Abstract
Wound healing after corneal injury typically involves fibrosis, with transforming growth factor β1 (TGF-β1) as one of its strongest mediators. A class of small molecules—peroxisome proliferator–activated receptor γ (PPARγ) ligands—exert potent antifibrotic effects in the cornea by blocking phosphorylation of p38 mitogen-activated protein kinase (MAPK). However, why this blocks fibrosis remains unknown. Herein, we show that PPARγ ligands (rosiglitazone, troglitazone, and 15-deoxy-Δ12,14-prostaglandin J2) decrease levels of β-catenin. We also show that β-catenin siRNA and the Wingless/integrated (Wnt) inhibitor pyrvinium block the ability of corneal fibroblasts to up-regulate synthesis of α-smooth muscle actin (α-SMA), collagen 1 (COL1), and fibronectin (FN) in response to TGF-β1. Activation of TGF-β receptors and p38 MAPK increased glycogen synthase kinase 3β (GSK3β) phosphorylation, whereas a chemical inhibitor of p38 MAPK (SB203580) reduced the phosphorylation of GSK3β, decreasing active β-catenin levels in both cytoplasmic and nuclear fractions. Finally, lithium chloride, a GSK3 inhibitor, also attenuated the TGF-β1–induced increase in α-SMA, COL1, and FN expression. All in all, our results suggest that TGF-β1 stimulation increases active β-catenin concentration in cultured corneal fibroblasts through p38 MAPK regulation of canonical Wnt/β-catenin signaling, increasing α-SMA, COL1, and FN synthesis. Thus, PPARγ ligands, by blocking TGF-β1–induced p38 MAPK phosphorylation, prevent increases in both total and active β-catenin through p38 MAPK-GSK3β signaling.
Injury to the corneal surface causes the release of multiple wound healing modulators,1, 2 followed by apoptosis of keratocytes in the wound area.3 Surviving keratocytes are induced to proliferate, migrate, and transform into fibroblasts4 (and then into myofibroblasts)5 that occupy the stromal wound.6 Among the many regulators of keratocyte activity in such wounds, transforming growth factor β1 (TGF-β1) is probably the strongest known profibrotic agent.1, 7, 8 TGF-β binds to TGF-β receptors, which exert their cellular responses by activating both Smad and non-Smad pathways.9, 10 These pathways regulate multiple genes that are essential for the differentiation of fibroblasts into myofibroblasts, as well as for the actions of myofibroblasts within their immediate extracellular matrix environment.6, 7, 11, 12
Prior work in our laboratory showed peroxisome proliferator-activated receptor γ (PPARγ) ligands to have potent antifibrotic effects in corneal fibroblasts primarily by blocking phosphorylation of p38 mitogen-activated protein kinase (MAPK).13 However, the upstream and downstream components of p38 MAPK signaling mediating these antifibrotic effects remained undefined, representing an important knowledge gap. In human dermal fibroblasts, the β-catenin pathway is up-regulated by TGF-β1 through both p38 MAPK and Smad3, contributing to the formation of hypertrophic scars and keloids.14 Moreover, some of the TGF-β1–activated MAPKs, such as p3814 and protein kinase B (AKT),15 influence the activity of glycogen synthase kinase (GSK) 3α/β, a key β-catenin regulator.16, 17 GSK3α/β is a constitutively active, ubiquitously expressed serine/threonine kinase, whose inhibitory activity is regulated by serine phosphorylation (S21 at GSK3α/S9 at GSK3β) of its N-terminal region.18, 19, 20 Lithium chloride, best known for its therapeutic use as an antipsychotic agent,21 was also the first-described GSK3α/β inhibitor.22 In this context, it regulates canonical Wnt signaling through increased phosphorylation of GSK3α/β23, 24 and inhibits TGF-β1–induced myofibroblast differentiation.25, 26, 27, 28
β-Catenin is best known as an effector of Wnt,29 a large family of lipid-modified glycoproteins, which control diverse aspects of embryonic development and adult homeostasis.29 They exert this control through at least three known pathways, but the most canonical is the Wnt/β-catenin signaling pathway,30 which plays a detrimental role in congenital malformations, cancer, and osteoporosis,29 as well as in abnormal wound repair and fibrogenesis.31 Indeed, sustained activation of Wnt/β-catenin signaling is associated with a number of human fibroproliferative disorders in lung,32, 33 liver,34 skin,35, 36 and kidney.37, 38, 39 Information regarding Wnt/β-catenin signaling in the cornea is limited, but also suggestive of a potential role in fibrosis.40, 41 Thus, a reasonable question in the context of corneal wound healing and stromal fibrosis is whether the interaction between TGF-β1 and β-catenin signaling might be a key site mediating the antifibrotic effects of PPARγ ligands.
Generally, there are two pools of β-catenin in cells: one tightly linked to cadherins at cell-cell junctions (Figure 1) and another that exists in free form in the cytosol and nucleus, implicated in transcriptional regulation.42, 43 In the resting state, cytosolic/nuclear free β-catenin is maintained at low levels through rapid turnover via a multiprotein complex, consisting of GSK3α/β, adenomatous polyposis coli, Axin, and casein kinase 1. Casein kinase 1 and GSK3α/β sequentially phosphorylate β-catenin, causing it to be targeted for degradation (Figure 1). Alternatively, when the canonical Wnt/β-catenin pathway is activated by the appropriate Wnt ligands (Figure 1), this triggers a cascade of signaling events, which inhibit GSK3α/β kinase activity.43 As such, GSK3-dependent phosphorylation of β-catenin at Ser33/37/Thr41 is suppressed. Un-phosphorylated β-catenin becomes stable and accumulates in the cytoplasm in its active form. This cytoplasmic pool of active β-catenin can then translocate to the nucleus, where it functions as a transcriptional activator for subsets of genes, in a cell-context specific manner42 (Figure 1). With respect to corneal wound healing and fibrosis, we asked the following key questions: i) Does β-catenin mediate the TGF-β1–induced increase in expression of α-smooth muscle actin (α-SMA), fibronectin (FN), and collagen 1 (COL1) in corneal fibroblasts? ii) Does TGF-β1 regulate the expression of β-catenin in corneal fibroblasts; if so, is it via a canonical Wnt/β-catenin signaling pathway (ie, by controlling phosphorylation of GSK3β), and what role (if any) does p38 MAPK play in this? iii) Finally, do PPARγ ligands block TGF-β1–induced increases in total β-catenin expression in corneal fibroblasts? Answering these questions is important to properly understand the mechanisms of action of PPARγ ligands, which are critical as we begin to consider using these small molecules as antiscarring agents for the eye.
Figure 1.
Proposed mechanism of action of PPARγ ligands and p38 MAPK on transforming growth factor (TGF)-β1–induced β-catenin signaling in corneal fibroblasts. The left side of the panel illustrates a hypothetical resting state, where cytoplasmic levels of β-catenin are low because β-catenin exists primarily in its phosphorylated form. Phosphorylated (p) β-catenin is associated with a multiprotein complex that includes GSK3α/β, adenomatous polyposis coli (APC), Axin, and casein kinase (CK) 1, and is readily degraded via ubiquitin-mediated proteolysis. TGF-β1 stimulation of TGF-β receptor I/II (TGFRI/TGFRII) complexes and/or stimulation of Wnt receptors, such as Frizzle (Fz) and lipoprotein receptor–related proteins (LRPs), both activate the canonical Wnt/β-catenin signaling pathway, which appears to be a key regulator of α-smooth muscle actin (α-SMA), collagen 1 (COL1), and fibronectin (FN) synthesis. In the case of TGF-β1 stimulation, this appears to depend on phosphorylation of p38 MAPK, which induces phosphorylation of GSK3β, which prevents phosphorylation of β-catenin. This active (un-phosphorylated) β-catenin is able to travel into the nucleus and stimulate synthesis of profibrotic molecules (α-SMA, COL1, and FN). PPARγ ligands (red) inhibit TGF-β1–induced p38 MAPK activity, which blocks phosphorylation of GSK3β, decreasing levels of active (un-phosphorylated) β-catenin, and by consequence, blocking increased synthesis of α-SMA, COL1, and FN. cat, catenin.
Materials and Methods
Ethical Statement
All animal procedures were conducted according to the guidelines of the University of Rochester (Rochester, NY) Committee on Animal Research, the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Vision Research, and the NIH Guide for the Care and Use of Laboratory Animals.44
Isolation and Culture of Cat Corneal Fibroblasts
Primary feline corneal fibroblasts were generated as previously described.13 In brief, fresh corneas were obtained immediately post-mortem from young, adult, domestic short-hair cats (Felis catus). The epithelium and endothelium were scraped off, and the corneal stroma underwent double enzyme digestion. Isolated cells were grown in Fibroblast Growth Factor Medium (FGFM; PromoCell GmbH, Heidelberg, Germany) and refreshed every second day until they reached confluence. After passage 2, the medium was changed to Dulbecco's Modified Eagle's Medium (DMEM)/F12 (Cellgro, Manassas, VA) with 5% of mitogen-poor horse serum (HS; catalog number P5552; Sigma Aldrich, St. Louis, MO) instead of fetal bovine serum in an attempt to keep cells in a more quiescent state. Prior work had shown that cells cultured in HS retain their keratocyte morphological features longer, express keratocan and CD34, and secrete highly sulfated forms of keratin sulfate proteoglycan, another keratocyte marker.45, 46, 47, 48 However, all cells used in the present experiments were passaged six to seven times to generate sufficient numbers. After this many passages, isolated cells take on fibroblast and even myofibroblast phenotypes, likely because of a combination of factors, including the presence of latent TGF-β in culture serum48 and cell density.49 However, by culturing cells in 5% HS and maintaining cell density within a consistent range, we generated basic cultures that were relatively free of α-SMA and thus myofibroblasts. Finally, all baseline controls were treated with dimethyl sulfoxide (DMSO) unless otherwise stated, and all experiments were performed in triplicate.
Impact of PPARγ Ligands and the p38 Inhibitor on TGF-β1–Induced β-Catenin Expression
Passage 6 to 7 corneal fibroblasts were seeded at a density of 1 × 105 to 2 × 105 cells/6-cm dish containing DMEM/F12 plus 5% HS, which was then changed to DMEM/F12 plus 1% HS for 1 day to promote quiescence. The cells were treated with the PPARγ ligands rosiglitazone (Cayman, Ann Arbor, MI), troglitazone (Cayman), or 15-deoxy-Δ12,14-prostaglandin J2 (Enzo, Plymouth Meeting, PA), or with the p38 inhibitor SB203580 at previously determined optimal doses for blocking fibrosis in our culture system.13 After 30 minutes, 1 ng/mL recombinant human TGF-β1 (R&D Systems Inc., Minneapolis, MN) was added to the medium and incubated for 3 days. Polyclonal rabbit anti–β-catenin antibody (1:1000; Santa Cruz Biotechnology Inc., Dallas, TX) was used to detect the expression of β-catenin relative to that of β-tubulin (1:5000; Santa Cruz Biotechnology Inc.), our loading control. The expression of α-SMA (1:10,000; Thermo Fisher Scientific, Pittsburgh, PA) was used as an indicator of myofibroblast differentiation.
Effect of β-Catenin siRNA and a Wnt Inhibitor on TGF-β1–Induced Protein Expression
To evaluate the role of β-catenin in TGF-β1–induced corneal myofibroblast differentiation and the associated increased expression of α-SMA, COL1, and FN, we used β-catenin siRNA (catalog number J-003482-11-0020; GE Dharmacon, Lafayette, CO) and a Wnt inhibitor, pyrvinium pamoate50, 51 (Sigma Aldrich). Lipofectamine RNAiMAX (Invitrogen, Carlsbad, CA) reagent was used to perform siRNA transfections, as per the manufacturer's instructions. Briefly, 1 × 105 cat corneal fibroblasts/6-well plate were seeded in DMEM/F12 with 5% HS. After attachment, cells were changed into reduced-serum medium without antibiotics for 1 day, then transfected with β-catenin siRNA or control siRNA. Approximately 24 hours after transfection, cells were cultured with or without 1 ng/mL of TGF-β1 for 3 days. Nontargeting siRNA (catalog number D-001810-01-05; GE Dharmacon) was used as negative control. For the Wnt inhibitor experiments, corneal fibroblasts were seeded at a density of 5 × 104 cells/6-well plate, and changed to DMEM/F12 plus 1% HS for 1 day to promote quiescence. Cells were then treated with pyrvinium and after 30 minutes, recombinant human TGF-β1 was added to the medium and incubated for 3 days. Western blots were used to estimate the expression of β-catenin, α-SMA, COL1 (1:5000; a gift from Dr. Larry Fisher, NIH, Bethesda, MD), and FN (1:5000; Santa Cruz Biotechnology Inc.) relative to that of β-tubulin.
Effect of TGF-β1 on Phosphorylation of GSK3β
To verify that TGF-β1's impact on pGSK3β levels was critically dependent on its activation of the TGF-β receptor, we added SB431542 (Calbiochem, San Diego, CA), a potent TGF-β receptor blocker, to cultured feline fibroblasts. Different doses (1, 5, and 10 μmol/L) of the p38 inhibitor SB203580 (Calbiochem) were also added to the medium, and incubation was continued for 16 hours. A 10% gel was run using 20 to 30 μg of cell lysates separated by electrophoresis, transferred to a nitrocellulose membrane, and probed with primary antibodies against rabbit polyclonal anti–phospho-GSK3β (Ser9) (1:1000; Cell Signaling Technology Inc., Danvers, MA). The expression of GSK3β (1:1000; Cell Signaling Technology Inc.) was used as a loading control.
Effect of TGF-β1 and p38 Inhibitor on Cytoplasmic and Nuclear Levels of Active β-Catenin
Feline corneal fibroblasts were seeded at a density of 1.5 × 106 cells into 10-cm dishes. After 24 hours, they were pretreated with 10 μmol/L of the p38 inhibitor SB203580 (the optimal dose for achieving maximal GSK3β inactivation) for 30 minutes and then treated with and without TGF-β1 for 18 hours. After incubation, cells were washed with 1X Dulbecco Phosphate Buffered Saline (DPBS; Hyclone Laboratories, Logan, UT) containing 1 mmol/L sodium orthovanadate (Sigma Aldrich). Nuclear and cytoplasmic fractions were separated, as previously described.13 To detect the un-phosphorylated, active β-catenin, we used anti–non-phospho-β-catenin (Ser33/37/Thr41) antibody (1:500; Cell Signaling Technology, Inc.). Typical cross contamination between cytosolic and nuclear fractions was assayed with anti–β-tubulin and purified mouse anti-SC35 (BD Pharmingen, San Jose, CA), respectively.
Effect of Lithium Chloride on TGF-β1–Induced Myofibroblast Differentiation
To further investigate the critical role of GSK-3β in TGF-β1–induced myofibroblast differentiation, we measured the effects of lithium chloride, a GSK3β inhibitor,52, 53 on TGF-β1–induced increases in β-catenin, α-SMA, COL1, and FN. Corneal fibroblasts were seeded at a density of 5 × 104 cells/6-well plate containing DMEM/F12 plus 5% HS. Cells were transferred to DMEM/F12 plus 1% HS for 1 day to promote quiescence before being treated with 10 mmol/L lithium chloride (LiCl; Sigma Aldrich) for 30 minutes and being incubated with or without TGF-β1 for 3 days. Western blots were used to estimate the expression of total β-catenin, α-SMA, COL1, and FN relative to that of β-tubulin.
Statistical Analysis
Statistical significance was assessed for raw expression levels only, using a two-way analysis of variance, a one-way analysis of variance, or a two-tailed t-test, as appropriate. A probability of error of P < 0.05 was considered statistically significant. All statistical tests were performed using VassarStats (http://vassarstats.net, last accessed March 20, 2017). Because there were no differences between the baseline and DMSO/TGF-β1 conditions for β-catenin expression, we combined these densitometric results, so that the sample size was n = 9 for β-catenin expression and n = 6 for α-SMA, COL1, and FN expression levels.
Results
PPARγ Ligands and p38 Inhibitor Block TGF-β1–Induced Increases in β-Catenin Expression
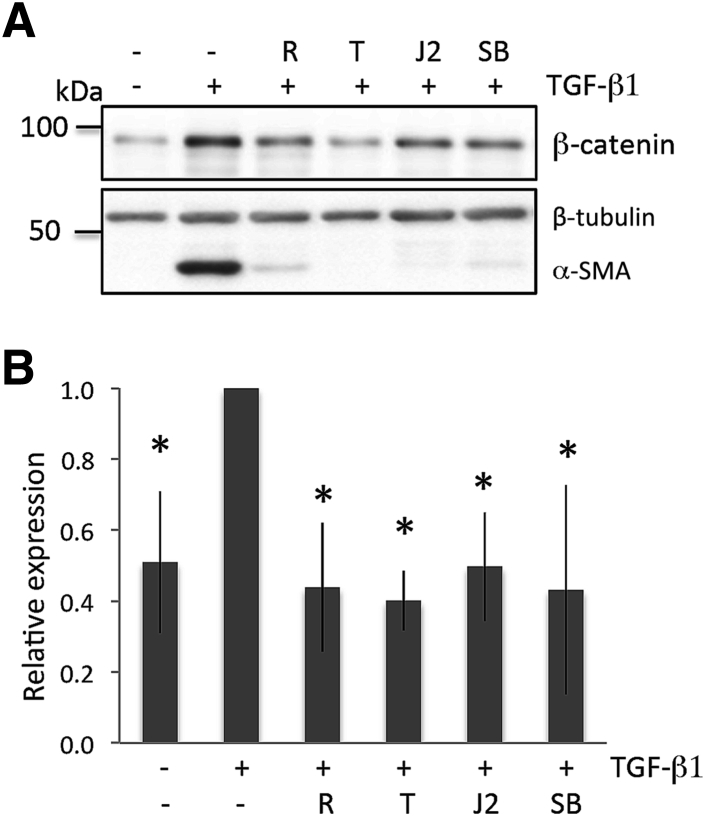
Basal levels of β-catenin were observed in un-stimulated, cultured corneal fibroblasts (Figure 2), suggesting that low levels of this molecule are constitutively synthesized. Addition of TGF-β1 at doses sufficient to stimulate expression of α-SMA increased levels of β-catenin (independent sample t-test: t16 = 3.06, P = 0.0075) (Figure 2). All three PPARγ ligands inhibited the TGF-β1–induced, increased expression of β-catenin at doses that blocked up-regulation of α-SMA (Figure 2). Resulting β-catenin levels were not significantly different from baseline [one-way analysis of variance: F(3,17) = 0.19, P = 0.9014]. Administration of 10 μmol/L SB203580 (a p38 MAPK inhibitor) also blocked TGF-β1–induced increases in β-catenin (together with α-SMA expression), with β-catenin levels remaining similar to baseline (t-test: t10 = 0.22, P = 0.830) (Figure 2). Total β-tubulin levels were unaffected by the addition of TGF-β1, PPARγ ligands, or the p38 inhibitor, and were used as loading controls.
Figure 2.
PPARγ ligands and p38 inhibitor block transforming growth factor (TGF)-β1–induced β-catenin expression in cultured cat corneal fibroblasts. A: Representative Western blots showing protein levels for β-catenin and α-smooth muscle actin (α-SMA) after TGF-β1 stimulation for 3 days. Basal levels of β-catenin were distinctly above zero. After 3 days in culture, TGF-β1 increases the expression of β-catenin and α-SMA. As previously reported for this cell culture system,13 75 μmol/L rosiglitazone (R), 15 μmol/L troglitazone (T), 5 μmol/L 15-deoxy-Δ12,14-prostaglandin J2 (J2), and 10 μmol/L p38 inhibitor (SB; SB203580) all block the expression of TGF-β1–induced β-catenin and α-SMA. Total β-tubulin levels are unaffected by the addition of TGF-β1, PPARγ ligands, or the p38 inhibitor, and are used as loading controls. Data shown are representative of three separate experiments. B: Plot of relative expression of β-catenin normalized to densitometric values obtained in cells treated with dimethyl sulfoxide (DMSO)/ng/mL TGF-β1. Data shown are averaged over three experiments, except for the baseline and DMSO/TGF-β1 stimulation conditions, which were averaged over nine experiments. TGF-β1 stimulation increases levels of β-catenin. Addition of the three PPARγ ligands (R, T, and J2) or of the p38 inhibitor (SB) inhibits TGF-β1's ability to up-regulate β-catenin expression, whose level remains at baseline. Data are expressed as means ± SD (B). ∗P < 0.05 versus the TGF-β1 only condition.
β-Catenin siRNA and Pyrvinium Block TGF-β1–Induced Protein Expression in Corneal Fibroblasts
Two approaches were used to test whether β-catenin mediates TGF-β1–induced increases in expression of α-SMA, COL1, and FN. First, corneal fibroblasts were transfected with β-catenin siRNA. In the absence of TGF-β1, basal levels of β-catenin were significantly decreased in these cells relative to control siRNA-transfected cells (independent sample t-test: t4 = 6, P = 0.0039) (Figure 3, A and B). In addition, β-catenin siRNA completely blocked TGF-β1–induced increases in β-catenin expression (Figure 3, A and B). More important, in β-catenin siRNA–transfected cells, TGF-β1 also failed to induce the usual increases in α-SMA, COL1, and FN expression (Figure 3, A and B); a two-way analysis of variance yielded a significant effect of transfection [control or β-catenin, F(1,23) = 175, P < 0.0001], a significant effect of molecule [β-catenin, α-SMA, COL1, and FN, F(3,23) = 6.22, P = 0.0053], but no significant interaction [F(3,23) = 2, P = 0.1546]. More important, control siRNA treatment did not impair the ability of TGF-β1 to increase the expression of α-SMA, COL1, and FN, all of which exhibited significantly higher levels of expression than without TGF-β1 stimulation [two-way analysis of variance: F(1,17) = 148, P < 0.0001] (Figure 3, A and B). This general phenomenon was verified using a pharmacological approach. Application of pyrvinium 30 minutes before TGF-β1 decreased the ability of the latter to up-regulate β-catenin, α-SMA, COL1, and FN in a dose-dependent manner (Figure 3, C and D). Under control conditions, TGF-β1 induced a nearly twofold increase in β-catenin levels and, as mentioned above, a significant change from baseline; after treatment with 100 nmol/L pyrvinium, however, β-catenin expression increased to only 1.2-fold baseline (Figure 3C) (independent t-test: t10 = 0.67, P = 0.518). Levels of α-SMA, FN, and COL1 were also elevated relative to baseline [two-way analysis of variance: F(1,26) = 28.74, P < 0.0001], but they remained 1.6-fold (FN and COL1) and 4.3-fold (α-SMA) lower than in the TGF-β1 stimulated (no pyrvinium) control condition (Figure 3D). Treatment with 50 mmol/L pyrvinium before TGF-β1 stimulation also trended toward decreased expression of all four molecules of interest compared to the DMSO/TGF-β1 condition, but this did not reach significance (Figure 3D).
Figure 3.
β-Catenin controls increased expression of α-smooth muscle actin (α-SMA), collagen 1 (COL1), and fibronectin (FN) after transforming growth factor (TGF)-β1 stimulation. A: Representative Western blot showing protein levels for β-catenin, α-SMA, COL1, and FN in cat corneal fibroblasts transfected with either β-catenin siRNA or control (nontargeting) siRNA. β-Tubulin levels were assayed as a loading control. β-Catenin siRNA (lanes 3 to 4) blocks TGF-β1–induced increases in β-catenin, α-SMA, COL1, and FN proteins compared with control siRNA (lanes 1 to 2). In addition, β-catenin siRNA decreases basal levels of β-catenin and COL1. β-Tubulin levels remain stable throughout. B: Plot of relative expression of β-catenin, α-SMA, FN, and COL1 in cells transfected with control siRNA or β-catenin siRNA. Values were normalized to those obtained in the control siRNA plus 1 ng/mL TGF-β1 condition. In the absence of TGF-β1, basal levels of β-catenin and COL1 significantly decrease in β-catenin-siRNA–transfected cells relative to control siRNA-transfected cells (β-catenin: two-tailed, independent sample t-test: t4 = 6, P = 0.0037; COL1: two-tailed, independent sample t-test: t4 = 4.33, P = 0.0124). Control siRNA treatment does not impair the ability of TGF-β1 to increase expression of α-SMA, COL1, and FN, all of which are more highly expressed than without TGF-β1 stimulation. C: Representative Western blot showing protein levels for β-catenin, α-SMA, COL1, and FN in cat corneal fibroblasts treated with dimethyl sulfoxide (DMSO; control) and 50 or 100 nmol/L pyrvinium for 3 days. β-Tubulin levels were assayed as a loading control. Pyrvinium (lanes 4 and 6) decreases TGF-β1–induced increases in β-catenin, α-SMA, COL1, and FN proteins compared with TGF-β1 alone (lane 2) in a dose-dependent manner. β-Tubulin levels remain stable throughout. D: Plot of relative expression of β-catenin, α-SMA, FN, and COL1 in cells treated with pyrvinium/TGF-β1 or DMSO/TGF-β1, normalized to values obtained after stimulation with DMSO plus 1 ng/mL TGF-β1. Data shown are representative of three separate experiments for the pyrvinium/TGF-β1 conditions, and six to nine experiments for the DMSO/TGF-β1 conditions. Neither dose of pyrvinium alters baseline levels of β-catenin, α-SMA, FN, and COL1. However, 100 nmol/L pyrvinium prevents TGF-β1 from significantly changing relative β-catenin expression from baseline. Although relative levels of α-SMA, FN, and COL1 do increase from baseline, they remain lower than in the TGF-β1–stimulated, control (no pyrvinium) condition. Data are expressed as means ± SD. ∗P < 0.05 versus control siRNA + TGF-β1; †P < 0.05 versus control siRNA baseline; ‡P < 0.05 versus DMSO/TGF-β1 condition; §P < 0.05 versus DMSO baseline.
TGF-β1 Stimulation Increases Levels of pGSK3β (Ser9) via TGF-β Receptor Activation, p38 MAPK
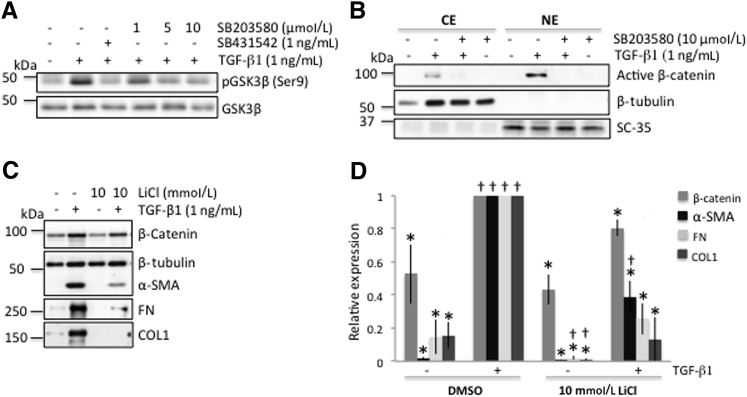
Next, we sought to determine whether TGF-β1 exerted its effects by regulating levels of active β-catenin in cat corneal fibroblasts. One way to achieve this is to inhibit GSK3α/β kinase activity via phosphorylation of Ser21 in GSK3α or Ser9 in GSK3β.43 This would suppress GSK3β-dependent β-catenin phosphorylation at Ser33/37/Thr41, and β-catenin would remain un-phosphorylated (ie, in its active form). Consistent with this hypothesis, addition of TGF-β1 to the culture medium induced an increase in levels of phosphorylated GSK3β (pGSK3β) at serine 9 relative to the untreated condition (Figure 4A). This increase in pGSK3β (Ser9) was completely blocked by addition of 1 ng SB431542, a TGF-β receptor inhibitor (Figure 4A), confirming that this effect was mediated through TGF-β receptor activation. The TGF-β1–induced increase in pGSK3β (Ser9) was also blocked in a dose-dependent manner by addition of SB203580, a p38 MAPK inhibitor (Figure 4A). Total GSK3β levels remained relatively constant throughout, and were used as a loading control.
Figure 4.
Transforming growth factor (TGF)-β1 stimulates canonical Wnt/β-catenin signaling via p38 MAPK in cat corneal fibroblasts. A: Representative Western blots showing that administration of 1 ng/mL TGF-β1 increases the phosphorylation of GSK3β (Ser9) (lane 2), which is blocked by addition of 1 ng TGF-β receptor inhibitor (lane 3). The p38 inhibitor (SB203580) blocks the effect of TGF-β1 on pGSK3β (Ser9) in a dose-dependent manner (lanes 4 to 6). Total GSK3β levels remained relatively stable throughout and were used as a loading control. B: Representative Western blots showing effect of p38 inhibitor on TGF-β1–induced levels of active (phosphorylated) β-catenin protein. The cultured fibroblasts were harvested and fractionated for isolation of the cytoplasmic extract (CE) and nuclear extract (NE). To check for cross contamination between cytoplasmic and nuclear fractions, anti–β-tubulin was used as a cytoplasmic marker and mouse anti-SC35 was used as a nuclear marker when running Western blots. C: Representative Western blot showing protein levels for β-catenin, α-smooth muscle actin (α-SMA), collagen 1 (COL1), and fibronectin (FN) in cat corneal fibroblasts treated with 10 mmol/L lithium chloride (LiCl) for 3 days. β-Tubulin levels were assayed as a loading control. The 10 mmol/L LiCl (lane 4) blocks TGF-β1–induced increases in β-catenin, α-SMA, COL1, and FN proteins compared with TGF-β1 alone (lane 2). β-Tubulin levels remain stable throughout. D: Plot of relative expression of β-catenin, α-SMA, COL1, and FN in cells treated with LiCl ± TGF-β1 or dimethyl sulfoxide (DMSO) ± TGF-β1. Data shown are representative of three separate experiments for the LiCl/TGF-β1 conditions, and six to nine experiments for the DMSO/TGF-β1 conditions. Values are expressed as a ratio to values obtained under the DMSO + TGF-β1 condition. Although LiCl does not change relative expression of β-catenin and α-SMA at baseline, after TGF-β1 stimulation, both proteins are up-regulated. However, neither reaches levels attained with DMSO/TGF-β1. With respect to FN and COL1, LiCl completely blocks TGF-β1's effects, so that overall, relative levels of these molecules remain similar to the DMSO baseline condition. Data are expressed as means ± SD (D). ∗P < 0.05 versus DMSO/TGF-β1 condition; †P < 0.05 versus DMSO baseline.
Inhibition of p38 MAPK Blocks TGF-β1–Induced Increases in Active β-Catenin
Levels of un-phosphorylated β-catenin were increased in both the cytoplasmic and nuclear fractions of cultured cat corneal fibroblasts after exposure to TGF-β1 (Figure 4B) compared with no TGF-β1 (Figure 4B). Because p38 MAPK activity appeared to regulate levels of pGSK3β (Figure 4A),16, 17 we asked whether it also affected levels of active β-catenin. Preincubation of cat corneal fibroblasts with 10 μmol/L SB203580 before adding TGF-β1 completely blocked any positive effects of TGF-β1 on active β-catenin levels in either the cytoplasmic or nuclear fractions (Figure 4B). In contrast, neither the vehicle (DMSO) (Figure 4B) nor the p38 inhibitor alone (Figure 4B) influenced levels of un-phosphorylated β-catenin, including its presence in the nucleus.
Inhibition of GSK3β Suppresses TGF-β1–Induced Increases in β-Catenin, α-SMA, COL1, and FN
To confirm the importance of GSK3β in profibrotic molecule expression under TGF-β1 stimulation, a general GSK-3β inhibitor, lithium chloride, was used. Application of 10 mmol/L LiCl 30 minutes before TGF-β1 treatment decreased the ability of the latter to up-regulate β-catenin, α-SMA, COL1, and FN (Figure 4, C and D). After treatment with 10 mmol/L LiCl + TGF-β1, β-catenin levels were not significantly different from baseline (unpaired two-tailed t-test: t7 = −0.22, P = 0.8321). In addition, after treatment with 10 mmol/L LiCl, TGF-β1 stimulation no longer elevated levels of FN and COL1 above baseline [two-way analysis of variance: F(1,17) = 0.29, P = 0.5987] (Figure 4D); levels of α-SMA were higher than baseline (unpaired t-test, unequal sample variances: t2.05 = −7.54, P = 0.0086), but remained 3.4-fold lower than in the DMSO + TGF-β1 condition.
Discussion
Injury to the cornea can trigger scar formation via fibrosis, which involves the differentiation of keratocytes into α-SMA–positive myofibroblasts. Myofibroblasts have increased synthesis of COL1 and FN, and lay down these extracellular matrix components in a disorganized manner, decreasing corneal transparency.54 TGF-β1 is perhaps the most important profibrotic growth factor to stimulate myofibroblast differentiation7 and increase synthesis of α-SMA, COL1, and FN.13 Recently, we reported that three different PPARγ ligands—two nonelectrophilic, synthetic compounds (troglitazone and rosiglitazone) and one electrophilic, natural PPARγ ligand (15-deoxy-Δ12,14-prostaglandin J2)—could act as effective corneal antifibrotics in vitro and in vivo.13, 55 Moreover, phosphorylation of p38 MAPK13 appeared critical to their antifibrotic actions. The present study identified components of p38 MAPK signaling mediating these antifibrotic effects, thus providing important mechanistic insights into this phenomenon. Multiple lines of evidence suggest that β-catenin signaling mediates fibrosis in different organ systems, offering new therapeutic possibilities for fibrotic diseases.31 However, to date, there are little data supporting a specific role for Wnt/β-catenin signaling in corneal fibrosis. Herein, we report that β-catenin is up-regulated by TGF-β1 stimulation in cat corneal fibroblasts and that it may be critical for TGF-β1's ability to increase expression of profibrotic molecules. To our knowledge, our siRNA experiments and the use of pyrvinium constitute the first critical evidence that β-catenin mediates increased production of α-SMA, COL1, and FN after TGF-β1 stimulation.
PPARγ is a ligand-activated nuclear hormone receptor that regulates genes important in cell differentiation and metabolism.56 An opposing interplay between PPARγ and canonical Wnt/β-catenin signaling has been described.57, 58 However, not all PPARγ ligands can exert this negative influence on β-catenin signaling. Indeed, among 15 synthetic PPARγ ligands examined, only troglitazone, rosiglitazone, pioglitazone, and a non-thiazolidinedione (GW1929) were able to inhibit β-catenin–mediated signaling,59 albeit via different targets.60 Our prior work showed that troglitazone, rosiglitazone, and 15-deoxy-Δ12,14-prostaglandin J2 effectively blocked the phosphorylation of p38 MAPK in corneal fibroblasts.13 Herein, we show that these three PPARγ ligands decrease total cellular levels of β-catenin to a similar extent. Together with the observation that β-catenin seemed necessary for the typical fibrotic phenotype induced by TGF-β1 stimulation in corneal fibroblasts, our next step was to determine whether TGF-β1 exerted its effects by activating the canonical Wnt/β-catenin signaling pathway. One way for TGF-β1 to do this is to phosphorylate GSK3α/β kinase, which then suppresses GSK3β-dependent β-catenin phosphorylation, causing the accumulation of un-phosphorylated (ie, active) β-catenin inside the cell (Figure 1). Active β-catenin is able to translocate to the nucleus, where it then stimulates gene transcription (eg, of α-SMA, COL1, and FN). Our data confirmed that in cat corneal fibroblasts, TGF-β1 activation via the TGF-β receptor led to increased nuclear levels of un-phosphorylated β-catenin. However, we also showed herein that this process depended on p38 MAPK activity, because blocking p38 MAPK with SB203580 prevented the TGF-β1–induced accumulation of active β-catenin in both the cytoplasm and nucleus. More important, we showed that p38 MAPK likely exerts this effect because it is critical for the TGF-β1–induced phosphorylation of the GSK3β kinase at Ser9. GSK3α/β is normally phosphorylated by growth factor–activated protein kinase B61 or p90 ribosomal S6 kinase62 at a conserved N-terminal serine (Ser21 in GSK3α and Ser9 in GSK3β). Recently, there have been suggestions of a potentially novel role for p38 MAPK in regulating GSK3β inactivation in other cell systems.16, 17, 63 Our data add to this body of evidence with respect to corneal fibroblasts. However, Thornton et al16 found p38 MAPK to also phosphorylate GSK3β at Thr390 (corresponding to Ser389 in the mouse), eventually inactivating GSK3β's kinase activity. However, phospho-GSK3β (Thr390) is mainly detected in the brain, thymocytes, and spleen cells.16 Despite several attempts (data not shown), we failed to detect it in corneal fibroblasts under our specific culture conditions. We also tried using lithium chloride to block GSK3β activity, and our results and other evidence27, 28, 64 suggest that it disrupts cross talk between signaling pathways regulated by TGF-β1 and GSK3β. Thus, although we cannot completely rule out the possibility that TGF-β1 activates other kinases, which partially inactivate GSK3β, it seems that p38 MAPK is the critical one controlling activity of GSK3β in TGF-β1–stimulated corneal fibroblasts.
All in all, our results lead us to propose that when PPARγ ligands block p38 MAPK, phosphorylation of GSK3β is decreased, increasing its activity, which reduces levels of active β-catenin in the cells (Figure 1), preventing them from adopting a scarring phenotype. These novel insights into the mechanisms of action of PPARγ ligands in corneal fibroblasts are directly relevant to their potential clinical use as topical antiscarring agents in the cornea. Moreover, the present study revealed an unexpectedly fundamental role of Wnt/β-catenin signaling in corneal fibrosis, potentially opening new avenues for controlling this detrimental consequence of ocular wounding.
Acknowledgments
We thank Dr. Larry W. Fisher (NIH, Bethesda, MD) for providing the anti–collagen 1 antibody and Dr. Keith W. Nehrke for constructive comments on the manuscript and experimental design.
Footnotes
Supported by NIH grants R01 EY015836 (K.R.H.), EY023239 (R.P.P.), and core grant P30 EY001319 to the Center for Visual Science and a Research to Prevent Blindness Foundation unrestricted grant to the Department of Ophthalmology at the University of Rochester.
Disclosures: None declared.
References
- 1.Vesaluoma M., Teppo A.M., Gronhagen-Riska C., Tervo T. Release of TGF-beta 1 and VEGF in tears following photorefractive keratectomy. Curr Eye Res. 1997;16:19–25. doi: 10.1076/ceyr.16.1.19.5119. [DOI] [PubMed] [Google Scholar]
- 2.Etheredge L., Kane B.P., Hassell J.R. The effect of growth factor signaling on keratocytes in vitro and its relationship to the phases of stromal wound repair. Invest Ophthalmol Vis Sci. 2009;50:3128–3136. doi: 10.1167/iovs.08-3077. [DOI] [PubMed] [Google Scholar]
- 3.Wilson S.E. Analysis of the keratocyte apoptosis, keratocyte proliferation, and myofibroblast transformation responses after photorefractive keratectomy and laser in situ keratomileusis. Trans Am Ophthalmol Soc. 2002;100:411–433. [PMC free article] [PubMed] [Google Scholar]
- 4.Fini M.E. Keratocyte and fibroblast phenotypes in the repairing cornea. Prog Retin Eye Res. 1999;18:529–551. doi: 10.1016/s1350-9462(98)00033-0. [DOI] [PubMed] [Google Scholar]
- 5.Jester J.V., Rodrigues M.M., Herman I.M. Characterization of avascular corneal wound healing fibroblasts: new insights into the myofibroblast. Am J Pathol. 1987;127:140–148. [PMC free article] [PubMed] [Google Scholar]
- 6.Jester J.V., Petroll W.M., Barry P.A., Cavanagh H.D. Expression of alpha-smooth muscle (alpha-SM) actin during corneal stromal wound healing. Invest Ophthalmol Vis Sci. 1995;36:809–819. [PubMed] [Google Scholar]
- 7.Desmouliere A., Geinoz A., Gabbiani F., Gabbiani G. Transforming growth factor-beta 1 induces alpha-smooth muscle actin expression in granulation tissue myofibroblasts and in quiescent and growing cultured fibroblasts. J Cell Biol. 1993;122:103–111. doi: 10.1083/jcb.122.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jester J.V., Barry-Lane P.A., Cavanagh H.D., Petroll W.M. Induction of alpha-smooth muscle actin expression and myofibroblast transformation in cultured corneal keratocytes. Cornea. 1996;15:505–516. [PubMed] [Google Scholar]
- 9.Tandon A., Tovey J.C., Sharma A., Gupta R., Mohan R.R. Role of transforming growth factor Beta in corneal function, biology and pathology. Curr Mol Med. 2010;10:565–578. doi: 10.2174/1566524011009060565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mu Y., Gudey S.K., Landstrom M. Non-Smad signaling pathways. Cell Tissue Res. 2012;347:11–20. doi: 10.1007/s00441-011-1201-y. [DOI] [PubMed] [Google Scholar]
- 11.Hassell J.R., Birk D.E. The molecular basis of corneal transparency. Exp Eye Res. 2010;91:326–335. doi: 10.1016/j.exer.2010.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wilson S.E. Corneal myofibroblast biology and pathobiology: generation, persistence, and transparency. Exp Eye Res. 2012;99:78–88. doi: 10.1016/j.exer.2012.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jeon K.I., Kulkarni A., Woeller C.F., Phipps R.P., Sime P.J., Hindman H.B., Huxlin K.R. Inhibitory effects of PPARgamma ligands on TGF-beta1-induced corneal myofibroblast transformation. Am J Pathol. 2014;184:1429–1445. doi: 10.1016/j.ajpath.2014.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sato M. Upregulation of the Wnt/beta-catenin pathway induced by transforming growth factor-beta in hypertrophic scars and keloids. Acta Derm Venereol. 2006;86:300–307. doi: 10.2340/00015555-0101. [DOI] [PubMed] [Google Scholar]
- 15.Bakin A.V., Tomlinson A.K., Bhowmick N.A., Moses H.L., Arteaga C.L. Phosphatidylinositol 3-kinase function is required for transforming growth factor beta-mediated epithelial to mesenchymal transition and cell migration. J Biol Chem. 2000;275:36803–36810. doi: 10.1074/jbc.M005912200. [DOI] [PubMed] [Google Scholar]
- 16.Thornton T.M., Pedraza-Alva G., Deng B., Wood C.D., Aronshtam A., Clements J.L., Sabio G., Davis R.J., Matthews D.E., Doble B., Rincon M. Phosphorylation by p38 MAPK as an alternative pathway for GSK3beta inactivation. Science. 2008;320:667–670. doi: 10.1126/science.1156037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bikkavilli R.K., Feigin M.E., Malbon C.C. p38 Mitogen-activated protein kinase regulates canonical Wnt-beta-catenin signaling by inactivation of GSK3beta. J Cell Sci. 2008;121:3598–3607. doi: 10.1242/jcs.032854. [DOI] [PubMed] [Google Scholar]
- 18.Sutherland C., Leighton I.A., Cohen P. Inactivation of glycogen synthase kinase-3 beta by phosphorylation: new kinase connections in insulin and growth-factor signalling. Biochem J. 1993;296:15–19. doi: 10.1042/bj2960015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stambolic V., Woodgett J.R. Mitogen inactivation of glycogen synthase kinase-3 beta in intact cells via serine 9 phosphorylation. Biochem J. 1994;303:701–704. doi: 10.1042/bj3030701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sutherland C., Cohen P. The alpha-isoform of glycogen synthase kinase-3 from rabbit skeletal muscle is inactivated by p70 S6 kinase or MAP kinase-activated protein kinase-1 in vitro. FEBS Lett. 1994;338:37–42. doi: 10.1016/0014-5793(94)80112-6. [DOI] [PubMed] [Google Scholar]
- 21.Alda M. Lithium in the treatment of bipolar disorder: pharmacology and pharmacogenetics. Mol Psychiatry. 2015;20:661–670. doi: 10.1038/mp.2015.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kramer T., Schmidt B., Lo Monte F. Small-molecule inhibitors of GSK-3: structural insights and their application to Alzheimer's disease models. Int J Alzheimers Dis. 2012;2012:381029. doi: 10.1155/2012/381029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang F., Phiel C.J., Spece L., Gurvich N., Klein P.S. Inhibitory phosphorylation of glycogen synthase kinase-3 (GSK-3) in response to lithium: evidence for autoregulation of GSK-3. J Biol Chem. 2003;278:33067–33077. doi: 10.1074/jbc.M212635200. [DOI] [PubMed] [Google Scholar]
- 24.Galli C., Piemontese M., Lumetti S., Manfredi E., Macaluso G.M., Passeri G. GSK3b-inhibitor lithium chloride enhances activation of Wnt canonical signaling and osteoblast differentiation on hydrophilic titanium surfaces. Clin Oral Implants Res. 2013;24:921–927. doi: 10.1111/j.1600-0501.2012.02488.x. [DOI] [PubMed] [Google Scholar]
- 25.Caraci F., Gili E., Calafiore M., Failla M., La Rosa C., Crimi N., Sortino M.A., Nicoletti F., Copani A., Vancheri C. TGF-beta1 targets the GSK-3beta/beta-catenin pathway via ERK activation in the transition of human lung fibroblasts into myofibroblasts. Pharmacol Res. 2008;57:274–282. doi: 10.1016/j.phrs.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 26.Chung E.J., Sohn Y.H., Kwon S.H., Jung S.A., Lee J.H. Lithium chloride inhibits TGF-beta1-induced myofibroblast transdifferentiation via PI3K/Akt pathway in cultured fibroblasts from Tenon's capsule of the human eye. Biotechnol Lett. 2014;36:1217–1224. doi: 10.1007/s10529-014-1487-4. [DOI] [PubMed] [Google Scholar]
- 27.Choi S.I., Kim B.Y., Dadakhujaev S., Jester J.V., Ryu H., Kim T.I., Kim E.K. Inhibition of TGFBIp expression by lithium: implications for TGFBI-linked corneal dystrophy therapy. Invest Ophthalmol Vis Sci. 2011;52:3293–3300. doi: 10.1167/iovs.10-6405. [DOI] [PubMed] [Google Scholar]
- 28.Michalik M., Wojcik K.A., Jakiela B., Szpak K., Pierzchalska M., Sanak M., Madeja Z., Czyz J. Lithium attenuates TGF-beta(1)-induced fibroblasts to myofibroblasts transition in bronchial fibroblasts derived from asthmatic patients. J Allergy (Cairo) 2012;2012:206109. doi: 10.1155/2012/206109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Clevers H., Nusse R. Wnt/beta-catenin signaling and disease. Cell. 2012;149:1192–1205. doi: 10.1016/j.cell.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 30.Chien A.J., Conrad W.H., Moon R.T. A Wnt survival guide: from flies to human disease. J Invest Dermatol. 2009;129:1614–1627. doi: 10.1038/jid.2008.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lam A.P., Gottardi C.J. β-catenin signaling: a novel mediator of fibrosis and potential therapeutic target. Curr Opin Rheumatol. 2011;23:562–567. doi: 10.1097/BOR.0b013e32834b3309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Konigshoff M., Balsara N., Pfaff E.M., Kramer M., Chrobak I., Seeger W., Eickelberg O. Functional Wnt signaling is increased in idiopathic pulmonary fibrosis. PLoS One. 2008;3:e2142. doi: 10.1371/journal.pone.0002142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lam A.P., Flozak A.S., Russell S., Wei J., Jain M., Mutlu G.M., Budinger G.R., Feghali-Bostwick C.A., Varga J., Gottardi C.J. Nuclear beta-catenin is increased in systemic sclerosis pulmonary fibrosis and promotes lung fibroblast migration and proliferation. Am J Respir Cell Mol Biol. 2011;45:915–922. doi: 10.1165/rcmb.2010-0113OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li W., Zhu C., Chen X., Li Y., Gao R., Wu Q. Pokeweed antiviral protein down-regulates Wnt/beta-catenin signalling to attenuate liver fibrogenesis in vitro and in vivo. Dig Liver Dis. 2011;43:559–566. doi: 10.1016/j.dld.2011.02.016. [DOI] [PubMed] [Google Scholar]
- 35.Bayle J., Fitch J., Jacobsen K., Kumar R., Lafyatis R., Lemaire R. Increased expression of Wnt2 and SFRP4 in Tsk mouse skin: role of Wnt signaling in altered dermal fibrillin deposition and systemic sclerosis. J Invest Dermatol. 2008;128:871–881. doi: 10.1038/sj.jid.5701101. [DOI] [PubMed] [Google Scholar]
- 36.Wei J., Melichian D., Komura K., Hinchcliff M., Lam A.P., Lafyatis R., Gottardi C.J., MacDougald O.A., Varga J. Canonical Wnt signaling induces skin fibrosis and subcutaneous lipoatrophy: a novel mouse model for scleroderma? Arthritis Rheum. 2011;63:1707–1717. doi: 10.1002/art.30312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.He W., Kang Y.S., Dai C., Liu Y. Blockade of Wnt/beta-catenin signaling by paricalcitol ameliorates proteinuria and kidney injury. J Am Soc Nephrol. 2011;22:90–103. doi: 10.1681/ASN.2009121236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Heikkila E., Juhila J., Lassila M., Messing M., Perala N., Lehtonen E., Lehtonen S., Sjef Verbeek J., Holthofer H. Beta-Catenin mediates adriamycin-induced albuminuria and podocyte injury in adult mouse kidneys. Nephrol Dial Transplant. 2010;25:2437–2446. doi: 10.1093/ndt/gfq076. [DOI] [PubMed] [Google Scholar]
- 39.von Toerne C., Bedke J., Safi S., Porubsky S., Gretz N., Loewe R., Nelson P.J., Grone H.J. Modulation of Wnt and Hedgehog signaling pathways is linked to retinoic acid-induced amelioration of chronic allograft dysfunction. Am J Transplant. 2012;12:55–68. doi: 10.1111/j.1600-6143.2011.03776.x. [DOI] [PubMed] [Google Scholar]
- 40.Zhang Y., Call M.K., Yeh L.K., Liu H., Kochel T., Wang I.J., Chu P.H., Taketo M.M., Jester J.V., Kao W.W., Liu C.Y. Aberrant expression of a beta-catenin gain-of-function mutant induces hyperplastic transformation in the mouse cornea. J Cell Sci. 2010;123:1285–1294. doi: 10.1242/jcs.063321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang L., Zhang M.C., Zhang Y., He Z., Zhang L., Xiao S.Y. Beta-Catenin expression in rat neovascularized cornea after alkali burn. Int J Ophthalmol. 2010;3:304–307. doi: 10.3980/j.issn.2222-3959.2010.04.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bowley E., O'Gorman D.B., Gan B.S. Beta-catenin signaling in fibroproliferative disease. J Surg Res. 2007;138:141–150. doi: 10.1016/j.jss.2006.07.026. [DOI] [PubMed] [Google Scholar]
- 43.Wu D., Pan W. GSK3: a multifaceted kinase in Wnt signaling. Trends Biochem Sci. 2010;35:161–168. doi: 10.1016/j.tibs.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Committee for the Update of the Guide for the Care and Use of Laboratory Animals; National Research Council . ed 8. National Academies Press; Washington, DC: 2011. Guide for the Care and Use of Laboratory Animals. [Google Scholar]
- 45.Funderburgh J.L., Funderburgh M.L., Mann M.M., Prakash S., Conrad G.W. Synthesis of corneal keratan sulfate proteoglycans by bovine keratocytes in vitro. J Biol Chem. 1996;271:31431–31436. doi: 10.1074/jbc.271.49.31431. [DOI] [PubMed] [Google Scholar]
- 46.Beales M.P., Funderburgh J.L., Jester J.V., Hassell J.R. Proteoglycan synthesis by bovine keratocytes and corneal fibroblasts: maintenance of the keratocyte phenotype in culture. Invest Ophthalmol Vis Sci. 1999;40:1658–1663. [PubMed] [Google Scholar]
- 47.Chen Y.H., Wang I.J., Young T.H. Formation of keratocyte spheroids on chitosan-coated surface can maintain keratocyte phenotypes. Tissue Eng Part A. 2009;15:2001–2013. doi: 10.1089/ten.tea.2008.0251. [DOI] [PubMed] [Google Scholar]
- 48.Oida T., Weiner H.L. Depletion of TGF-beta from fetal bovine serum. J Immunol Methods. 2010;362:195–198. doi: 10.1016/j.jim.2010.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Masur S.K., Dewal H.S., Dinh T.T., Erenburg I., Petridou S. Myofibroblasts differentiate from fibroblasts when plated at low density. Proc Natl Acad Sci U S A. 1996;93:4219–4223. doi: 10.1073/pnas.93.9.4219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Thorne C.A., Hanson A.J., Schneider J., Tahinci E., Orton D., Cselenyi C.S., Jernigan K.K., Meyers K.C., Hang B.I., Waterson A.G., Kim K., Melancon B., Ghidu V.P., Sulikowski G.A., LaFleur B., Salic A., Lee L.A., Miller D.M., 3rd, Lee E. Small-molecule inhibition of Wnt signaling through activation of casein kinase 1alpha. Nat Chem Biol. 2010;6:829–836. doi: 10.1038/nchembio.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Saraswati S., Alfaro M.P., Thorne C.A., Atkinson J., Lee E., Young P.P. Pyrvinium, a potent small molecule Wnt inhibitor, promotes wound repair and post-MI cardiac remodeling. PLoS One. 2010;5:e15521. doi: 10.1371/journal.pone.0015521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Klein P.S., Melton D.A. A molecular mechanism for the effect of lithium on development. Proc Natl Acad Sci U S A. 1996;93:8455–8459. doi: 10.1073/pnas.93.16.8455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ryves W.J., Harwood A.J. Lithium inhibits glycogen synthase kinase-3 by competition for magnesium. Biochem Biophys Res Commun. 2001;280:720–725. doi: 10.1006/bbrc.2000.4169. [DOI] [PubMed] [Google Scholar]
- 54.Torricelli A.A., Santhanam A., Wu J., Singh V., Wilson S.E. The corneal fibrosis response to epithelial-stromal injury. Exp Eye Res. 2016;142:110–118. doi: 10.1016/j.exer.2014.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Huxlin K.R., Hindman H.B., Jeon K.I., Buhren J., MacRae S., DeMagistris M., Ciufo D., Sime P.J., Phipps R.P. Topical rosiglitazone is an effective anti-scarring agent in the cornea. PLoS One. 2013;8:e70785. doi: 10.1371/journal.pone.0070785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Simpson-Haidaris P.J., Pollock S.J., Ramon S., Guo N., Woeller C.F., Feldon S.E., Phipps R.P. Anticancer role of PPARgamma agonists in hematological malignancies found in the vasculature, marrow, and eyes. PPAR Res. 2010;2010:814609. doi: 10.1155/2010/814609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Takada I., Kouzmenko A.P., Kato S. Wnt and PPARgamma signaling in osteoblastogenesis and adipogenesis. Nat Rev Rheumatol. 2009;5:442–447. doi: 10.1038/nrrheum.2009.137. [DOI] [PubMed] [Google Scholar]
- 58.Lecarpentier Y., Vallee A. Opposite interplay between PPAR gamma and canonical Wnt/beta-catenin pathway in amyotrophic lateral sclerosis. Front Neurol. 2016;7:100. doi: 10.3389/fneur.2016.00100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lu D., Carson D.A. Repression of beta-catenin signaling by PPAR gamma ligands. Eur J Pharmacol. 2010;636:198–202. doi: 10.1016/j.ejphar.2010.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kajabadi N.S., Ghoochani A., Peymani M., Ghaedi K., Kiani-Esfahani A., Hashemi M.S., Nasr-Esfahani M.H., Baharvand H. The synergistic enhancement of cloning efficiency in individualized human pluripotent stem cells by peroxisome proliferative-activated receptor-gamma (PPARgamma) activation and rho-associated kinase (ROCK) inhibition. J Biol Chem. 2015;290:26303–26313. doi: 10.1074/jbc.M114.624841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cross D.A., Alessi D.R., Cohen P., Andjelkovich M., Hemmings B.A. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature. 1995;378:785–789. doi: 10.1038/378785a0. [DOI] [PubMed] [Google Scholar]
- 62.Eldar-Finkelman H., Seger R., Vandenheede J.R., Krebs E.G. Inactivation of glycogen synthase kinase-3 by epidermal growth factor is mediated by mitogen-activated protein kinase/p90 ribosomal protein S6 kinase signaling pathway in NIH/3T3 cells. J Biol Chem. 1995;270:987–990. doi: 10.1074/jbc.270.3.987. [DOI] [PubMed] [Google Scholar]
- 63.Bikkavilli R.K., Malbon C.C. Mitogen-activated protein kinases and Wnt/beta-catenin signaling: molecular conversations among signaling pathways. Commun Integr Biol. 2009;2:46–49. doi: 10.4161/cib.2.1.7503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Liang M.H., Wendland J.R., Chuang D.M. Lithium inhibits Smad3/4 transactivation via increased CREB activity induced by enhanced PKA and AKT signaling. Mol Cell Neurosci. 2008;37:440–453. doi: 10.1016/j.mcn.2007.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]