Abstract
Background
Patients on chronic dialysis have among the highest mortality and hospitalization rates. In the non-renal literature, functional dependence is recognized as a contributor to subsequent disability, recurrent hospitalization, and increased mortality. A higher burden of functional dependence with progressive worsening of renal function has been observed in several studies, suggesting functional dependence may contribute to both morbidity and mortality in dialysis patients.
Study Design
Prospective cohort study
Setting & Participants
7,226 hemodialysis patients from 12 countries in the Dialysis Outcomes and Practice Patterns Study (DOPPS) phase 4 (2009–2011) with self-reported data on functional status (FS).
Predictor
Patients’ ability to perform 13 basic and instrumental Activities of Daily Living (ADL) was summarized to create an overall FS score ranging from 1.25 (most dependent) to 13 (functionally independent).
Outcome
Cox regression was used to estimate the association between FS and all-cause mortality, adjusting for several demographic and clinical risk factors for mortality. Median follow-up was 17.2 months.
Results
The proportion of patients who could perform each ADL task without assistance ranged from 97% (eating) to 47% (doing housework). 36% of patients could perform all 13 tasks without assistance (FS=13), and 14% of patients had high functional dependence (FS < 8). Functionally independent patients were younger and had many indicators of better health status including higher quality of life. Compared with functionally independent patients, the adjusted hazard ratio for mortality was 2.37 (95% confidence interval =1.92–2.94) for patients with FS < 8.
Limitations
Possible non-response bias and residual confounding
Conclusions
We found a high burden of functional dependence across all age groups and across all DOPPS countries. When adjusting for several known mortality risk factors, including age, access type, cachexia and multi-morbidity, functional dependence was a strong, consistent predictor of mortality.
Index Words: chronic kidney disease, dependence, dialysis, hospitalization, independence, morbidity, mortality, physical activity, quality of life
INTRODUCTION
Patients established on dialysis have amongst the highest mortality and hospitalization rates of all chronic conditions1–3. One potential contributor may be the high burden of functional dependence. In the general geriatric literature, functional dependence is recognized as a contributor to subsequent disability, recurrent hospitalization, and increased mortality4–9. Functional dependence can be measured using a variety of validated scales which assess the individual’s ability to perform tasks associated with personal care (such as grooming, toileting, eating and dressing), and those associated with maintaining a household (such as grocery shopping, meal preparation, and household chores). In contrast to “leisure” activities (e.g., gardening or sports), these tasks are often regarded as fundamental for day-to-day life and loss of independence may contribute to the reduced quality of life seen in both patients and their caregivers. Predictors of functional loss include age, chronic diseases, multiple comorbidities, and recurrent hospitalization10–14.
In older patients with earlier stages of chronic kidney disease (CKD), multicenter studies have shown that CKD places patients at increased risk of functional dependence even after adjustment for the higher prevalence of predisposing comorbidities15–21. Recent estimates suggest individuals with CKD stage 3b have a threefold increased risk of developing dependence in daily activities such as bathing, dressing, and personal care compared to individuals without renal impairment16. Furthermore, there appears to be a faster rate of functional decline17. However, studies in the dialysis population are limited to small single center studies of older patients with little information on the difficulties that younger individuals report15, 16. Dialysis-related factors that may predispose to functional decline have not been characterized. In this study we evaluated the proportion of patients, across all age groups, established on dialysis that reported functional dependence, and questioned whether the presence of functional dependence would be associated with a higher mortality and hospitalization rate independent of clinical and demographic variables. In addition we evaluated whether the burden of functional dependence would vary with age and across the countries participating in the Dialysis Outcomes and Practice Patterns (DOPPS) Study.
METHODS
Data Source
The DOPPS is an international prospective cohort study of in-center hemodialysis (HD) patients ≥ 18 years of age. Patients were randomly selected from a representative sample of dialysis facilities within each country22, 23. In this analysis, data from participants in DOPPS phase 4 (2009–2011) in Australia, Belgium, Canada, France, Germany, Italy, Japan, New Zealand, Spain, Sweden, the United Kingdom, and the United States were used. Demographics, comorbid conditions, and laboratory values at study entry were abstracted from medical records. All variables were collected using uniform and standardized data collection tools for all DOPPS participants in all countries.
Variables
Functional status (FS) was assessed on the DOPPS self-reported patient questionnaire (PQ). Patients indicated their level of ability to perform 5 Activities of Daily Living (ADL) tasks and 8 instrumental ADL (IADL) tasks using the Katz24 and Lawton-Brody25 questionnaires respectively. Both questionnaires have been validated in the general population. In keeping with previous research regarding the psychometric properties of these scales, the scales were combined to create an overall FS score26. To score individual items, IADL responses of “need no help” were scored 1, “need some help” were scored 0.5, and “unable to do at all” were scored 0. On the ADL, responses of “yes” were scored 1. A response of “no” could not distinguish between performing a task with some help or unable to perform the task at all; thus, a score of 0.25 was assigned rather than 0. Functional status score was defined as the sum of the 13 item scores and ranged from 1.25 (most dependent) to 13 (functionally independent). To examine the shape of the association between FS and outcomes, FS score was categorized into four groups: (1) FS < 8, (2) 8 ≤ FS < 11, (3) 11 ≤ FS < 13, (4) FS = 13. Patients with full independence (FS=13) were categorized separately; the remaining patients were categorized into increasingly smaller groups. Quality of life (QOL) was also assessed on the PQ using the SF-12, a subset of the KDQOL-3627, and summarized into a physical (PCS) and mental (MCS) component summary score. Cachexia was clinically defined as undernourished or cachectic (malnourished) at enrollment date.
Study Population
This analysis included patients with complete self-reported FS data on a PQ completed within 6 months of DOPPS enrollment (median time to questionnaire completion: 1.0 months, IQR: 0.5 to 1.7 months). Of the 17,297 patients enrolled in the DOPPS 4 study, 5,074 (29%) were excluded as they underwent dialysis in a US large dialysis organization; comorbidity data, felt to be key to evaluating the FS-mortality relationship, were not available for many of these individuals. Of the remaining 12,223 patients, 2,391 (20%) did not return a PQ, 1,952 (16%) returned a PQ but had missing data on one or more ADL questions, and an additional 654 (5%) completed their PQ > 6 months after study entry. As a result, data for 7,226 patients were considered for analysis.
Statistical Analysis
Characteristics of included patients were summarized descriptively and compared to the remaining patients in the DOPPS 4 study sample. Differences in patient characteristics among included patients were examined descriptively by FS score. To test whether 5 potentially modifiable patient characteristics (pre-dialysis systolic blood pressure [SBP], treatment time, hemoglobin, vascular access, body mass index) were associated with FS, FS was treated as a 4-category ordinal outcome variable. Proportional odds logistic regression models based on generalized estimating equations were used, assuming an independent working correlation to account for clustering within facilities. Using the proportional odds model, we estimated the adjusted common odds ratio for each predictor, comparing patients with a low FS score to patients with a higher score, assuming that the odds ratio is the same for each possible cutpoint when the FS score is dichotomized (i.e., < 13, < 11, or < 8). This assumption was assessed by comparing odds-ratio estimates for all three possible cutpoints of each predictor.
Cox regression was used to estimate the association between FS and mortality, incorporating stratification by country and accounting for facility clustering using robust sandwich covariance estimators. Models were left-truncated, with time from DOPPS enrollment to death or censoring as the time axis and time at risk beginning at the PQ completion date. Adjustment was made for expanding sets of covariates: (1) crude analysis, (2) age, (3) gender, black race, body mass index, years on dialysis, (4) 13 summary comorbidities, (5) serum albumin, creatinine, phosphorus, hemoglobin, single pool Kt/V, (6) vascular access, (7) cachexia. Multivariable Cox regression was also used to estimate the association between FS and (1) first hospitalization for any reason, and (2) withdrawal from dialysis. Time at risk ended at the time of death, seven days after leaving the facility due to transfer or change in renal replacement therapy modality, loss to follow-up, transplantation, end of study phase, or the most recent date of data availability (whichever event occurred first). The median length of follow-up from PQ completion was 17.2 months (interquartile range: 8.0 to 28.9 months).
Interactions between FS and age, gender, diabetes, catheter use, vintage, black race, and region were assessed in Cox models using product terms, adjusted for all of the variables previously described in the step-wise adjustment analysis. The interaction between FS and age was further investigated using a discrete survival method (with a binomial distribution, logit link function, and log(follow-up) offset) to model the risk of dying in one year at each age. Age was included as a continuous covariate, squared term, and cubic term to maintain flexibility with the functional form. The product of each age term with each FS category indicator (except for the reference group) was included to allow for effect modification; country was included as an adjustment covariate. Predicted probabilities were output from the model, which approximate the 1 year mortality risk at each FS-age combination, and the 1 year mortality rate in cases that the event is rare (e.g., < 10%).
Linear regression, clustering for facility, with PCS and MCS as outcomes was used to estimate the association between FS and QOL, adjusting for country and age. To investigate possible effect modification by age, we used two separate linear regression models (for PCS and MCS) and modeled age as a cubic term similar to the mortality analysis. Predicted values for age*FS combinations were used to estimate PCS and MCS.
As patients who completed a PQ may not be representative of all sampled DOPPS patients, we performed a weighted sensitivity analysis using inverse probability weighting. Note that the excluded patients from US large dialysis organizations are not represented in the “Excluded” group in this analysis. We calculated the predicted probability (range 17% to 88%) of a patient being included in our analysis (N=7,226, vs. N=4,997 excluded) using a logistic regression model adjusted for country and other variables associated with exclusion: age, gender, black race, BMI, vintage, cancer, diabetes, neurologic disease, psychiatric disorder, serum calcium, serum creatinine, vascular access, and cachexia. The inverse of this probability was then used as the weight in a Cox model as described above. For primary analyses among the included patients, missing covariate values were imputed multiply using the chained equation method28 by IVEWARE29. Results from five imputed data sets were combined for the final analysis using Rubin’s formula30. The proportion of missing data was below 10% for all imputed covariates, with the exception of Kt/V (26%) and QOL (PCS/MCS, 19%). All analyses used SAS software, version 9.3 (SAS institute, Cary, NC).
RESULTS
Functional status distribution
The analysis included 7,226 participants in DOPPS phase 4 (2009–2011). The distribution of ADL and IADL items among these patients is shown in Table 1. The proportion of patients who could perform each task without assistance ranged from 97% (eating) to 47% (doing housework or handyman work). 81% of patients reported the ability to perform the 5 ADL tasks without assistance, but only 36% of patients reported the ability to perform all 13 tasks without assistance (FS = 13). Among patients who could perform all but one task without assistance, this task was most likely housework or handyman work (43%), getting to places beyond walking distance (18%), or doing laundry (13%). The skewed distribution of FS scores is illustrated in Table 1; the mean and median FS scores were 10.9 and 12.0, respectively. The distribution of FS varied widely across DOPPS countries. Japan had the highest proportion of patients with FS=13 (57%) and the UK had the lowest (19%), indicating 81% of patients in the UK had some functional dependence (Figure 1A). Higher FS scores (i.e., more functional independence) were present among younger patients, males, and non-diabetics (Figure 1B).
Table 1.
Distribution of tasks included in the Activities of Daily Living (ADL) and Instrumental Activities of Daily Living (IADL) in the study sample (N, %).
| Activities of Daily Living (ADL) | |||
| Able to perform the task without assistance | Yes (1) | No (0.25) | |
| Eating | 7026 (97%) | 200 (3%) | |
| Getting dressed | 6553 (91%) | 673 (9%) | |
| Bathing | 5943 (82%) | 1283 (18%) | |
| Using the toilet | 6839 (95%) | 387 (5%) | |
| Transferring from bed to chair | 6812 (94%) | 414 (6%) | |
|
| |||
| Instrumental Activities of Daily Living (IADL) | |||
| Ability to perform the task | Need no help (1) | Need some help (0.5) | Unable to do at all (0) |
| Using the telephone | 6572 (91%) | 506 (7%) | 148 (2%) |
| Getting places beyond walking distance | 4230 (59%) | 1979 (27%) | 1017 (14%) |
| Grocery shopping | 4238 (59%) | 1802 (25%) | 1186 (16%) |
| Preparing meals | 4657 (64%) | 1516 (21%) | 1053 (15%) |
| Doing housework or handyman work | 3379 (47%) | 2364 (33%) | 1483 (21%) |
| Doing laundry | 4223 (58%) | 1503 (21%) | 1500 (21%) |
| Taking medications | 6082 (84%) | 887 (12%) | 257 (4%) |
| Managing money | 5845 (81%) | 938 (13%) | 443 (6%) |
|
| |||
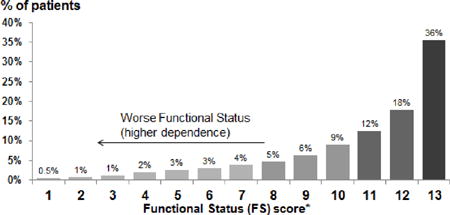
| |||
FS truncated at the integer value
Figure 1A. Functional Status (FS) score by country.
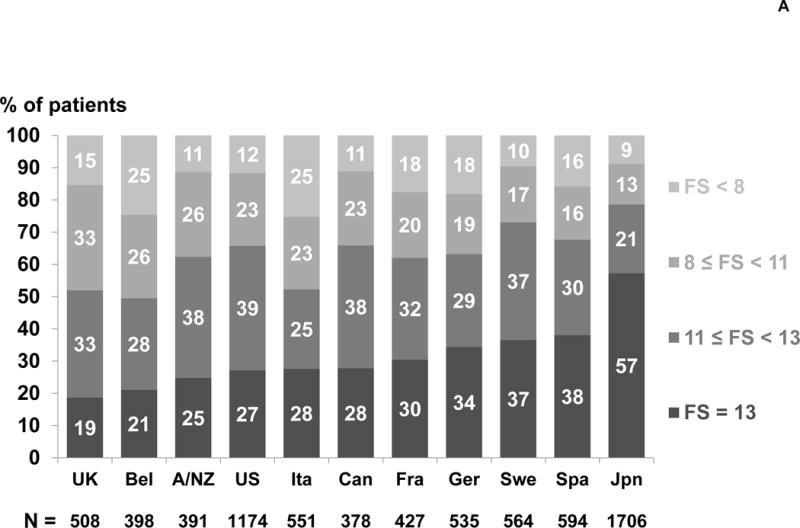
Percents were rounded to the nearest integer and thus may not sum to 100%.
Figure 1B. Functional Status (FS) score by age, gender, and diabetes status.
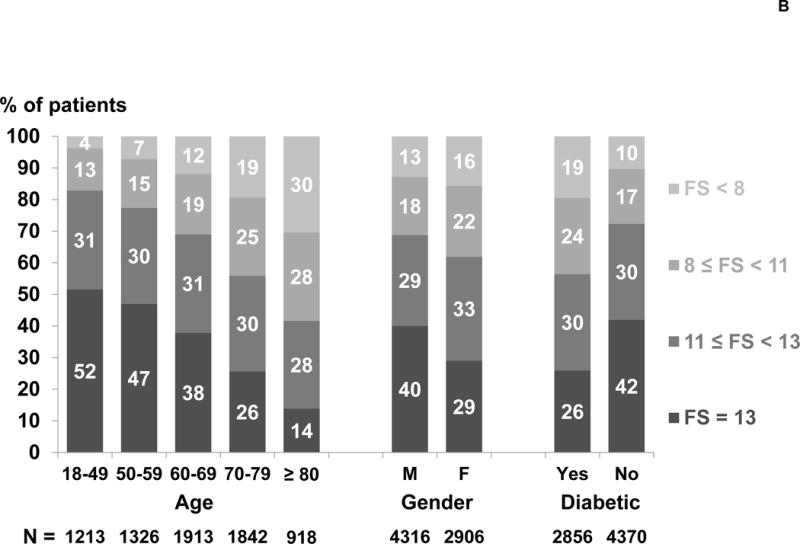
Percents were rounded to the nearest integer and thus may not sum to 100%.
Patient characteristics by functional status levels
Table 2 shows patient characteristics, both for the overall population and by FS category. Fully independent patients (FS=13) were much younger than patients in the lowest FS category (FS < 8) (mean age 58.8 versus 71.7 years). Three percent of patients (n=203) lived in assisted living or nursing homes; among these patients, 79% had high dependency with FS score < 11. As expected, functionally independent patients had many indicators of better patient health status: higher serum albumin, creatinine, phosphorus, were less likely to have a catheter, and had a lower prevalence of several summary comorbid conditions. Overall, patients who responded to the FS questions on the patient questionnaire and who were included in the analysis, had generally better health status with fewer comorbidities and lower proportion of catheter usage than sampled patients excluded from the analysis due to missing data. In adjusted analyses, low blood pressure (SBP < 130 mmHg), catheter or arteriovenous graft use, and high BMI (BMI ≥ 30) were associated with a worse FS (Supplementary Figure 1).
Table 2.
Patient characteristics by Functional Status (FS) score.
| Patient characteristic (mean ± SD or %) | All included patients | Excluded patients | Functional Status (FS) score of included patients | |||
|---|---|---|---|---|---|---|
| FS < 8 | 8 ≤ FS < 11 | 11 ≤ FS < 13 | FS = 13 | |||
| N patients | 7226 | 4997 | 1008 (14%) | 1446 (20%) | 2197 (30%) | 2575 (36%) |
| Age (years) | 63.6 ± 14.4 | 66.0 ± 14.9 | 71.7 ± 11.6 | 67.2 ± 13.3 | 63.2 ± 14.6 | 58.8 ± 13.9 |
| Gender (% male) | 4316 (60%) | 3032 (61%) | 554 (55%) | 793 (55%) | 1241 (57%) | 1728 (67%) |
| Vintage (years) | 2.2 (0.5, 5.9) | 1.5 (0.3, 4.8) | 2.2 (0.5, 5.4) | 2.1 (0.5, 5.3) | 2.0 (0.5, 5.6) | 2.4 (0.5, 7.0) |
| Residual Kidney Function* (%) | 2647 (39%) | 1762 (38%) | 316 (33%) | 516 (38%) | 804 (39%) | 1011 (42%) |
| Body mass index (kg/m2) | 25.7 ± 6.2 | 26.0 ± 6.0 | 26.1 ± 6.8 | 26.5 ± 6.5 | 26.3 ± 6.5 | 24.5 ± 5.4 |
| Albumin (g/dL) | 3.7 ± 0.6 | 3.7 ± 0.6 | 3.5 ± 0.7 | 3.7 ± 0.6 | 3.7 ± 0.5 | 3.8 ± 0.5 |
| Creatinine (mg/dL) | 8.5 ± 3.1 | 7.7 ± 2.9 | 7.0 ± 2.3 | 7.7 ± 2.7 | 8.3 ± 2.9 | 9.7 ± 3.2 |
| Hemoglobin (g/dL) | 11.1 ± 1.4 | 11.1 ± 1.5 | 11.1 ± 1.5 | 11.2 ± 1.5 | 11.2 ± 1.4 | 11.1 ± 1.4 |
| White Blood Cells (1000/mm3) | 6.9 ± 2.3 | 7.2 ± 2.4 | 7.3 ± 2.7 | 7.2 ± 2.4 | 7.0 ± 2.2 | 6.5 ± 2.1 |
| Phosphorus (mg/dL) | 5.2 ± 1.6 | 5.0 ± 1.6 | 4.8 ± 1.5 | 5.1 ± 1.7 | 5.3 ± 1.6 | 5.4 ± 1.6 |
| Calcium (mg/dL) | 8.9 ± 0.9 | 9.0 ± 1.2 | 8.9 ± 0.8 | 8.9 ± 1.0 | 8.9 ± 0.9 | 9.0 ± 1.0 |
| Predialysis SBP (mm Hg) | 142 ± 22 | 142 ± 22 | 137 ± 24 | 141 ± 24 | 143 ± 22 | 143 ± 21 |
| Single Pool Kt/V | 1.46 ± 0.33 | 1.48 ± 0.34 | 1.50 ± 0.34 | 1.47 ± 0.33 | 1.48 ± 0.33 | 1.43 ± 0.32 |
| Treatment time (min) | 236 ± 38 | 233 ± 36 | 233 ± 32 | 233 ± 35 | 236 ± 40 | 238 ± 40 |
| Catheter use (%) | 1657 (24%) | 1550 (33%) | 349 (37%) | 411 (30%) | 499 (24%) | 398 (17%) |
| Cachexia (%) | 575 (8%) | 615 (13%) | 181 (18%) | 149 (10%) | 136 (6%) | 109 (4%) |
| Comorbid conditions (%) | ||||||
| Coronary artery disease | 2583 (36%) | 2098 (43%) | 488 (49%) | 626 (44%) | 776 (36%) | 693 (27%) |
| Cancer (non-skin) | 1067 (15%) | 747 (15%) | 165 (17%) | 239 (17%) | 333 (15%) | 330 (13%) |
| Other cardiovascular disease | 2093 (29%) | 1668 (33%) | 416 (41%) | 485 (34%) | 624 (28%) | 568 (22%) |
| Cerebrovascular disease | 1102 (15%) | 932 (19%) | 307 (31%) | 276 (19%) | 304 (14%) | 215 (8%) |
| Congestive heart failure | 1594 (22%) | 1344 (27%) | 337 (34%) | 379 (26%) | 476 (22%) | 402 (16%) |
| Diabetes | 2856 (40%) | 2262 (46%) | 556 (55%) | 689 (48%) | 870 (40%) | 741 (29%) |
| Gastrointestinal bleeding | 348 (5%) | 286 (6%) | 69 (7%) | 79 (6%) | 89 (4%) | 111 (4%) |
| Hypertension | 6115 (85%) | 4325 (88%) | 838 (84%) | 1201 (84%) | 1875 (86%) | 2201 (86%) |
| Lung disease | 863 (12%) | 792 (16%) | 179 (18%) | 228 (16%) | 272 (12%) | 184 (7%) |
| Neurologic disease | 665 (9%) | 778 (16%) | 242 (24%) | 176 (12%) | 149 (7%) | 98 (4%) |
| Psychiatric disorder | 1203 (17%) | 1027 (21%) | 250 (25%) | 308 (21%) | 365 (17%) | 280 (11%) |
| Peripheral vascular disease | 1969 (27%) | 1660 (34%) | 466 (46%) | 508 (35%) | 597 (27%) | 398 (16%) |
| Gangrene/recurrent cellulitis | 653 (9%) | 550 (11%) | 202 (20%) | 188 (13%) | 177 (8%) | 86 (3%) |
| Physical component summary (PCS) | 37.1 ± 11.0 | – | 26.4 ± 7.4 | 30.6 ± 8.9 | 36.2 ± 9.3 | 44.9 ± 8.5 |
| Mental component summary (MCS) | 45.2 ± 11.6 | – | 38.4 ± 12.8 | 42.4 ± 11.8 | 46.0 ± 11.0 | 48.3 ± 10.1 |
RKF defined as urine output >200 mL/day on or before the enrollment date; Summary statistics reported as Mean ± SD, Median (IQR), or N(%); Note for N(%) reported, numbers may be not extrapolate to 100% due to missingness
Functional status and adverse clinical outcomes
Over the follow-up period, 1,140 (16%) patients died; 85 (1%) switched modality; 800 (11%) transferred to another facility; 438 (6%) received a kidney transplant; nearly all other patients were censored at the end of follow-up. Table 3 shows the crude association between FS and mortality (Model 1) and adjusted associations with expanding sets of covariates (Models 2–7). The association was attenuated most by adjustment for age (Model 2) and comorbidities (Model 4). When adjusting for many potential confounders (Model 7), there remained a strong dose-response association between FS and mortality. Compared to patients with FS=13 (functionally independent), the adjusted hazard ratio (HR) for patients with FS < 8 was 2.37 (95% CI = 1.92–2.94). Model 7 in Table 3 was also applied to two other outcomes: first hospitalization during follow-up, and withdrawal from dialysis. Compared to patients with FS=13: the adjusted HR for hospitalization in patients with FS < 8 was 1.28 (95% CI = 1.14–1.44), and the adjusted HR for dialysis withdrawal in patients with FS < 8 was 2.02 (95% CI = 1.45–2.80).
Table 3.
Hazard ratios (95% CI) for Functional Status (FS) score and survival, by various levels of adjustment.
| Model 1: Crude | Model 2: + age | Model 3: + demographics | Model 4: + comorbidities | Model 5: + labs | Model 6:+ vascular access | Model 7: + cachexia | |
|---|---|---|---|---|---|---|---|
| Functional Status (FS) score | |||||||
| FS < 8 | 4.56 (3.80–5.47) | 3.34 (2.76–4.03) | 3.63 (3.00–4.39) | 2.65 (2.16–3.26) | 2.46 (2.00–3.02) | 2.46 (1.99–3.04) | 2.37 (1.92–2.94) |
| 8 ≤ FS < 11 | 2.62 (2.20–3.11) | 2.07 (1.74–2.47) | 2.23 (1.87–2.66) | 1.81 (1.50–2.17) | 1.69 (1.41–2.03) | 1.70 (1.41–2.04) | 1.65 (1.38–1.99) |
| 11 ≤ FS < 13 | 1.52 (1.27–1.82) | 1.33 (1.11–1.60) | 1.39 (1.16–1.67) | 1.26 (1.06–1.51) | 1.24 (1.03–1.49) | 1.24 (1.04–1.49) | 1.24 (1.03–1.48) |
| FS = 13 | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) |
All models stratified by country, and accounting for facility clustering effects; Demographics: gender, black race, body mass index, years on dialysis; Comorbidities: listed in Table 2, Labs: serum albumin, creatinine, phosphorus, hemoglobin, single pool Kt/V.
The association of FS score with mortality appeared stronger in younger patients than older patients when comparing the HR (P value for age-FS interaction = 0.01). Figure 2 shows the mortality risk for patients, interacting FS with age. In part because the baseline mortality risk among younger patients is low, we observed a higher risk ratio (RR) but only modest absolute risk difference (RD) when comparing FS < 8 vs. FS = 13 in younger patients. For example: the estimated mortality risk for patients with FS=13 was 0.028 (95% CI: 0.020–0.038) at age 50 and 0.098 (95% CI: 0.079–0.121) at age 80, while the estimated mortality risk for patients with FS < 8 was 0.114 (95% CI: 0.081– 0.159) at age 50 and 0.284 (95% CI: 0.252–0.317) at age 80. Thus the calculated RR of FS < 8 vs. FS = 13 was 4.12 (0.114/0.028) at age 50 and 2.90 (0.284/0.098) at age 80 years. In contrast the RD of FS < 8 vs. FS = 13 was 0.086 (0.114 minus 0.028) and 0.186 (0.284 minus 0.098) at ages 50 and 80 years respectively. We did not find a monotonic relation between any other tested covariate (gender, diabetes, catheter use, vintage, black race, region) and the estimated HR for the effect of FS on mortality (p > 0.15 for heterogeneity of the HR).
Figure 2. Mortality risk (per year) by age and functional status (FS).
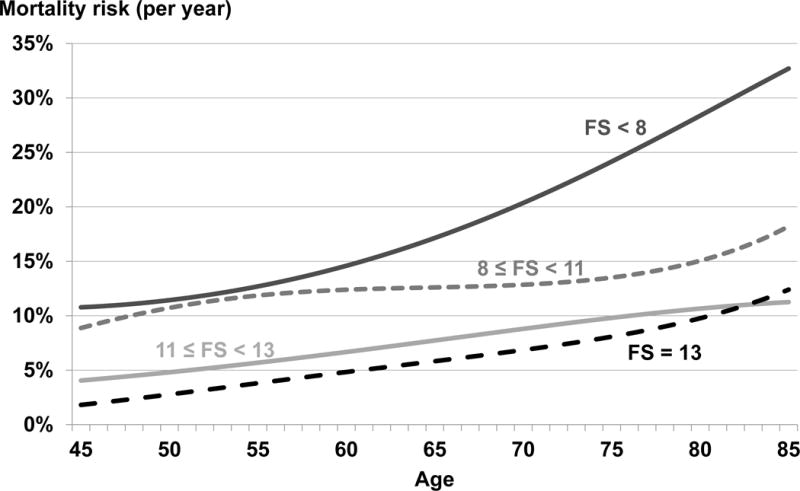
Discrete survival model with binomial distribution, logit link function, and log(follow-up) offset. Model was adjusted for country and included age as a cubic term and an interaction between FS category and age. Predicted probabilities for age*FS combinations used to estimate mortality risk.
Functional status and quality of life
Patients with FS=13 reported higher physical (difference=16.2, 95% CI: 15.5–16.9) and mental (difference=11.7, 95% CI: 10.8–12.6) QOL than patients with FS < 8, after adjustment for country and age. Results were consistent across all ages (Supplementary Figure 2). Furthermore, the results suggested that QOL was more strongly correlated with functional status than with age such that older patients with full independence (FS=13) had higher PCS and MCS than younger patients with mild degrees of functional dependence (11 ≤ FS < 13).
Sensitivity analyses
A sensitivity analysis weighted by the inverse probability of being included in the main analysis was performed to help account for potential bias arising from the observation that patients who responded to the PQ and self-reported their FS had fewer comorbidities than those who were excluded (Table 2). Sensitivity tests suggested results were consistent with the main analysis; the adjusted HR was 1.21 (95% CI = 1.00–1.46) for 11 ≤ FS < 13, 1.60 (95% CI = 1.33–1.92) for 8 ≤ FS < 11, and 2.37 (95% CI = 1.91–2.95) for FS < 8 when compared to patients who were fully independent (FS=13).
DISCUSSION
Using data from the international DOPPS sample, we demonstrated that worldwide a very high proportion of dialysis patients, of all ages, experience difficulty with routine daily tasks, and that the need for help with daily tasks (as measured by functional dependence) was strongly associated with increased mortality.
Overall, the majority of HD patients presented some level of functional dependence with the highest burden being seen in older diabetic women and those with the highest comorbidity burden. Both the prevalence and the burden of functional dependence were higher than expected compared to age-matched data from the general population. For example, the proportion of non-institutionalized patients aged 70 years or more who reported dependency in the DOPPS study population was 77% (data not shown) while in older populations, such as that from the community-based National Health and Nutrition Examination Survey (NHANES) and the Cardiovascular Health Study (CHS), less than one-third of older adults (aged 65 years or more) had functional dependence in ADL31 or IADL activities32–35. Perhaps even more striking was the observation that almost half of younger patients reported needing help with at least one IADL activity. This degree of dependence is in stark contrast to studies of the general population that suggest functional independence is normally preserved until ages into the 60s or 70s.32, 33 Previous studies in the dialysis population have shown high levels of functional dependence in IADL in prevalent HD patients aged 65 years or more15, in octogenarians36 and in those who were residing in a nursing home at the time of dialysis initiation,37 but most reports of dependence in the younger population have focused only on physical activity38, employment status39, and self-reported physical health15, 40–42.
Multiple factors are likely to contribute to the high prevalence of functional dependence. We found a non-linear relation between FS score and BMI that suggests decreased independence in those who are obese; and we found a relation between FS and both low blood pressure and access type, suggesting that those with vascular disease may be more dependent. While potentially amenable to modifiable clinical practice, this relation may also reflect residual confounding. Other potential factors may include the chronic progressive nature of kidney failure, multi-morbidity, the high prevalence of depression and cognitive disorders, and the repeated need for hospitalization that may contribute to the high rate of functional dependencies7–9, 35, 43. In addition, post-dialysis fatigue and rapid volume shifts may have an impact on overall health and functionality44, 45 while the observation that both caregivers and health care workers facilitate patients taking on a learned helplessness46, 47 may perpetuate the decline in physical health and self-care ability.
Studies from the general population suggest that cross-cultural differences, while present, are generally relatively small48, 49. In contrast, we found that the prevalence of functional dependence differed considerably across the DOPPS countries, possibly reflecting not only differences in patient characteristics and comorbidities, but also in their reporting behavior. These differences were preserved even after adjustment for other factors, suggesting that cultural and other societal factors may play an important part in the selection of patients for dialysis and, or, the patient perception of dependence.
We demonstrated a strong association between greater functional dependence and mortality, dialysis withdrawal, and time to first hospitalization. These findings are consistent with those of previous studies showing higher mortality in dialysis patients who have either an observed low FS at the time of admission to acute care50 or poor function as reported by low self-rated physical health40–42 or sedentary lifestyles31, 38. They are also consistent with data from large community-based, non-renal population studies where recurrent transient episodes of disability in ADL activities portended a high risk of mortality, subsequent catastrophic disability, or likelihood of admission to long-term care5, 7, 9, 10, 12, 13, 35, 51.
The association between functional dependence and mortality may in part be explained by demographic and clinical factors. For example patients who are older or have more severe disease, and thus at higher risk of mortality, are more likely to have difficulty with daily activities. To address this, we adjusted for several known risk factors for mortality such as age, gender, race, and comorbidities. We used expanding sets of covariate adjustment to allow a better understanding of the impact of these potential confounders. We proposed that both cachexia and the use of a central venous catheter would be important clinical factors reflecting patients who were medically unwell and therefore at higher risk of death. However, in our analyses we found that, conditional on the other covariates in Models 2–5, the addition of either type of vascular access or cachexia did little to change the estimated hazard ratio for FS.
Our data add further support to those advocating for a change in the approach as to how chronic dialysis care is provided to individuals with complex multimorbidity, those at the extremes of age and those with high dependency.52–55 They argue in favor of care that includes close attention to modifiable symptoms, such as pain or weakness, in an attempt to improve functional status, as well as, perhaps furthering discussions about the value of care that prioritizes symptom management over laboratory-target driven dialysis care. We also observed that patients with advanced functional dependence were more likely to withdraw from dialysis, a finding that is consistent with the clinical impression that both patients and caregivers experience a low QOL when a large amount of assistance with daily tasks are required. However we also noted that there is a stronger correlation between functional status and QOL measures than between age and QOL. This observation may be clinically important for two reasons. As the renal community shifts increasingly towards evaluating the quality of care based on patient-reported outcomes, ongoing assessment for functional status may be important; it may be appropriate to incorporate functional status (as a marker of future mortality risk and QOL) when assessing appropriateness of chronic dialytic care.
One limitation of this analysis is that while the DOPPS is designed to be nationally representative of in-center adult HD patients, those who responded to the patient questionnaire and self-reported their FS tended to be somewhat younger and healthier than excluded patients. A sensitivity analysis giving more weight to patients more likely to be non-responders, however, suggested minimal change of the hazard ratios, suggesting the observation is likely robust across the wider dialysis population. Separately, two-thirds of the US DOPPS cohort was not eligible for the analysis due to missing information on comorbidity history – a key confounder in the FS-mortality relationship. Dialyzing in a US large dialysis organization and not responding to the ADL questions on the PQ are two very different reasons for exclusion from our study population: the former reflects a type of administrative exclusion; the latter reflects exclusions based on patient differences. The propensity score weighted sensitivity analysis attempts to address the latter of these; it does not address the large dialysis organization exclusion, which is not likely to be a major source of bias in estimating the effect of functional status on mortality, but which might limit generalization of our findings to US patients dialyzing in large dialysis organization facilities. As with many observational studies, it is not possible to speculate as to reasons for the high rate of dependence or to why dependence is associated with increased mortality. Further, we are unable to address the effects of residual confounding due to unknown or unmeasured factors, nor to provide meaningful insight into the trajectory of functional decline over time.
In conclusion, we observed a high level of functional dependence in daily activities in dialysis patients across a wide range of age groups and countries, and we found a strong dose-response association between functional dependence and adverse clinical outcomes. It remains to be shown whether rehabilitation or interventional programs can prevent or reverse functional dependence and thereby improve patient outcomes.
Supplementary Material
Modifiable predictors of Functional Status (FS) score. Ordinal logistic regression (proportional odds) models showing the odds ratio (95% CI) of having one category lower FS (e.g., an OR > 1 indicates association with lower FS); 5 separate models adjusted for country, age, gender, black race, BMI, years on dialysis, 13 comorbidities listed in Table 2, serum albumin, creatinine, calcium, phosphorus, hemoglobin, single pool Kt/V, vascular access, cachexia, and accounting for facility clustering effects; Model with treatment time as the exposure was not adjusted for Kt/V.
Two separate linear regression models, with PCS (A) and MCS (B) as the outcome. Models were adjusted for country and included age as a cubic term and an interaction between FS category and age. Predicted values for age*FS combinations used to estimate PCS and MCS.
Acknowledgments
The DOPPS program is supported by Amgen, Kyowa Hakko Kirin, AbbVie Inc., Sanofi Renal, Baxter Healthcare, and Vifor Fresenius Medical Care Renal Pharma, Ltd. Additional support for specific projects and countries is also provided in Canada by Amgen, BHC Medical, Janssen, Takeda, Kidney Foundation of Canada (for logistics support); in Germany by Hexal, DGfN, Shire, WiNe Institute; for PDOPPS in Japan by the Japanese Society for Peritoneal Dialysis (JSPD). All support is provided without restrictions on publications. F.T. is supported, in part, by National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) Award K01DK087762. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIDDK or the National Institutes of Health. All support is provided without restrictions on publications.
F.T. has received honoraria from Amgen, Dialysis Clinic Inc., and Renal Research Institute. R.L.P. has received speaker fees from Amgen, Kyowa Hakko Kirin, and Vifor; served as a consultant for Pursuit Vascular; and served on an advisory panel for Merck. B.M.R. has received speaker fees for Kyowa Hakko Kirin. MRM is an employee of Baxter Healthcare.
The authors wish to thank Shauna Leighton for providing editorial assistance for this manuscript.
Footnotes
The remaining authors have no conflicts to report.
References
- 1.USRDS. Chapter 3: Hospitalization. 2013 http://www.usrds.org/2013/pdf/v2_ch3_13.pdf last accessed 6 August 2015.
- 2.Fischer MJ, Ho PM, McDermott K, Lowy E, Parikh CR. Chronic kidney disease is associated with adverse outcomes among elderly patients taking clopidogrel after hospitalization for acute coronary syndrome. BMC Nephrol. 2013;14:107. doi: 10.1186/1471-2369-14-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ariyaratne TV, Ademi Z, Duffy SJ, Andrianopoulos N, Billah B, Brennan AL, et al. Cardiovascular readmissions and excess costs following percutaneous coronary intervention in patients with chronic kidney disease: data from a large multi-centre Australian registry. Int J Cardiol. 2013;168:2783–2790. doi: 10.1016/j.ijcard.2013.03.128. [DOI] [PubMed] [Google Scholar]
- 4.Sands LP, Xu H, Craig BA, Eng C, Covinsky KE. Predicting change in functional status over quarterly intervals for older adults enrolled in the PACE community-based long-term care program. Aging Clin Exp Res. 2008;20:419–427. doi: 10.1007/BF03325147. [DOI] [PubMed] [Google Scholar]
- 5.Carey EC, Covinsky KE, Lui LY, Eng C, Sands LP, Walter LC. Prediction of mortality in community-living frail elderly people with long-term care needs. J Am Geriatr Soc. 2008;56:68–75. doi: 10.1111/j.1532-5415.2007.01496.x. [DOI] [PubMed] [Google Scholar]
- 6.Carey EC, Walter LC, Lindquist K, Covinsky KE. Development and validation of a functional morbidity index to predict mortality in community-dwelling elders. J Gen Intern Med. 2004;19:1027–1033. doi: 10.1111/j.1525-1497.2004.40016.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Covinsky KE, Palmer RM, Fortinsky RH, Counsell SR, Stewart AL, Kresevic D, et al. Loss of independence in activities of daily living in older adults hospitalized with medical illnesses: increased vulnerability with age. J Am Geriatr Soc. 2003;51:451–458. doi: 10.1046/j.1532-5415.2003.51152.x. [DOI] [PubMed] [Google Scholar]
- 8.Gill TM, Kurland B. The burden and patterns of disability in activities of daily living among community-living older persons. J Gerontol A Biol Sci Med Sci. 2003;58:70–75. doi: 10.1093/gerona/58.1.m70. [DOI] [PubMed] [Google Scholar]
- 9.Onder G, Penninx BW, Ferrucci L, Fried LP, Guralnik JM, Pahor M. Measures of physical performance and risk for progressive and catastrophic disability: results from the Women’s Health and Aging Study. J Gerontol A Biol Sci Med Sci. 2005;60:74–79. doi: 10.1093/gerona/60.1.74. [DOI] [PubMed] [Google Scholar]
- 10.Boyd CM, Landefeld CS, Counsell SR, Palmer RM, Fortinsky RH, Kresevic D, et al. Recovery of activities of daily living in older adults after hospitalization for acute medical illness. J Am Geriatr Soc. 2008;56:2171–2179. doi: 10.1111/j.1532-5415.2008.02023.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buurman BM, Hoogerduijn JG, de Haan RJ, Abu-Hanna A, Lagaay AM, Verhaar HJ, et al. Geriatric conditions in acutely hospitalized older patients: prevalence and one-year survival and functional decline. PLoS ONE. 2011;6:e26951. doi: 10.1371/journal.pone.0026951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Covinsky KE, Justice AC, Rosenthal GE, Palmer RM, Landefeld CS. Measuring prognosis and case mix in hospitalized elders. The importance of functional status. J Gen Intern Med. 1997;12:203–208. doi: 10.1046/j.1525-1497.1997.012004203.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gill TM, Allore H, Guo Z. Restricted activity and functional decline among community-living older persons. Arch Intern Med. 2003;163:1317–1322. doi: 10.1001/archinte.163.11.1317. [DOI] [PubMed] [Google Scholar]
- 14.McClure JA, Salter K, Meyer M, Foley N, Kruger H, Teasell R. Predicting length of stay in patients admitted to stroke rehabilitation with high levels of functional independence. Disabil Rehabil. 2011;33(23–24):2356–61. doi: 10.3109/09638288.2011.572225. [DOI] [PubMed] [Google Scholar]
- 15.Cook WL, Jassal SV. Functional dependencies among the elderly on hemodialysis. Kidney Int. 2008;73:1289–1295. doi: 10.1038/ki.2008.62. [DOI] [PubMed] [Google Scholar]
- 16.Bowling CB, Sawyer P, Campbell RC, Ahmed A, Allman RM. Impact of chronic kidney disease on activities of daily living in community-dwelling older adults. J Gerontol A Biol Sci Med Sci. 2011;66:689–694. doi: 10.1093/gerona/glr043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lattanzio F, Corsonello A, Abbatecola AM, Volpato S, Pedone C, Pranno L, et al. Relationship between renal function and physical performance in elderly hospitalized patients. Rejuvenation Res. 2012;15:545–552. doi: 10.1089/rej.2012.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shlipak MG, Stehman-Breen C, Fried LF, Song X, Siscovick D, Fried LP, et al. The presence of frailty in elderly persons with chronic renal insufficiency. Am J Kidney Dis. 2004;43:861–867. doi: 10.1053/j.ajkd.2003.12.049. [DOI] [PubMed] [Google Scholar]
- 19.Fried LF, Lee JS, Shlipak M, Chertow GM, Green C, Ding J, et al. Chronic Kidney Disease and Functional Limitation in Older People: Health, Aging and Body Composition Study. J Am Geriatr Soc. 2006;54:750–756. doi: 10.1111/j.1532-5415.2006.00727.x. [DOI] [PubMed] [Google Scholar]
- 20.Odden MC, Shlipak MG, Tager IB. Serum creatinine and functional limitation in elderly persons. J Gerontol A Biol Sci Med Sci. 2009;64(3):370–6. doi: 10.1093/gerona/gln037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kurella M, Ireland C, Hlatky MA, Shlipak MG, Yaffe K, Hulley SB, et al. Physical and sexual function in women with chronic kidney disease. Am J Kidney Dis. 2004;43(5):868–76. doi: 10.1053/j.ajkd.2003.12.050. [DOI] [PubMed] [Google Scholar]
- 22.Pisoni RL, Gillespie BW, Dickinson DM, Chen K, Kutner MH, Wolfe RA. The Dialysis Outcomes and Practice Patterns Study (DOPPS): design, data elements, and methodology. Am J Kidney Dis. 2004;44:7–15. doi: 10.1053/j.ajkd.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 23.Young EW, Goodkin DA, Mapes DL, Port FK, Keen ML, Chen K, et al. The Dialysis Outcomes and Practice Patterns Study: an international hemodialysis study. Kidney Int. 2000;57:S74–S81. [Google Scholar]
- 24.Katz S, Downs TD, Cash HR, Grotz RC. Progress in development of the index of ADL. Gerontologist. 1970;10:20–30. doi: 10.1093/geront/10.1_part_1.20. [DOI] [PubMed] [Google Scholar]
- 25.Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9:179–186. [PubMed] [Google Scholar]
- 26.LaPlante MP. The classic measure of disability in activities of daily living is biased by age but an expanded IADL/ADL measure is not. J Gerontol B Psychol Sci Soc Sci. 2010;65:720–732. doi: 10.1093/geronb/gbp129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hays RD, Kallich JD, Mapes DL, Coons SJ, Carter WB. Development of the kidney disease quality of life (KDQOL) instrument. Quality of life research: an international journal of quality of life aspects of treatment, care and rehabilitation. 1994;3:329–338. doi: 10.1007/BF00451725. [DOI] [PubMed] [Google Scholar]
- 28.van Buuren S, Boshuizen HC, Knook DL. Multiple imputation of missing blood pressure covariates in survival analysis. Stat Med. 1999;18:681–694. doi: 10.1002/(sici)1097-0258(19990330)18:6<681::aid-sim71>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 29.Trivellore E, Raghunathan PWS, Van Hoewyk John. IVEware: Imputation and Variance Estimation Software. Available at: http://www.isr.umich.edu/src/smp/ive/. Aceessed 12/12/14.
- 30.Little RJAR, DB . Statistical analysis with missing data. New York: Wiley; 1987. [Google Scholar]
- 31.Tentori F, Mapes DL. Health-related quality of life and depression among participants in the DOPPS: predictors and associations with clinical outcomes. Semin Dial. 2010;23:14–16. doi: 10.1111/j.1525-139X.2009.00677.x. [DOI] [PubMed] [Google Scholar]
- 32.Sonn U. Longitudinal studies of dependence in daily life activities among elderly persons. Scand J Rehabil Med Suppl. 1996;34:1–35. [PubMed] [Google Scholar]
- 33.Lin SF, Beck AN, Finch BK, Hummer RA, Masters RK. Trends in US older adult disability: exploring age, period, and cohort effects. Am J Public Health. 2012;102:2157–2163. doi: 10.2105/AJPH.2011.300602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kuo HK, Al Snih S, Kuo YF, Raji MA. Chronic inflammation, albuminuria, and functional disability in older adults with cardiovascular disease: the National Health and Nutrition Examination Survey, 1999–2008. Atherosclerosis. 2012;222:502–508. doi: 10.1016/j.atherosclerosis.2012.03.004. [DOI] [PubMed] [Google Scholar]
- 35.Picavet HS, Hoeymans N. Physical disability in The Netherlands: prevalence, risk groups and time trends. Public Health. 2002;116:231–237. doi: 10.1038/sj.ph.1900864. [DOI] [PubMed] [Google Scholar]
- 36.Jassal SV, Chiu E, Hladunewich M. Loss of independence in patients starting dialysis at 80 years of age or older. New Engl J Med. 2009;361:1612–1613. doi: 10.1056/NEJMc0905289. [DOI] [PubMed] [Google Scholar]
- 37.Kurella Tamura M, Covinsky KE, Chertow GM, Yaffe K, Landefeld CS, McCulloch CE. Functional status of elderly adults before and after initiation of dialysis. N Engl J Med. 2009;361:1539–1547. doi: 10.1056/NEJMoa0904655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.O’Hare AM, Tawney K, Bacchetti P, Johansen KL. Decreased survival among sedentary patients undergoing dialysis: results from the dialysis morbidity and mortality study wave 2. Am J Kidney Dis. 2003;41(2):447–54. doi: 10.1053/ajkd.2003.50055. [DOI] [PubMed] [Google Scholar]
- 39.Kutner NG, Zhang R, Huang Y, Johansen KL. Depressed mood, usual activity level, and continued employment after starting dialysis. Clin J Am Soc Nephrol. 2010;5:2040–2045. doi: 10.2215/CJN.03980510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.DeOreo PB. Hemodialysis patient-assessed functional health status predicts continued survival, hospitalization, and dialysis-attendance compliance. Am J Kidney Dis. 1997;30(2):204–12. doi: 10.1016/s0272-6386(97)90053-6. [DOI] [PubMed] [Google Scholar]
- 41.McClellan WM, Anson C, Birkeli K, Tuttle E. Functional status and quality of life: predictors of early mortality among patients entering treatment for end stage renal disease. J Clin Epidemiol. 1991;44(1):83–9. doi: 10.1016/0895-4356(91)90204-m. [DOI] [PubMed] [Google Scholar]
- 42.Knight EL, Ofsthun N, Teng M, Lazarus JM, Curhan GC. The association between mental health, physical function, and hemodialysis mortality. Kidney Int. 2003;63:1843–1851. doi: 10.1046/j.1523-1755.2003.00931.x. [DOI] [PubMed] [Google Scholar]
- 43.Lo D, Chiu E, Jassal SV. A prospective pilot study to measure changes in functional status associated with hospitalization in elderly dialysis-dependent patients. Am J Kidney Dis. 2008;52:956–961. doi: 10.1053/j.ajkd.2008.04.010. [DOI] [PubMed] [Google Scholar]
- 44.Lindsay RM, Heidenheim PA, Nesrallah G, Garg AX, Suri R. Minutes to recovery after a hemodialysis session: a simple health-related quality of life question that is reliable, valid, and sensitive to change. Clin J Am Soc Nephrol. 2006;1:952–959. doi: 10.2215/CJN.00040106. [DOI] [PubMed] [Google Scholar]
- 45.Thong MS, Kaptein AA, Krediet RT, Boeschoten EW, Dekker FW. Social support predicts survival in dialysis patients. Nephrol Dial Transplant. 2007;22:845–850. doi: 10.1093/ndt/gfl700. [DOI] [PubMed] [Google Scholar]
- 46.Miller WR, Seligman ME. Depression and learned helplessness in man. J Abnorm Psychol. 1975;84:228–238. doi: 10.1037/h0076720. [DOI] [PubMed] [Google Scholar]
- 47.Farragher J, Jassal SV. Rehabilitation of the geriatric dialysis patient. Semin Dial. 2012;25:649–656. doi: 10.1111/sdi.12014. [DOI] [PubMed] [Google Scholar]
- 48.Skevington SM, Lotfy M, O’Connell KA. The World Health Organization’s WHOQOL-BREF quality of life assessment: psychometric properties and results of the international field trial. A report from the WHOQOL group. Qual Life Res. 2004;13:299–310. doi: 10.1023/B:QURE.0000018486.91360.00. [DOI] [PubMed] [Google Scholar]
- 49.Molzahn AE, Kalfoss M, Schick Makaroff K, Skevington SM. Comparing the importance of different aspects of quality of life to older adults across diverse cultures. Age Ageing. 2011;40:192–199. doi: 10.1093/ageing/afq156. [DOI] [PubMed] [Google Scholar]
- 50.Sood MM, Rigatto C, Bueti J, Jassal SV, Miller L, Verrelli M, et al. Functional status, discharge to a long-term care facility and in-hospital death among dialysis patients. Am J Kid Dis. 2011;58:804–812. doi: 10.1053/j.ajkd.2011.06.017. [DOI] [PubMed] [Google Scholar]
- 51.Rudberg MA, Sager MA, Zhang J. Risk factors for nursing home use after hospitalization for medical illness. J Gerontol A Biol Sci Med Sci. 1996;51(5):M189–94. doi: 10.1093/gerona/51a.5.m189. [DOI] [PubMed] [Google Scholar]
- 52.Jassal SV. Four plus forty-four: hours to modify, theirs to enjoy. Clin J Am Soc Nephrol. 2015;10(2):169–71. doi: 10.2215/CJN.12681214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vandecasteele SJ, Kurella Tamura M. A patient-centered vision of care for ESRD: dialysis as a bridging treatment or as a final destination? J Am Soc Nephrol. 2014;25(8):1647–51. doi: 10.1681/ASN.2013101082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Churchill DN, Jassal SV. Dialysis: destination or journey. J Am Soc Nephrol. 2014;25(8):1609–11. doi: 10.1681/ASN.2014040390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Grubbs V, Moss AH, Cohen LM, Fischer MJ, Germain MJ, Jassal SV, Perl J, Weiner DE, Mehrotra R, Dialysis Advisory Group of the American Society of Nephrology A palliative approach to dialysis care: a patient-centered transition to the end of life. Clin J Am Soc Nephrol. 2014;9(12):2203–9. doi: 10.2215/CJN.00650114. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Modifiable predictors of Functional Status (FS) score. Ordinal logistic regression (proportional odds) models showing the odds ratio (95% CI) of having one category lower FS (e.g., an OR > 1 indicates association with lower FS); 5 separate models adjusted for country, age, gender, black race, BMI, years on dialysis, 13 comorbidities listed in Table 2, serum albumin, creatinine, calcium, phosphorus, hemoglobin, single pool Kt/V, vascular access, cachexia, and accounting for facility clustering effects; Model with treatment time as the exposure was not adjusted for Kt/V.
Two separate linear regression models, with PCS (A) and MCS (B) as the outcome. Models were adjusted for country and included age as a cubic term and an interaction between FS category and age. Predicted values for age*FS combinations used to estimate PCS and MCS.


