Abstract
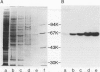
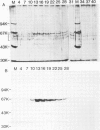
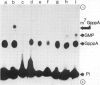
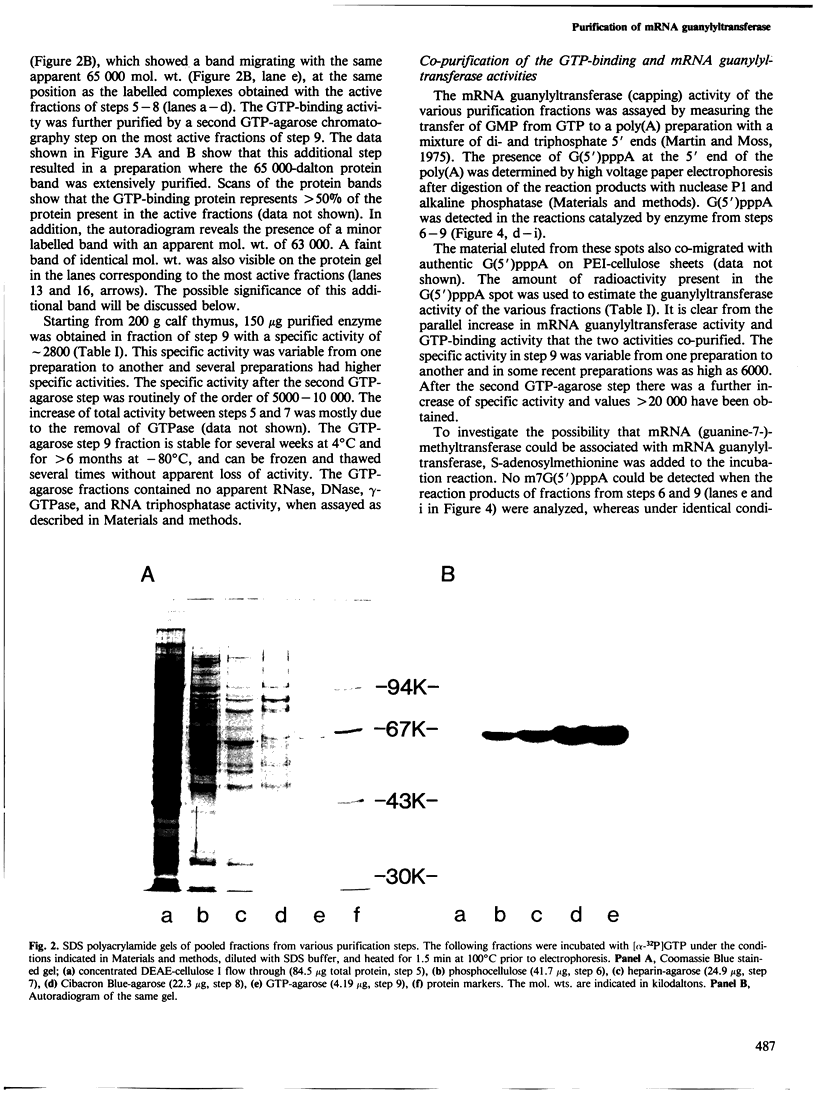
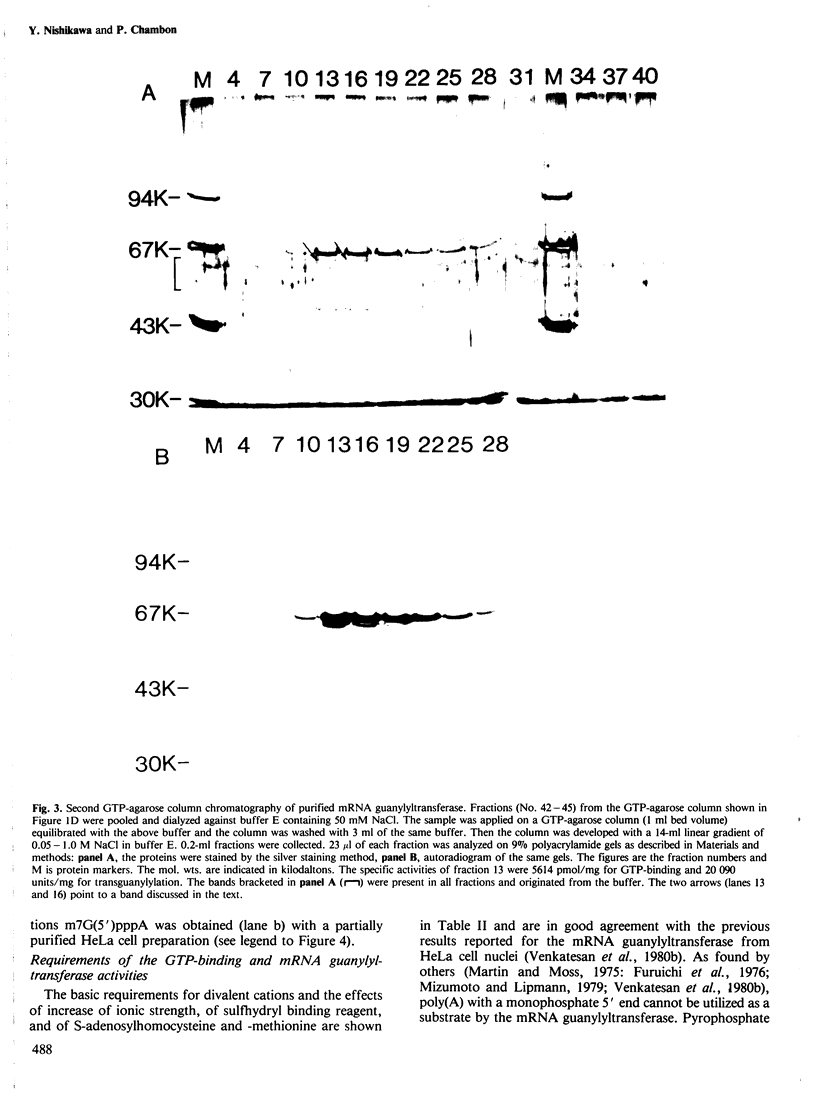
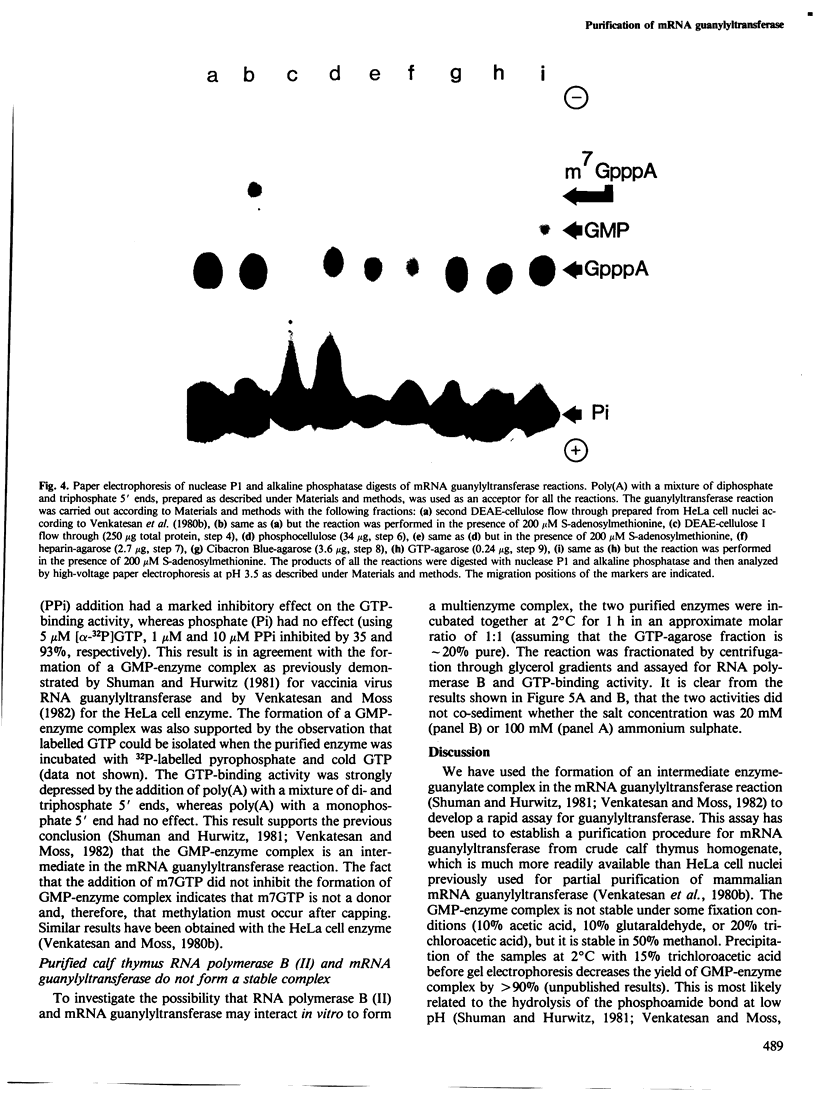
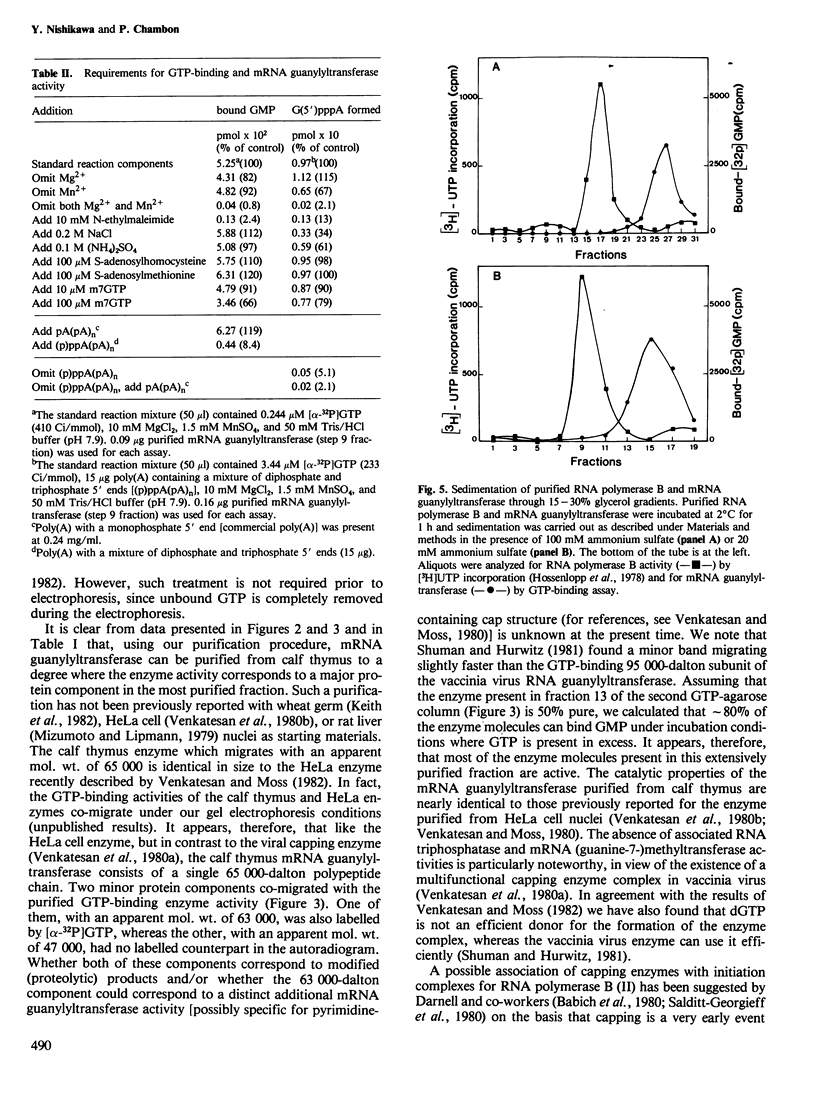
mRNA guanylyltransferase has been extensively purified from calf thymus. A GTP-binding assay was used based on the observations by Shuman and Hurwitz (1981) and Venkatesan and Moss (1982) that vaccinia virus and HeLa cell mRNA guanylyltransferases bind the GMP moiety from GTP in the absence of an acceptor RNA. The mol. wt. of the purified enzyme from calf thymus, estimated by polyacrylamide gel electrophoresis in the presence of SDS, is 65 000. The major protein in the purified enzyme fraction comigrates with the peptide labelled with GMP. Based on scans of silver-stained polyacrylamide gels, mRNA guanylyltransferase constitutes greater than 50% of the protein in these fractions. The enzyme catalyzed the guanylylation at the 5' end of poly(A) with a mixture of diphosphate and triphosphate ends. No evidence was obtained for a direct interaction between mRNA guanylyltransferase and RNA polymerase B (II).
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Babich A., Nevins J. R., Darnell J. E., Jr Early capping of transcripts from the adenovirus major late transcription unit. Nature. 1980 Sep 18;287(5779):246–248. doi: 10.1038/287246a0. [DOI] [PubMed] [Google Scholar]
- Bajszár G., Szabó G., Simoncsits A., Molnár J. Methylated cap formation by enzymes bound to nuclear informofer particles. Mol Biol Rep. 1978 Jun 16;4(2):93–96. doi: 10.1007/BF00775967. [DOI] [PubMed] [Google Scholar]
- Banerjee A. K. 5'-terminal cap structure in eucaryotic messenger ribonucleic acids. Microbiol Rev. 1980 Jun;44(2):175–205. doi: 10.1128/mr.44.2.175-205.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donis-Keller H., Maxam A. M., Gilbert W. Mapping adenines, guanines, and pyrimidines in RNA. Nucleic Acids Res. 1977 Aug;4(8):2527–2538. doi: 10.1093/nar/4.8.2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuichi Y., Muthukrishnan S., Tomasz J., Shatkin A. J. Mechanism of formation of reovirus mRNA 5'-terminal blocked and methylated sequence, m7GpppGmpC. J Biol Chem. 1976 Aug 25;251(16):5043–5053. [PubMed] [Google Scholar]
- Hodo H. G., 3rd, Blatti S. P. Purification using polyethylenimine precipitation and low molecular weight subunit analyses of calf thymus and wheat germ DNA-dependent RNA polymerase II. Biochemistry. 1977 May 31;16(11):2334–2343. doi: 10.1021/bi00630a005. [DOI] [PubMed] [Google Scholar]
- Hossenlopp P., Oudet P., Chambon P. Animal DNA-dependent RNA polymerases. Studies on the binding of mammalian RNA polymerases AI and B to Simian virus 40 DNA. Eur J Biochem. 1974 Jan 16;41(2):397–411. doi: 10.1111/j.1432-1033.1974.tb03281.x. [DOI] [PubMed] [Google Scholar]
- Hossenlopp P., Sümegi J., Chambon P. Transcription in vitro of adenovirus-2 DNA by RNA polymerases class C purified from uninfected and adenovirus-infected HeLa cells. Eur J Biochem. 1978 Oct 16;90(3):615–631. doi: 10.1111/j.1432-1033.1978.tb12642.x. [DOI] [PubMed] [Google Scholar]
- Kedinger C., Chambon P. Animal DNA-dependent RNA polymerases. 3. Purification of calf-thymus BI and BII enzymes. Eur J Biochem. 1972 Jul 13;28(2):283–290. doi: 10.1111/j.1432-1033.1972.tb01912.x. [DOI] [PubMed] [Google Scholar]
- Keith J. M., Venkatesan S., Gershowitz A., Moss B. Purification and characterization of the messenger ribonucleic acid capping enzyme GTP:RNA guanylyltransferase from wheat germ. Biochemistry. 1982 Jan 19;21(2):327–333. doi: 10.1021/bi00531a020. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Langberg S. R., Moss B. Post-transcriptional modifications of mRNA. Purification and characterization of cap I and cap II RNA (nucleoside-2'-)-methyltransferases from HeLa cells. J Biol Chem. 1981 Oct 10;256(19):10054–10060. [PubMed] [Google Scholar]
- Martin S. A., Moss B. Modification of RNA by mRNA guanylyltransferase and mRNA (guanine-7-)methyltransferase from vaccinia virions. J Biol Chem. 1975 Dec 25;250(24):9330–9335. [PubMed] [Google Scholar]
- McKnight G. S. A colorimetric method for the determination of submicrogram quantities of protein. Anal Biochem. 1977 Mar;78(1):86–92. doi: 10.1016/0003-2697(77)90011-2. [DOI] [PubMed] [Google Scholar]
- Mizumoto K., Lipmann F. Transmethylation and transguanylylation in 5'-RNA capping system isolated from rat liver nuclei. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4961–4965. doi: 10.1073/pnas.76.10.4961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss B., Gershowitz A., Wei C. M., Boone R. Formation of the guanylylated and methylated 5'-terminus of vaccinia virus mRNA. Virology. 1976 Jul 15;72(2):341–351. doi: 10.1016/0042-6822(76)90163-x. [DOI] [PubMed] [Google Scholar]
- Palmiter R. D. Magnesium precipitation of ribonucleoprotein complexes. Expedient techniques for the isolation of undergraded polysomes and messenger ribonucleic acid. Biochemistry. 1974 Aug 13;13(17):3606–3615. doi: 10.1021/bi00714a032. [DOI] [PubMed] [Google Scholar]
- Salditt-Georgieff M., Harpold M., Chen-Kiang S., Darnell J. E., Jr The addition of 5' cap structures occurs early in hnRNA synthesis and prematurely terminated molecules are capped. Cell. 1980 Jan;19(1):69–78. doi: 10.1016/0092-8674(80)90389-x. [DOI] [PubMed] [Google Scholar]
- Schaffner W., Weissmann C. A rapid, sensitive, and specific method for the determination of protein in dilute solution. Anal Biochem. 1973 Dec;56(2):502–514. doi: 10.1016/0003-2697(73)90217-0. [DOI] [PubMed] [Google Scholar]
- Shuman S., Hurwitz J. Mechanism of mRNA capping by vaccinia virus guanylyltransferase: characterization of an enzyme--guanylate intermediate. Proc Natl Acad Sci U S A. 1981 Jan;78(1):187–191. doi: 10.1073/pnas.78.1.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatesan S., Gershowitz A., Moss B. Modification of the 5' end of mRNA. Association of RNA triphosphatase with the RNA guanylyltransferase-RNA (guanine-7-)methyltransferase complex from vaccinia virus. J Biol Chem. 1980 Feb 10;255(3):903–908. [PubMed] [Google Scholar]
- Venkatesan S., Gershowitz A., Moss B. Purification and characterization of mRNA guanylyltransferase from HeLa cell nuclei. J Biol Chem. 1980 Apr 10;255(7):2829–2834. [PubMed] [Google Scholar]
- Venkatesan S., Moss B. Donor and acceptor specificities of HeLa cell mRNA guanylyltransferase. J Biol Chem. 1980 Apr 10;255(7):2835–2842. [PubMed] [Google Scholar]
- Venkatesan S., Moss B. Eukaryotic mRNA capping enzyme-guanylate covalent intermediate. Proc Natl Acad Sci U S A. 1982 Jan;79(2):340–344. doi: 10.1073/pnas.79.2.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei C., Moss B. 5'-Terminal capping of RNA by guanylyltransferase from HeLa cell nuclei. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3758–3761. doi: 10.1073/pnas.74.9.3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weil P. A., Luse D. S., Segall J., Roeder R. G. Selective and accurate initiation of transcription at the Ad2 major late promotor in a soluble system dependent on purified RNA polymerase II and DNA. Cell. 1979 Oct;18(2):469–484. doi: 10.1016/0092-8674(79)90065-5. [DOI] [PubMed] [Google Scholar]
- Wray W., Boulikas T., Wray V. P., Hancock R. Silver staining of proteins in polyacrylamide gels. Anal Biochem. 1981 Nov 15;118(1):197–203. doi: 10.1016/0003-2697(81)90179-2. [DOI] [PubMed] [Google Scholar]