Abstract
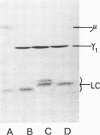
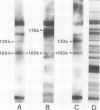
One IgM and three IgG monoclonal antibodies specific to band 1 of human erythrocyte spectrin have been characterised. The antigenic sites of the IgG antibodies have been identified and mapped by radioimmune labelling of tryptic fragments of spectrin fractionated by SDS slab gel electrophoresis and blotted onto nitrocellulose filters. The binding site of one of these antibodies has also been directly visualised in the electron microscope after low-angle shadowing of the antibody-spectrin dimer complex, and lies at that end of the dimer which is responsible for tetramer formation.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson J. M. Structural studies on human spectrin. Comparison of subunits and fragmentation of native spectrin. J Biol Chem. 1979 Feb 10;254(3):939–944. [PubMed] [Google Scholar]
- Bjerrum O. J., Bhakdi S., Bog-Hansen T. C., Knüfermann H., Wallach D. F. Quantitative immunoelectrophoresis of proteins in human erythrocyte membranes. Analysis of protein bands obtained by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Biochim Biophys Acta. 1975 Nov 3;406(4):489–504. doi: 10.1016/0005-2736(75)90027-9. [DOI] [PubMed] [Google Scholar]
- Bowen B., Steinberg J., Laemmli U. K., Weintraub H. The detection of DNA-binding proteins by protein blotting. Nucleic Acids Res. 1980 Jan 11;8(1):1–20. doi: 10.1093/nar/8.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burridge K. Changes in cellular glycoproteins after transformation: identification of specific glycoproteins and antigens in sodium dodecyl sulfate gels. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4457–4461. doi: 10.1073/pnas.73.12.4457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catt K., Tregear G. W. Solid-phase radioimmunoassay in antibody-coated tubes. Science. 1967 Dec 22;158(3808):1570–1572. doi: 10.1126/science.158.3808.1570. [DOI] [PubMed] [Google Scholar]
- Cohen C. M., Tyler J. M., Branton D. Spectrin-actin associations studied by electron microscopy of shadowed preparations. Cell. 1980 Oct;21(3):875–883. doi: 10.1016/0092-8674(80)90451-1. [DOI] [PubMed] [Google Scholar]
- Galfre G., Howe S. C., Milstein C., Butcher G. W., Howard J. C. Antibodies to major histocompatibility antigens produced by hybrid cell lines. Nature. 1977 Apr 7;266(5602):550–552. doi: 10.1038/266550a0. [DOI] [PubMed] [Google Scholar]
- Koprowski H., Gerhard W., Croce C. M. Production of antibodies against influenza virus by somatic cell hybrids between mouse myeloma and primed spleen cells. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2985–2988. doi: 10.1073/pnas.74.7.2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köhler G., Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975 Aug 7;256(5517):495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Morrow J. S., Speicher D. W., Knowles W. J., Hsu C. J., Marchesi V. T. Identification of functional domains of human erythrocyte spectrin. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6592–6596. doi: 10.1073/pnas.77.11.6592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralston G. B. The isolation of aggregates of spectrin from bovine erythrocyte membranes. Aust J Biol Sci. 1975 Jun;28(3):259–266. doi: 10.1071/bi9750259. [DOI] [PubMed] [Google Scholar]
- Ralston G., Dunbar J., White M. The temperature-dependent dissociation of spectrin. Biochim Biophys Acta. 1977 Mar 28;491(1):345–348. doi: 10.1016/0005-2795(77)90072-1. [DOI] [PubMed] [Google Scholar]
- Sheetz M. P., Painter R. G., Singer S. J. Relationships of the spectrin complex of human erythrocyte membranes to the actomyosins of muscle cells. Biochemistry. 1976 Oct 5;15(20):4486–4492. doi: 10.1021/bi00665a024. [DOI] [PubMed] [Google Scholar]
- Shotton D. M., Burke B. E., Branton D. The molecular structure of human erythrocyte spectrin. Biophysical and electron microscopic studies. J Mol Biol. 1979 Jun 25;131(2):303–329. doi: 10.1016/0022-2836(79)90078-0. [DOI] [PubMed] [Google Scholar]
- Shulman M., Wilde C. D., Köhler G. A better cell line for making hybridomas secreting specific antibodies. Nature. 1978 Nov 16;276(5685):269–270. doi: 10.1038/276269a0. [DOI] [PubMed] [Google Scholar]
- Speicher D. W., Morrow J. S., Knowles W. J., Marchesi V. T. Identification of proteolytically resistant domains of human erythrocyte spectrin. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5673–5677. doi: 10.1073/pnas.77.10.5673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steck T. L. The organization of proteins in the human red blood cell membrane. A review. J Cell Biol. 1974 Jul;62(1):1–19. doi: 10.1083/jcb.62.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler J. M., Hargreaves W. R., Branton D. Purification of two spectrin-binding proteins: biochemical and electron microscopic evidence for site-specific reassociation between spectrin and bands 2.1 and 4.1. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5192–5196. doi: 10.1073/pnas.76.10.5192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler J. M., Reinhardt B. N., Branton D. Associations of erythrocyte membrane proteins. Binding of purified bands 2.1 and 4.1 to spectrin. J Biol Chem. 1980 Jul 25;255(14):7034–7039. [PubMed] [Google Scholar]
- Ziparo E., Lemay A., Marchesi V. T. The distribution of spectrin along the membranes of normal and echinocytic human erythrocytes. J Cell Sci. 1978 Dec;34:91–101. doi: 10.1242/jcs.34.1.91. [DOI] [PubMed] [Google Scholar]