enzyme.
Kinetic, mass spectrometric, protein fluorescence, molecular modeling, and X-ray crystallographic data are all consistent with the proposed chemical mechanism of inactivation of ICL by 2-VIC shown in Fig. 3. Our results demonstrate that 2-VIC comprises a true mechanism-based inactivator of both ICL1 and ICL2, effectively providing an electrophile similar to the affinity label 3BP, namely, 2-vinylglyoxylate, almost exclusively confined to the active site of Mtb ICL, and which forms a covalent bond with its conserved active-site cysteine. We believe that this type of mechanism-based inactivation may be the first of its kind for an isocitrate lyase. As a drug discovery strategy for TB, mechanism-based enzyme inactivation of target enzymes in Mycobacteria offers significant advantages over competitive inhibition, which include the following: (i) a “precision” covalent reaction confined to active-site residues of the target enzyme; (ii) irreversible, or slowly reversible, covalent inactivation of the target enzyme; and (iii) the targeting of an invariant active-site catalytic group, Cys191, which should be less subject to the development of resistance due to mutation. Forthcoming analogs of 2-VIC, although they may bind to other human enzymes, should exert little toxicity in mammalian cells lacking an isocitrate lyase owing to their inability to generate 2-vinylglyoxylate. Recently, benzothiazinones have been shown to be potent antimycobacterial agents, and are mechanism-based inactivators of DprE1, an enzyme located in the cell wall of Mtb. The benzothiazinones undergo reduction of a nitro substituent, resulting in a nitroso group, which subsequently reacts with a cysteine residue in the active site of DprE1 (22). The inactivation kinetic parameters of these compounds are comparable to those of 2-VIC. Our future efforts will concern the development of new analogs of 2-VIC that likewise provide mechanism-based inactivation as well as antimycobacterial activity in cell culture and in in vivo models of TB infection.
Materials and Methods
dl-Isocitrate (trisodium salt), d-threo-isocitrate (potassium salt), NaCl, DMSO, sodium glyoxylate (monohydrate), succinic acid, d-malic acid, glutathione (reduced form), DTT, and all other chemicals were obtained from Sigma-Aldrich. Isocitrate dehydrogenase (IDH) and lactate dehydrogenase (LDH) from E. coli were prepared as described (13).
Enzymes.
Two constructs of recombinant ICL1 from Mtb were used in these studies: a tag-free form (ICL1-TF) and a hexahistidine-tagged form (ICL1), and their expression and purification are described in SI Appendix. A truncated form of Mtb ICL2 (amino acids 1–605) with a C-terminal His6 tag was expressed in E. coli BL21(DE3) as described in SI Appendix.
X-Ray Crystallography of 2-VIC–Treated ICL.
ICL1:2-VIC complexes were prepared by incubating ICL-TF (∼10 mg/mL) in 50 mM Tris (pH 8.0), 10 mM MgCl2, and 0.5 mM DTT with 3 mM 2-VIC overnight at 17 °C. A concentration of 0.5 mM DTT did not impede covalent inactivation of ICL1-TF but was sufficient to prevent oxidation of the cysteine residues of ICL1. The hanging drop vapor diffusion method was used to produce crystals. A 1:1 volume ratio of the protein with a solution of 0.1 M Tris⋅HCl (pH 8.0), 0.2 M sodium acetate, and 20–30% (wt/vol) PEG 4000 was used to crystalize the protein. Crystals appeared in 2–3 wk after setting ∼3-μL drops against 500 μL of mother liquor in a closed reservoir at 17 °C. Fresh mother liquor was used as the cryoprotectant, and crystals were flash-frozen in liquid nitrogen for X-ray data collection, and maintained chilled under a stream of liquid nitrogen (100 K) throughout data collection. High-resolution diffraction data for ICL–2-VIC cocrystals were collected on an ADSC Quantum 315 detector at a wavelength of 0.9793 Å from beamline 19ID of the Structural Biology Center (Advanced Photon Source, Argonne National Laboratory). Detailed data transformation and structure refinement are described in SI Appendix.
Enzyme Assays.
Unless otherwise specified, all assays were conducted in reaction mixtures of 50 mM Hepes (pH 7.5), 5 mM MgCl2, and 1 mM DTT at 37 °C. In the direction of isocitrate lysis, product glyoxylate was either converted to glycolate in a coupled-enzyme assay using E. coli lactate dehydrogenase (ε340 = 6,220 M−1⋅cm−1) or reacted with phenylhydrazine-HCl to form a phenylhydrazone product (ε324 = 17,000 M−1⋅cm−1). In the direction of isocitrate synthesis, isocitrate product was converted to α-ketoglutarate in a coupled-enzyme assay using E. coli isocitrate dehydrogenase (ε340 = 6,220 M−1⋅cm−1).
Quantification of Product Succinate in ICL Inactivation Studies.
Succinic acid formed from ICL1-catalyzed cleavage of 2-VIC was detected using the succinate colorimetric assay kit and protocol of Biovision.
Preincubation Inactivation Studies of ICL1 and ICL2.
The time-dependent inactivation of ICL1 by 2-VIC (5a) was assessed by preincubation studies, in which residual enzyme activity was measured as initial velocity of either the forward or reverse reactions after preincubation of enzyme with 2-VIC. For the reverse reaction of ICL, 0.6-mL reaction mixtures containing 50 mM Hepes (pH 7.5), 5 mM MgSO4, 0.8 µM ICL, and 0–40 μM inactivator were incubated in sealed Eppendorf tubes. Fifty-microliter samples were withdrawn from the preincubation mixtures (0–300 min) and diluted 50-fold, and residual ICL1 activity was measured by spectrophotometry (Cary 100 spectrophotometer; 340 nm) of the absorbance of NADPH formation using the isocitrate dehydrogenase assay. For measurement of residual ICL activity using the forward reaction, 1 μM ICL1 or 15 μM ICL2 was incubated with 0–3 mM 2-VIC in preincubation buffer containing 50 mM Hepes (pH 7.5), 5 mM MgCl2, in the presence or absence of DTT. Samples were withdrawn from preincubation mixtures, diluted 50-fold, and glyoxylate product from lysis of isocitrate was measured via the phenylhydrazine assay as described in SI Appendix. Initial rates were normalized to that of an enzyme sample with no inactivator added.
Analysis of Kinetic Data.
Data analysis was conducted by global fitting of nonlinear regression using SigmaPlot (Systat). Time-dependent inactivation of ICL was assessed by fitting of the preincubation data to Eq. 1 in which vi and v0 are the initial rates at time t and 0, respectively, t is preincubation time, [I] is the micromolar concentration of inactivator, kinact is the maximal rate constant of inactivation, Kinact is the concentration of inactivator at which the observed rate constant of inactivation is one-half that of kinact, and kbgd is the observed rate constant for background loss of ICL activity over the experimental time courses, independent of the inactivator:
| [1] |
In addition, we fitted the time courses at each concentration of inactivator to Eq. 2, where resulting values of kobs were then replotted using Eq. 3:
| [2] |
| [3] |
For the partition analysis of mechanism-based inactivation, data were fitted to Eqs. 4 and 5; vi and v0 are, respectively, ICL activity measured after preincubation with or without 2-VIC, [2-VIC] and [Et] are the micromolar concentrations of inactivator and enzyme, respectively, p is the partition coefficient (k9/k11) and K′d = Kd/(1 + p) = (k9k12/k10k11)/(1 + k9/k11):
| [4] |
| [5] |
Protein Fluorescence.
ICL1 (1 µM monomers) in a buffer mixture of 50 mM Hepes (pH 7.5) and 10 mM MgCl2 was incubated at room temperature for 50 min with 1 mM dl-isocitrate, 100 µM 2-VIC, or 100 µM 3BP. Reaction mixtures (200 μL) were excited at λex = 290 nm, and the intrinsic fluorescence emission spectra (λem = 315–400 nm) were measured at room temperature in a Biotek Synergy plate reader using 96-well Greiner black, clear-bottom plates. The intrinsic fluorescence of ICL1 was also recorded in the presence of 10 mM glyoxylate and/or 10 mM succinate.
Molecular Docking.
Molecular models of ICL1 binary and tertiary complexes were built by docking of 2-VIC and 2VG plus succinate, respectively, into the crystal structure of the C191S mutant Mtb ICL1 (PDB ID code 1F61) (10) using Chimera/AutoDock Vina (21), which performs fitting of small-molecule ligands with freely rotatable bonds separated by three consecutive covalent bonds or fewer. Its training set allows small-molecule rotational freedom for up to 35 atoms while fixing the macromolecular receptors in rigid conformations. 2-VIC was posed in the closed conformation of ICL1 based on the structure of the C191S ICL1–glyoxylate complex (10), in which its 1-carboxylate and 2-hydroxyl groups of 2-VIC are coordinated to the magnesium ion in a manner analogous to glyoxylate. The model of the ternary ICL1–2VG–succinate complex was based on C191S ICL1–glyoxylate–3NP complex (10).
Supplementary Material
Acknowledgments
We thank GlaxoSmithKline Pharmaceuticals for support of the synthesis of 2-VIC and for materials to prepare ICL2. We thank the Argonne National Laboratory for crystallographic data. We thank Kimberly Loesch for analysis of cellular toxicity. We thank Texas A&M AgriLife Research, the Welch Foundation (Grant A-0015), and the NIH (Grant P011AI095208) for providing funding for this research.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The atomic coordinates and structure factors have been deposited in the Protein Data Bank, www.pdb.org (PDB ID code 5DQL).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1706134114/-/DCSupplemental.
References
- 1. World Health Organization (2015) Global Tuberculosis Report 2015 (World Health Organization, Geneva), Report No. WHO/HTM/TB/2015.22.
- 2.Wayne LG, Lin KY. Glyoxylate metabolism and adaptation of Mycobacterium tuberculosis to survival under anaerobic conditions. Infect Immun. 1982;37:1042–1049. doi: 10.1128/iai.37.3.1042-1049.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wheeler PR, Ratledge C. Use of carbon sources for lipid biosynthesis in Mycobacterium leprae: A comparison with other pathogenic mycobacteria. J Gen Microbiol. 1988;134:2111–2121. doi: 10.1099/00221287-134-8-2111. [DOI] [PubMed] [Google Scholar]
- 4.McKinney JD, et al. Persistence of Mycobacterium tuberculosis in macrophages and mice requires the glyoxylate shunt enzyme isocitrate lyase. Nature. 2000;406:735–738. doi: 10.1038/35021074. [DOI] [PubMed] [Google Scholar]
- 5.Muñoz-Elías EJ, McKinney JD. Mycobacterium tuberculosis isocitrate lyases 1 and 2 are jointly required for in vivo growth and virulence. Nat Med. 2005;11:638–644. doi: 10.1038/nm1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kondrashov FA, Koonin EV, Morgunov IG, Finogenova TV, Kondrashova MN. Evolution of glyoxylate cycle enzymes in Metazoa: Evidence of multiple horizontal transfer events and pseudogene formation. Biol Direct. 2006;1:31. doi: 10.1186/1745-6150-1-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gould TA, van de Langemheen H, Muñoz-Elías EJ, McKinney JD, Sacchettini JC. Dual role of isocitrate lyase 1 in the glyoxylate and methylcitrate cycles in Mycobacterium tuberculosis. Mol Microbiol. 2006;61:940–947. doi: 10.1111/j.1365-2958.2006.05297.x. [DOI] [PubMed] [Google Scholar]
- 8.Dunn MF, Ramírez-Trujillo JA, Hernández-Lucas I. Major roles of isocitrate lyase and malate synthase in bacterial and fungal pathogenesis. Microbiology. 2009;155:3166–3175. doi: 10.1099/mic.0.030858-0. [DOI] [PubMed] [Google Scholar]
- 9.Eoh H, Rhee KY. Methylcitrate cycle defines the bactericidal essentiality of isocitrate lyase for survival of Mycobacterium tuberculosis on fatty acids. Proc Natl Acad Sci USA. 2014;111:4976–4981. doi: 10.1073/pnas.1400390111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sharma V, et al. Structure of isocitrate lyase, a persistence factor of Mycobacterium tuberculosis. Nat Struct Biol. 2000;7:663–668. doi: 10.1038/77964. [DOI] [PubMed] [Google Scholar]
- 11.Singh J, Petter RC, Baillie TA, Whitty A. The resurgence of covalent drugs. Nat Rev Drug Discov. 2011;10:307–317. doi: 10.1038/nrd3410. [DOI] [PubMed] [Google Scholar]
- 12.Bradshaw JM, et al. Prolonged and tunable residence time using reversible covalent kinase inhibitors. Nat Chem Biol. 2015;11:525–531. doi: 10.1038/nchembio.1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moynihan MM, Murkin AS. Cysteine is the general base that serves in catalysis by isocitrate lyase and in mechanism-based inhibition by 3-nitropropionate. Biochemistry. 2014;53:178–187. doi: 10.1021/bi401432t. [DOI] [PubMed] [Google Scholar]
- 14.Schloss JV, Cleland WW. Inhibition of isocitrate lyase by 3-nitropropionate, a reaction-intermediate analogue. Biochemistry. 1982;21:4420–4427. doi: 10.1021/bi00261a035. [DOI] [PubMed] [Google Scholar]
- 15.Quartararo CE, Hadi T, Cahill SM, Blanchard JS. Solvent isotope-induced equilibrium perturbation for isocitrate lyase. Biochemistry. 2013;52:9286–9293. doi: 10.1021/bi4013319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ko YH, McFadden BA. Alkylation of isocitrate lyase from Escherichia coli by 3-bromopyruvate. Arch Biochem Biophys. 1990;278:373–380. doi: 10.1016/0003-9861(90)90273-2. [DOI] [PubMed] [Google Scholar]
- 17.Silverman RB, Holladay MW. The Organic Chemistry of Drug Design and Drug Action. 3rd Ed. Elsevier; Amsterdam: 2014. pp. 247–261. [Google Scholar]
- 18.Walsh CT. Suicide substrates, mechanism-based enzyme inactivators: Recent developments. Annu Rev Biochem. 1984;53:493–535. doi: 10.1146/annurev.bi.53.070184.002425. [DOI] [PubMed] [Google Scholar]
- 19.Silverman RB. Mechanism-based enzyme inactivators. Methods Enzymol. 1995;249:240–283. doi: 10.1016/0076-6879(95)49038-8. [DOI] [PubMed] [Google Scholar]
- 20.Kitz R, Wilson IB. Esters of methanesulfonic acid as irreversible inhibitors of acetylcholinesterase. J Biol Chem. 1962;237:3245–3249. [PubMed] [Google Scholar]
- 21.Trott O, Olson AJ. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J Comput Chem. 2010;31:455–461. doi: 10.1002/jcc.21334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Trefzer C, et al. Benzothiazinones are suicide inhibitors of mycobacterial decaprenylphosphoryl-β-d-ribofuranose 2′-oxidase DprE1. J Am Chem Soc. 2012;134:912–915. doi: 10.1021/ja211042r. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.