Abstract
The objective of this study was to develop a diagnostic model that allows for a highly specific diagnosis of chronic hypersensitivity pneumonitis using clinical and radiological variables alone. Chronic hypersensitivity pneumonitis and other interstitial lung disease cases were retrospectively identified from a longitudinal database. High-resolution CT scans were blindly scored for radiographic features (eg, ground-glass opacity, mosaic perfusion) as well as the radiologist’s diagnostic impression. Candidate models were developed then evaluated using clinical and radiographic variables and assessed by the cross-validated C-statistic. Forty-four chronic hypersensitivity pneumonitis and eighty other interstitial lung disease cases were identified. Two models were selected based on their statistical performance, clinical applicability and face validity. Key model variables included age, down feather and/or bird exposure, radiographic presence of ground-glass opacity and mosaic perfusion and moderate or high confidence in the radiographic impression of chronic hypersensitivity pneumonitis. Models were internally validated with good performance, and cut-off values were established that resulted in high specificity for a diagnosis of chronic hypersensitivity pneumonitis.
INTRODUCTION
Chronic hypersensitivity pneumonitis (HP) is an interstitial lung disease (ILD) caused by long-standing exposure to an environmental antigen1 that can be challenging to distinguish from other forms of chronic ILD.2,3 Making an accurate diagnosis of chronic HP is essential as chronic HP requires a distinct management plan and informs prognosis.3,4 Previously proposed multidimensional diagnostic models for chronic HP have failed to gain wide-spread acceptance, perhaps due to their complexity and lack of specificity for chronic disease.5,6 The purpose of this study was to construct a clinically feasible, multidimensional diagnostic model for chronic HP that would allow for a highly specific diagnosis in the absence of surgical lung biopsy.
METHODS
Study population
Patients with chronic HP and other ILDs seen between 2006 and 2012 were identified from a longitudinal cohort at the University of California, San Francisco. All patients were required to have a high-resolution CT (HRCT) scan of the chest performed within 1 year of their baseline clinic visit. Chronic HP cases were required to have a surgical lung biopsy and the criteria to establish a diagnosis of HP were consistent with those previously published from our institution.4,7 Non-HP ILD cases were identified from the database by selecting the non-HP patients immediately before and after each HP case included, resulting in a 1:2 case: control ratio. All non-HP ILD diagnoses were allowed; only ‘unclassifiable ILD’ cases were excluded.
Study variables
Baseline demographic and clinical data included in the analyses were age, sex, smoking history and pulmonary function test values. Self-reported, standardised and prospectively collected exposure histories to birds, down feathers, moulds, water damage, indoor hot tubs, steam room/saunas, humidifiers or farming were also included. All HRCT scans included thin section (<1.25 mm collimation) inspiratory images. Each scan was scored for specific features (see online supplementary table S1) and overall diagnostic impression by an expert thoracic radiologist (BME) blinded to the patient’s clinical data and diagnosis.
Prediction models were derived in the subset of patients with expiratory images,8 and validated in the remainder. Predictor variables associated (p<0.10) with a diagnosis of chronic HP were considered for inclusion in multivariate models. Candidate models were compared using the C-statistic, estimated using 10-fold cross-validation. For the models with the highest cross-validated C-statistic (CV-C), goodness of fit (GOF) was assessed using the p value for the Hosmer-Lemeshow (H-L) statistic.9 Two diagnostic models were developed that varied by HRCT variables included. Model 1 included specific radiographic findings, while model 2 included the radiologist’s diagnostic impression (dichotomised into low vs moderate or high likelihood of chronic HP). Final models were selected for CV-C at or near the maximum observed value, H-L p value >0.10, clinical feasibility (ie, the availability and ease of use for included variables) and face validity (ie, the clinical appropriateness of included variables). Receiver operating characteristic curves were constructed for each model. Model performance was tested in the validation cohort, as well as among the subset of patients with either chronic HP or idiopathic pulmonary fibrosis (IPF). All analyses were done using Stata V.13.1 (Stata, College Station, Texas, USA).
RESULTS
Two hundred and sixty-one patients were identified from the database (87 chronic HP, 174 non-HP ILD) and 190 met eligibility criteria (66 HP, 124 non-HP ILD controls) (see online supplementary figure S2). The derivation cohort consisted of 124 patients (44 HP, 80 non-HP ILD controls). The validation cohort consisted of 66 patients (22 HP, 44 non-HP ILD controls). Baseline characteristics of the study population are presented in table 1. Diagnoses included in the control populations are presented in online supplementary table S2.
Table 1.
Baseline patient characteristics
| Derivation cohort (n=124) | Validation cohort (n=66) | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Variable | HP cases (n=44) |
Non-HP ILD controls (n=80) |
p Value | HP cases (n=22) | Non-HP ILD controls (n=44) |
p Value |
| Age, years mean (SD) | 59.7 (10.8) | 56.8 (11.1) | 0.38 | 60.7 (15.4) | 63.1 (12.7) | 0.04 |
| Male sex, n (%) | 13 (29.6) | 41 (51.3) | 0.02 | 6 (27.3) | 22 (50.0) | 0.11 |
| Smoking history | ||||||
| Never smoker (%) | 21 (47.7) | 37 (46.9) | 1.00 | 15 (68.2) | 19 (43.2) | 0.07 |
| Ex-smoker (%) | 22 (50.0) | 41 (51.9) | 1.00 | 7 (31.8) | 25 (56.8) | 0.07 |
| Current smoker (%) | 1 (2.27) | 1 (1.27) | 1.00 | 0 (0.0) | 0 (0.0) | n/a |
| Pulmonary physiology | ||||||
| FVC, % predicted mean (SD) | 67.3 (17.4) | 70.3 (22.3) | 0.99 | 63.0 (17.1) | 78.5 (20.5) | 0.005 |
| FEV1, % predicted mean (SD) | 72.9 (18.4) | 73.8 (21.5) | 0.71 | 67.1 (15.5) | 83.6 (23.8) | 0.004 |
| FEV1/FVC mean (SD) | 0.82 (0.06) | 0.80 (0.09) | 0.35 | 0.83 (0.09) | 0.81 (0.08) | 0.38 |
| Selected exposure history | ||||||
| Bird (%) | 13 (29.6) | 9 (11.4) | 0.01 | 6 (27.3) | 3 (6.8) | 0.02 |
| Down (%) | 25 (56.8) | 29 (36.7) | 0.03 | 13 (59.1) | 22 (50.0) | 0.49 |
| Mould (%) | 11 (25.0) | 13 (16.4) | 0.34 | 6 (27.3) | 10 (22.7) | 0.76 |
| HRCT features | ||||||
| Mosaic perfusion (%) | 27 (61.4) | 17 (21.3) | <0.001 | 10 (45.5) | 5 (11.4) | <0.01 |
| Diffuse GGO predominance (%) | 12 (27.3) | 4 (5.0) | <0.001 | 4 (18.2) | 1 (2.3) | 0.02 |
| Fibrosis | 33 (75.0) | 62 (77.5) | 0.76 | 17 (77.3) | 39 (88.6) | 0.28 |
| Air trapping (%) | 19 (44.2) | 12 (15.2) | <0.001 | n/a | n/a | n/a |
GGO, ground-glass opacity; HP, hypersensitivity pneumonitis; HRCT, high-resolution CT; ILD, interstitial lung disease.
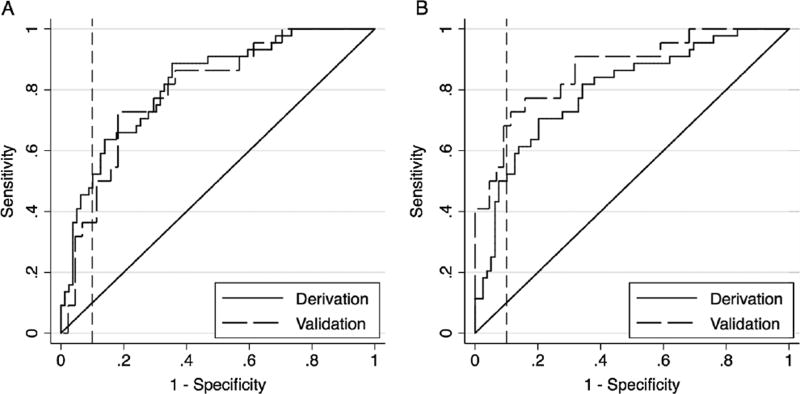
The top three candidates for model 1 and model 2 are presented in table 2. For model 1, the best overall model as defined by its performance, clinical feasibility and face validity included the following predictor variables: age, a history of down feather and/or bird exposure, the presence of diffuse craniocaudal ground-glass opacity on HRCT, and the presence of mosaic perfusion on HRCT (CV-C=77.8, H-L GOF p value=0.60) (figure 1A). This model demonstrated consistent performance in the validation cohort (CV-C=79.8, H-L GOF p value=0.89). Using model 1 to derive an ‘HP score’ point value (range 0–100), HP scores ≥63 demonstrated a specificity of 91% and sensitivity of 48% for the diagnosis of chronic HP. For model 2, the best overall model included the following: age, history of down feather and/or bird exposure and a moderate–high confidence in the radio-graphic diagnosis of HP (CV-C=75.8, H-L GOF p value=0.72) (figure 1B). This model also demonstrated consistent performance in the validation cohort (CV-C=86.9, H-L GOF p value=0.35). Using model 2, HP scores ≥57 demonstrated a specificity of 91% and sensitivity of 50% for the diagnosis of chronic HP. Details of both models, their performance in the subgroup of patients with chronic HP or IPF, and determining the performance characteristics of various cut-off values are provided in the online supplementary materials and supplementary tables S3a and S3b.
Table 2.
Clinical prediction model performance for diagnosis of HP
| CV-C | GOF | |
|---|---|---|
| Model 1 (clinical and individual radiological features) | ||
| Age, down, birds, diffuse craniocaudal GGO, mosaic perfusion | 77.8 | 0.60 |
| Age, down, any GGO, diffuse craniocaudal GGO, mosaic perfusion, air trapping | 78.0 | 0.47 |
| Age, down, birds, diffuse GGO in axial distribution, diffuse craniocaudal GGO, mosaic perfusion | 77.9 | 0.78 |
| Model 2 (clinical features and radiologist’s diagnostic impression) | ||
| Age, down, birds, moderate/high confidence HP | 75.8 | 0.72 |
| Age, down, birds, sex, moderate/high confidence HP | 75.6 | 0.27 |
| Age, down, sex, moderate/high confidence HP | 75.2 | 0.57 |
CV-C, cross-validated C-statistic; GGO, ground-glass opacity; GOF, Hosmer-Lemeshow goodness of fit p value; HP, chronic hypersensitivity pneumonitis.
Figure 1.
Receiver operating characteristic curve for (A) the derivation and validation prediction models incorporating clinical, exposure and individual radiological variables and (B) the derivation and validation prediction models incorporating clinical, exposure and radiologist’s confidence variables. The solid and dashed lines represent the derivation and validation cohorts, respectively. The curve areas to the left of the vertical line indicate diagnostic specificity ≥90%.
DISCUSSION
In this study, we developed multidimensional diagnostic models to identify patients with chronic HP with a high degree of specificity. In such patients, surgical lung biopsy can likely be avoided. While a clinical diagnosis of HP is already made in many ILD practices, these data are the first we are aware of that provide evidence to support this approach in selected patients.
There are several strengths to our models. First, they use readily available clinical parameters such as patient demographics, self-reported exposures and radiographic features. Second, they were derived in biopsy-proven patients with chronic HP, likely representative of those patients with increased diagnostic uncertainty (ie, those in whom a surgical biopsy would be considered), the most challenging patients encountered in clinical practice. Lastly, our models were internally validated.
mportantly, our models do not incorporate diagnostic tests that may have value in the diagnostic evaluation of chronic HP, as they were not routinely performed in our cohort. These include bronchoalveolar lavage differential cell counts, antigen-specific circulating antibodies and inhalational challenge.1,10 We excluded unclassifiable ILD and therefore cannot comment on model performance in this subset of patients. This represents a limitation to its application in clinical practice and requires further investigation. In addition, inciting antigens likely vary geographically, and specific exposures may be more or less predictive in other parts of the world. While this would be expected to primarily affect the sensitivity and not the specificity of the models, validation of these models in geographically diverse cohorts is essential.
Acknowledgments
We would like to thank the patients, providers and staff of the UCSF Interstitial Lung Disease Program for their time and commitment to clinical research, and Jane Berkeley for data management and retrieval. We would also like to thank Dr Cyrus Shariat, Dr W Richard Webb, Dr Theodore Lee and Dr Antonio Gomez for their thoughtful contributions to the development of this project.
Funding GSK/University of Calgary Advanced Fellowship in Respirology Grant (KAJ); Nina Ireland Program for Lung Health (HRC).
Competing interests
KAJ reports travel support from InterMune and personal fees from Boehringer Ingelheim and Hoffman-La Roche; KdB reports travel support from InterMune and personal fees from AstraZeneca and Boehringer Ingelheim; TEK reports personal fees from InterMune, ImmuneWorks, GlaxoSmithKline, Daiichi Sankyo and Boehringer Ingelheim; HRC reports personal fees from AstraZeneca, Bayer, Biogen, FibroGen, Genentech/InterMune, Genoa, Gilead, GlaxoSmithKline, Mesoblast, Moerae Matrix, Pfizer, Promedior, Prometic and PatientsLikeMe, grants from Boehringer Ingelheim, consultancy fees from the Pulmonary Fibrosis Foundation and grant funding from the NIH/NHLBI.
Footnotes
Contributors KAJ and HRC conceived of the project; KAJ, BME, DA, KdB, JAG, KDJ, LLK, JSL, BL, PJW and HRC generated data for the project; KAJ, EV and HRC analysed data for the project and wrote the manuscript; KAJ, BME, EV, DA, KdB, JAG, KDJ, TEK, LLK, JSL, BL, PJW and HRC revised the manuscript.
Ethics approval University of California, San Francisco’s Human Research Protection Program Committee on Human Research.
Provenance and peer review Not commissioned; externally peer reviewed.
References
- 1.Selman M, Pardo A, King TE., Jr Hypersensitivity pneumonitis: insights in diagnosis and pathobiology. Am J Respir Crit Care Med. 2012;186:314–24. doi: 10.1164/rccm.201203-0513CI. [DOI] [PubMed] [Google Scholar]
- 2.Lynch DA, Newell JD, Logan PM, et al. Can CT distinguish hypersensitivity pneumonitis from idiopathic pulmonary fibrosis? AJR Am J Roentgenol. 1995;165:807–11. doi: 10.2214/ajr.165.4.7676971. [DOI] [PubMed] [Google Scholar]
- 3.Raghu G, Collard HR, Egan JJ, et al. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med. 2011;183:788–824. doi: 10.1164/rccm.2009-040GL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mooney JJ, Elicker BM, Urbania TH, et al. Radiographic fibrosis score predicts survival in hypersensitivity pneumonitis. Chest. 2013;144:586–92. doi: 10.1378/chest.12-2623. [DOI] [PubMed] [Google Scholar]
- 5.Lacasse Y, Selman M, Costabel U, et al. Clinical diagnosis of hypersensitivity pneumonitis. Am J Respir Crit Care Med. 2003;168:952–8. doi: 10.1164/rccm.200301-137OC. [DOI] [PubMed] [Google Scholar]
- 6.Ramírez-Venegas A, Sansores RH, Pérez-Padilla R, et al. Utility of a provocation test for diagnosis of chronic pigeon Breeder’s disease. Am J Respir Crit Care Med. 1998;158:862–9. doi: 10.1164/ajrccm.158.3.9710036. [DOI] [PubMed] [Google Scholar]
- 7.Churg A, Muller NL, Flint J, et al. Chronic hypersensitivity pneumonitis. Am J Surg Pathol. 2006;30:201–8. doi: 10.1097/01.pas.0000184806.38037.3c. [DOI] [PubMed] [Google Scholar]
- 8.Silva CI, Müller NL, Lynch DA, et al. Chronic hypersensitivity pneumonitis: differentiation from idiopathic pulmonary fibrosis and nonspecific interstitial pneumonia by using thin-section CT. Radiology. 2008;246:288–97. doi: 10.1148/radiol.2453061881. [DOI] [PubMed] [Google Scholar]
- 9.Harrell FE, Jr, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. 1996;15:361–87. doi: 10.1002/(SICI)1097-0258(19960229)15:4<361::AID-SIM168>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 10.Meyer KC, Raghu G, Baughman RP, et al. An official American Thoracic Society clinical practice guideline: the clinical utility of bronchoalveolar lavage cellular analysis in interstitial lung disease. Am J Respir Crit Care Med. 2012;185:1004–14. doi: 10.1164/rccm.201202-0320ST. [DOI] [PubMed] [Google Scholar]