Abstract
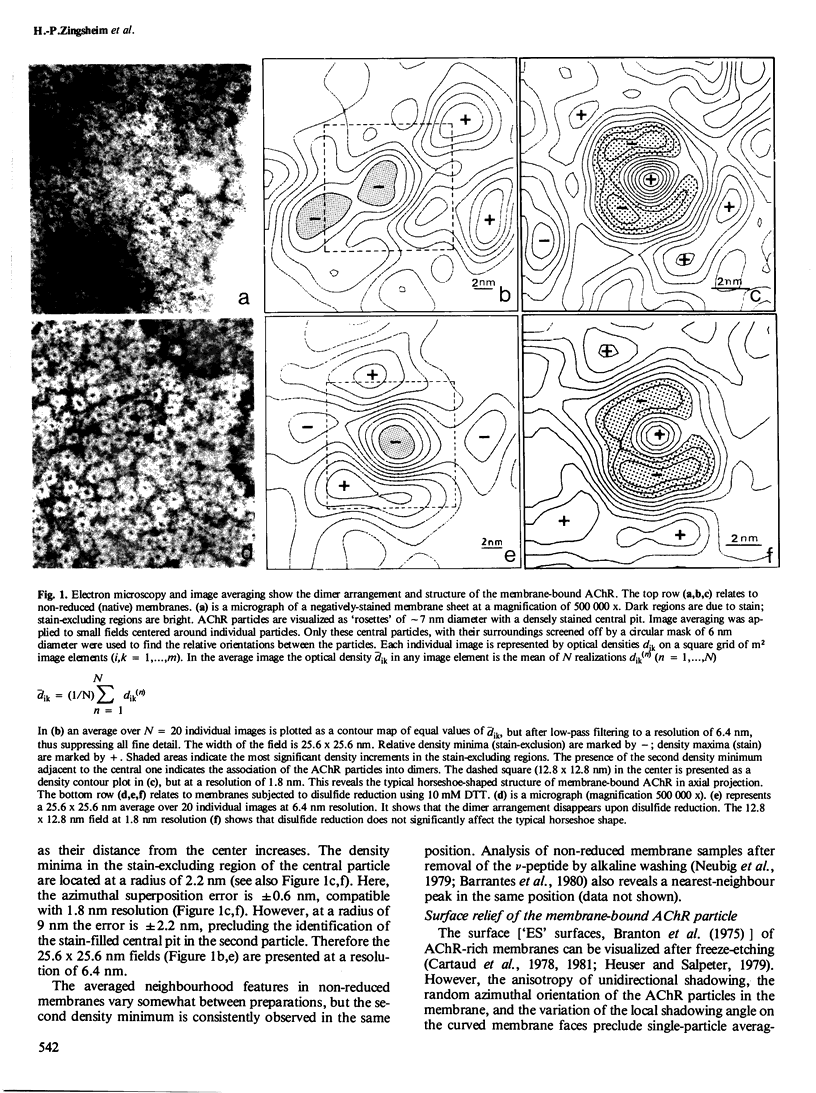
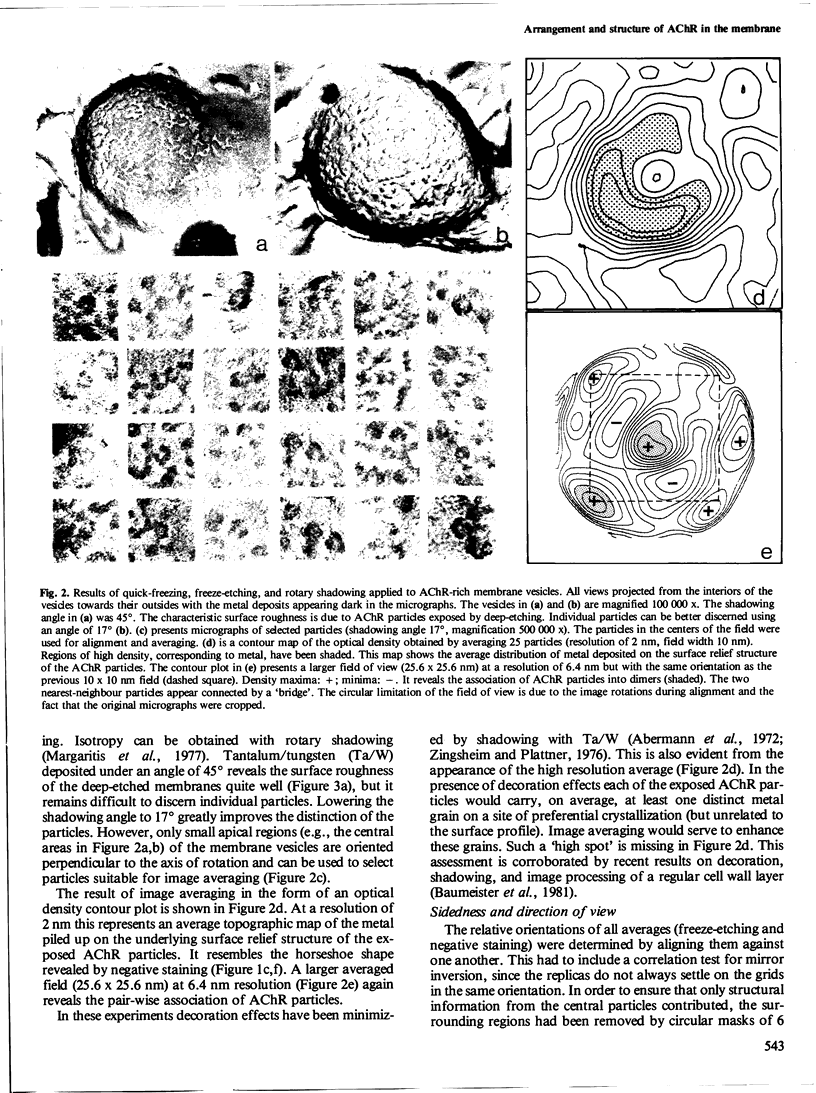
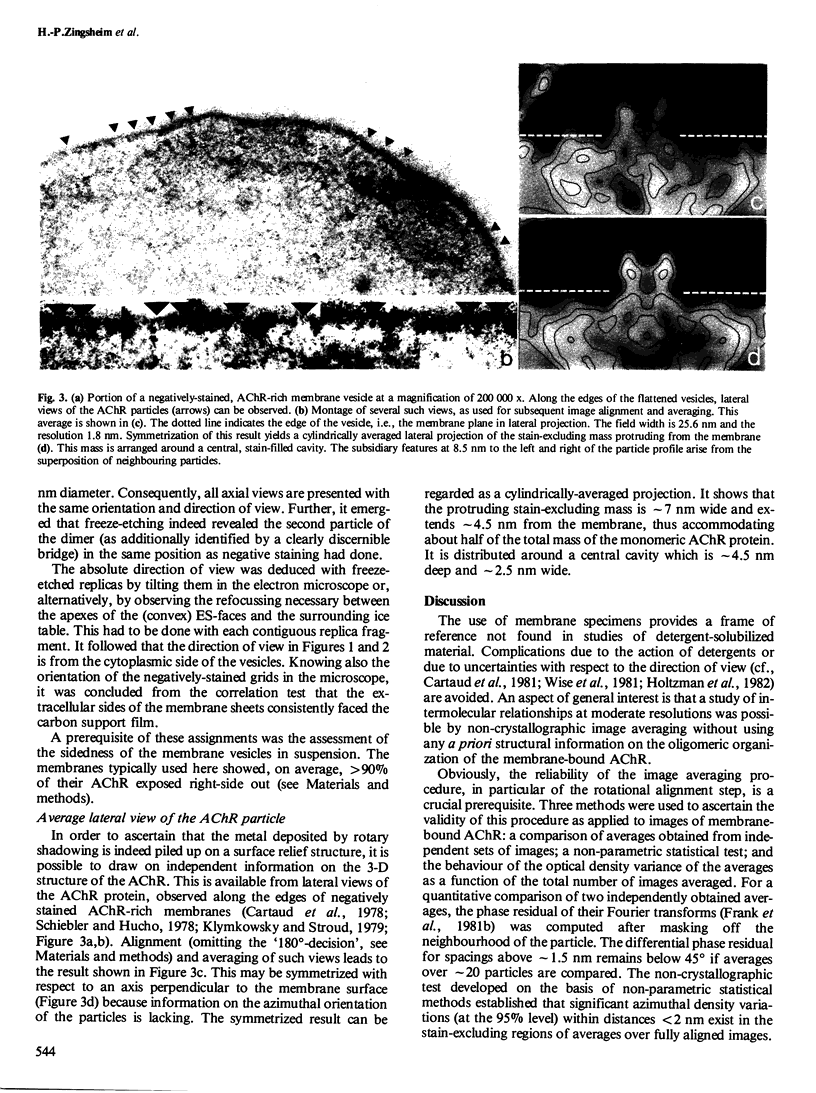
The acetylcholine receptor protein (AChR) from the electric organ of Torpedo marmorata is studied in its membrane-bound form by electron microscopy and single-particle image averaging. About half the molecule protrudes from the membrane surface by approximately 5 nm. The low-resolution 3-D structure of this hydrated portion, including its handedness, can be deduced from averaged axial and lateral projections and from freeze-etched membrane surfaces. In native membrane fragments, a dimeric form of the AChR is observed and the relative orientation of the AChR monomers within the dimer is established. The dimers disappear upon disulfide reduction of the membrane preparations, whereas the average axial projections of the AChR monomer remain unaffected. Since the existence of disulfide bonds linking AChR monomers between their respective delta-subunits is well documented, the approximate position of the delta-subunit within the low-resolution structure of the AChR molecule can be deduced from the structure of the dimers.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anholt R., Lindstrom J., Montal M. Functional equivalence of monomeric and dimeric forms of purified acetylcholine receptors from Torpedo californica in reconstituted lipid vesicles. Eur J Biochem. 1980 Aug;109(2):481–487. doi: 10.1111/j.1432-1033.1980.tb04819.x. [DOI] [PubMed] [Google Scholar]
- Bachmann L., Schmitt W. W. Improved cryofixation applicable to freeze etching. Proc Natl Acad Sci U S A. 1971 Sep;68(9):2149–2152. doi: 10.1073/pnas.68.9.2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrantes F. J. Endogenous chemical receptors: some physical aspects. Annu Rev Biophys Bioeng. 1979;8:287–321. doi: 10.1146/annurev.bb.08.060179.001443. [DOI] [PubMed] [Google Scholar]
- Barrantes F. J. Modulation of acetylcholine receptor states by thiol modification. Biochemistry. 1980 Jun 24;19(13):2957–2965. doi: 10.1021/bi00554a022. [DOI] [PubMed] [Google Scholar]
- Barrantes F. J., Neugebauer D. C., Zingsheim H. P. Peptide extraction by alkaline treatment is accompanied by rearrangement of the membrane-bound acetylcholine receptor from Torpedo marmorata. FEBS Lett. 1980 Mar 24;112(1):73–78. doi: 10.1016/0014-5793(80)80131-1. [DOI] [PubMed] [Google Scholar]
- Baumeister W., Kübler O., Zingsheim H. P. The structure of the cell envelope of Micrococcus radiodurans as revealed by metal shadowing and decoration. J Ultrastruct Res. 1981 Apr;75(1):60–71. doi: 10.1016/s0022-5320(81)80100-1. [DOI] [PubMed] [Google Scholar]
- Boheim G., Hanke W., Barrantes F. J., Eibl H., Sakmann B., Fels G., Maelicke A. Agonist-activated ionic channels in acetylcholine receptor reconstituted into planar lipid bilayers. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3586–3590. doi: 10.1073/pnas.78.6.3586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branton D., Bullivant S., Gilula N. B., Karnovsky M. J., Moor H., Mühlethaler K., Northcote D. H., Packer L., Satir B., Satir P. Freeze-etching nomenclature. Science. 1975 Oct 3;190(4209):54–56. doi: 10.1126/science.1166299. [DOI] [PubMed] [Google Scholar]
- Cartaud J., Benedetti E. L. A morphological study of the cholinergic receptor protein from Torpedo marmorata in its membrane environment and in its detergent-extracted purified form. J Cell Sci. 1978 Feb;29:313–337. doi: 10.1242/jcs.29.1.313. [DOI] [PubMed] [Google Scholar]
- Cartaud J., Sobel A., Rousselet A., Devaux P. F., Changeux J. P. Consequences of alkaline treatment for the ultrastructure of the acetylcholine-receptor-rich membranes from Torpedo marmorata electric organ. J Cell Biol. 1981 Aug;90(2):418–426. doi: 10.1083/jcb.90.2.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang H. W., Bock E. Molecular forms of acetylcholine receptor. Effects of calcium ions and a sulfhydryl reagent on the occurrence of oligomers. Biochemistry. 1977 Oct 4;16(20):4513–4520. doi: 10.1021/bi00639a028. [DOI] [PubMed] [Google Scholar]
- Fambrough D. M. Control of acetylcholine receptors in skeletal muscle. Physiol Rev. 1979 Jan;59(1):165–227. doi: 10.1152/physrev.1979.59.1.165. [DOI] [PubMed] [Google Scholar]
- Frank J., Goldfarb W., Eisenberg D., Baker T. S. Reconstruction of glutamine synthetase using computer averaging. Ultramicroscopy. 1978;3(3):283–290. doi: 10.1016/s0304-3991(78)80038-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank J., Verschoor A., Boublik M. Computer averaging of electron micrographs of 40S ribosomal subunits. Science. 1981 Dec 18;214(4527):1353–1355. doi: 10.1126/science.7313694. [DOI] [PubMed] [Google Scholar]
- Gulik-Krzywicki T., Costello M. J. The use of low temperature X-ray diffraction to evaluate freezing methods used in freeze-fracture electron microscopy. J Microsc. 1978 Jan;112(1):103–113. doi: 10.1111/j.1365-2818.1978.tb01158.x. [DOI] [PubMed] [Google Scholar]
- Hamilton S. L., McLaughlin M., Karlin A. Disulfide bond cross-linked dimer in acetylcholine receptor from Torpedo californica. Biochem Biophys Res Commun. 1977 Dec 7;79(3):692–699. doi: 10.1016/0006-291x(77)91167-6. [DOI] [PubMed] [Google Scholar]
- Hamilton S. L., McLaughlin M., Karlin A. Formation of disulfide-linked oligomers of acetylcholine receptor in membrane from torpedo electric tissue. Biochemistry. 1979 Jan 9;18(1):155–163. doi: 10.1021/bi00568a024. [DOI] [PubMed] [Google Scholar]
- Hartig P. R., Raftery M. A. Preparation of right-side-out, acetylcholine receptor enriched intact vesicles from Torpedo californica electroplaque membranes. Biochemistry. 1979 Apr 3;18(7):1146–1150. doi: 10.1021/bi00574a004. [DOI] [PubMed] [Google Scholar]
- Heidmann T., Changeux J. P. Structural and functional properties of the acetylcholine receptor protein in its purified and membrane-bound states. Annu Rev Biochem. 1978;47:317–357. doi: 10.1146/annurev.bi.47.070178.001533. [DOI] [PubMed] [Google Scholar]
- Heuser J. E., Salpeter S. R. Organization of acetylcholine receptors in quick-frozen, deep-etched, and rotary-replicated Torpedo postsynaptic membrane. J Cell Biol. 1979 Jul;82(1):150–173. doi: 10.1083/jcb.82.1.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtzman E., Wise D., Wall J., Karlin A. Electron microscopy of complexes of isolated acetylcholine receptor, biotinyl-toxin, and avidin. Proc Natl Acad Sci U S A. 1982 Jan;79(2):310–314. doi: 10.1073/pnas.79.2.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kistler J., Stroud R. M. Crystalline arrays of membrane-bound acetylcholine receptor. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3678–3682. doi: 10.1073/pnas.78.6.3678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kistler J., Stroud R. M., Klymkowsky M. W., Lalancette R. A., Fairclough R. H. Structure and function of an acetylcholine receptor. Biophys J. 1982 Jan;37(1):371–383. doi: 10.1016/S0006-3495(82)84685-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klymkowsky M. W., Stroud R. M. Immunospecific identification and three-dimensional structure of a membrane-bound acetylcholine receptor from Torpedo californica. J Mol Biol. 1979 Mar 5;128(3):319–334. doi: 10.1016/0022-2836(79)90091-3. [DOI] [PubMed] [Google Scholar]
- Lo M. M., Garland P. B., Lamprecht J., Barnard E. A. Rotational mobility of the membrane-bound acetylcholine receptor of Torpedo electric organ measured by phosphorescence depolarisation. FEBS Lett. 1980 Mar 10;111(2):407–412. doi: 10.1016/0014-5793(80)80838-6. [DOI] [PubMed] [Google Scholar]
- Margaritis L. H., Elgsaeter A., Branton D. Rotary replication for freeze-etching. J Cell Biol. 1977 Jan;72(1):47–56. doi: 10.1083/jcb.72.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neubig R. R., Krodel E. K., Boyd N. D., Cohen J. B. Acetylcholine and local anesthetic binding to Torpedo nicotinic postsynaptic membranes after removal of nonreceptor peptides. Proc Natl Acad Sci U S A. 1979 Feb;76(2):690–694. doi: 10.1073/pnas.76.2.690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pscheid P., Schudt C., Plattner H. Cryofixation of monolayer cell cultures for freeze-fracturing without chemical pre-treatments. J Microsc. 1981 Feb;121(Pt 2):149–167. doi: 10.1111/j.1365-2818.1981.tb01208.x. [DOI] [PubMed] [Google Scholar]
- Rousselet A., Cartaud J., Devaux P. F. Importance des interactions protéine-protéine dans les maintien de la structure des fragments excitables de l'organe électrique de Torpedo marmorata. C R Seances Acad Sci D. 1979 Sep 24;289(5):461–463. [PubMed] [Google Scholar]
- Rüchel R., Watters D., Maelicke A. Molecular forms and hydrodynamic properties of acetylcholine receptor from electric tissue. Eur J Biochem. 1981 Oct;119(2):215–223. doi: 10.1111/j.1432-1033.1981.tb05597.x. [DOI] [PubMed] [Google Scholar]
- Schiebler W., Hucho F. Membranes rich in acetylcholine receptor: characterization and reconstitution to excitable membranes from exogenous lipids. Eur J Biochem. 1978 Apr;85(1):55–63. doi: 10.1111/j.1432-1033.1978.tb12211.x. [DOI] [PubMed] [Google Scholar]
- Sobel A., Weber M., Changeux J. P. Large-scale purification of the acetylcholine-receptor protein in its membrane-bound and detergent-extracted forms from Torpedo marmorata electric organ. Eur J Biochem. 1977 Oct 17;80(1):215–224. doi: 10.1111/j.1432-1033.1977.tb11874.x. [DOI] [PubMed] [Google Scholar]
- Steinkilberg M., Schramm H. J. Eine verbesserte Drehkorrelationsmethode für die Strukturbestimmung biologischer Makromoleküle durch Mittelung elektronenmidroskopischer Bilder. Hoppe Seylers Z Physiol Chem. 1980 Sep;361(9):1363–1369. [PubMed] [Google Scholar]
- Suarez-Isla B. A., Hucho F. Acetylcholine receptor: SH group reactivity as indicator of conformational changes and functional states. FEBS Lett. 1977 Mar 15;75(1):65–69. doi: 10.1016/0014-5793(77)80054-9. [DOI] [PubMed] [Google Scholar]
- Van der Kloot W. G., Cohen I. Membrane surface potential changes may alter drug interactions: an example, acetylcholine and curare. Science. 1979 Mar 30;203(4387):1351–1352. doi: 10.1126/science.424757. [DOI] [PubMed] [Google Scholar]
- Wise D. S., Wall J., Karlin A. Relative locations of the beta and delta chains of the acetylcholine receptor determined by electron microscopy of isolated receptor trimer. J Biol Chem. 1981 Dec 25;256(24):12624–12627. [PubMed] [Google Scholar]
- Zingsheim H. P., Neugebauer D. C., Barrantes F. J., Frank J. Structural details of membrane-bound acetylcholine receptor from Tropedo marmorata. Proc Natl Acad Sci U S A. 1980 Feb;77(2):952–956. doi: 10.1073/pnas.77.2.952. [DOI] [PMC free article] [PubMed] [Google Scholar]






