Abstract
Background
Salmonella has significant public health implications causing food borne and zoonotic diseases in humans. Treatment of infections due to Salmonella is becoming difficult due to emergence of drug resistant strains. There is therefore need to characterize the circulating non-typhoidal Salmonella (NTS) serovars in domestic animals and animal products in Kenya as well as determine their antibiotic resistance profiles.
Methods
A total of 740 fecal samples were collected from cows (n = 150), pigs (n = 182), chicken (n = 191) and chicken eggs (n = 217) from various markets and abattoirs in Nairobi. The prevalence of NTS serovars using culture techniques and biochemical tests, antimicrobial sensitivity testing using disc diffusion method of the commonly prescribed antibiotics and phylogenetic relationships using 16S rRNA were determined.
Results
The results showed that the overall prevalence of Salmonella was 3.8, 3.6, 5.9 and 2.6% for pigs, chicken, eggs and cows respectively. Two serovars were isolated S. Typhimurium (85%) and S. Enteritidis (15%) and these two serovars formed distinct clades on the phylogenetic tree. Forty percent of the isolates were resistant to one or more antibiotics.
Conclusion
The isolation of Salmonella Typhimurium and Salmonella Enteritidis that are resistant to commonly used antibiotics from seemingly healthy animals and animal products poses a significant public health threat. This points to the need for regular surveillance to be carried out and the chain of transmission should be viewed to ascertain sources of contamination.
Keywords: Salmonella, Domestic animals, Phylogeny, Resistance
Background
Salmonella is a gram-negative enteric bacteria and is one of the major zoonotic foodborne pathogens worldwide [1]. Non typhoidal Salmonella (NTS) is responsible for about 93.8 million cases and causes approximately 155,000 deaths [2] as well as economic losses in the agricultural sector [3]. Non typhoidal serovars comprise of host generalists serovars such as S. Typhimurium and S. Enteritidis that induce a self-limiting gastroenteritis in a broad range of unrelated host species or can be adapted to a particular host such as S. Gallinarium in poultry and S. Dublin in cattle [4]. Non typhoidal salmonellosis has been associated with consumption of contaminated foods of animal origin, such as poultry, swine, dairy products [5] as well as person to person contact [6]. In developed countries, NTS in humans generally causes self-limiting gastroenteritis, in sub-Saharan Africa however NTS causes an invasive kind of infection that is common in infants, young children and adults. Salmonellosis also shares co-morbidities with tropical infectious diseases like malaria and Human Immunodeficiency Virus [7]. The invasive type is mainly caused by a variant ST313 that occurs exclusively in sub Saharan Africa [8]. The situation is further being complicated by emergence of drug resistant strains compromising the clinical treatment of the disease [9]. Drug resistance occurs as a result of the unmonitored use of antibiotics in farms for prophylaxis or as growth promoters (feed additives) [10, 11]. Multi drug resistance to commonly available drugs used as first line drugs which include chloramphenicol, trimethoprim/sulphamethoxazole and selected beta lactamases as well as fluoroquinolones is widespread in Kenya and Malawi [7]. The resistance observed among the invasive strains is conferred largely through a plasmid [11].
In Kenya, the prevalence of NTS serovars circulating in farms and animal products is unknown. [12]. Of the few studies done in Kenya, none has determined prevalence of Salmonella in eggs. The present study therefore sought to determine prevalence of Salmonella serovars circulating in cows, pigs, chicken and eggs across Kenya using molecular tools as well as determine the antibiotic resistant profiles of commonly available drugs to the Salmonella isolates namely; tetracycline, nitrofurantoin, nalidixic acid, streptomycin, sulphamethoxazole, cotrimaxazole, gentamycin and ampicillin.
Methods
Approximately 5 g of fresh fecal samples from cows (n = 150) and pigs (n = 182), chicken cloacal swabs (n = 191) and eggs (n = 217) were collected for this study from December 2013 to October 2014. Five grams of faeces from the cows and pigs were collected aseptically from the rectum immediately after the animals were slaughtered. Cloacal swabs were collected asceptically from chicken both in the slaughter houses and in the local markets in Nairobi County. The eggs were collected from the same markets that the chicken sampling was carried out and put in a sterile jar and transported to the lab for further processing.
Bovine fecal matter was collected at Nairobi’s Dagoretti slaughter house complex. Cattle slaughtered here originated from different parts of the country [13]. The pigs slaughtered at Nairobi’s Ndumbuini abattoir originate from Nairobi and Kiambu counties which are among the main pig farming counties in Kenya [14]. Grade and indigenous chicken samples as well as eggs were collected from various markets and slaughterhouses in Nairobi and its environs that receive chicken and eggs from all parts of the country. The sampling was done according to Daniel et al. [15]:
Where n = sample size, Z = Z statistic for a level of confidence, P = expected prevalence or proportion, d = precision
Using this formula the sample sizes was obtained: Pigs = 182 [16], Chicken- 191 [17], Eggs = 217 [18], Cattle = 148 [19].
Isolation and identification of Salmonella
In the laboratory, 1 g of the fecal matter from cows and pigs as well as cloacal swabs of chicken was inoculated in 10 ml of selenite broth (Oxoid, UK) and incubated at 37 °C for 24 h. After 24 h, a loopful of the enriched sample was plated on XLD agar plates (Oxoid, UK) and incubated at 37 °C for 24–48 h.
The eggs shells were cleaned thoroughly with soap and wiped with 70% ethanol. After air drying them, the eggs were cracked open using a pen knife and its contents thoroughly mixed after which 1 ml of the contents was cultured in 25 ml buffered peptone water (International Diagnostic Group, Lancashire, UK) and incubated at 37 °C for 24 h. An aliquot of 1 ml of the pre enriched sample was re-cultured in 10 ml of selenite F broth incubated 37 ° C for 24 h. After 24 h of incubation, a loopful of the selenite F broth culture was streaked on XLD agar plates and incubated at 37 °C for 24 to 48 h. The XLD plates were examined for the presence of Salmonella colonies. Positive samples were subsequently to biochemical tests using the API Biomerieux 20E strips, (Marcy-l’Etoile, France).
Extraction of genomic DNA
Pure colonies of Salmonella cultured in nutrient broth (Oxoid, UK) was used for DNA extraction using the QIAprep miniprep kit (Qiagen Valencia CA, USA) according to the manufacturers’ instructions.
Polymerase chain reaction (PCR)
Forward primer 16SF1 (5′-TGTTGTGGTTAATAACCGCA-3′) and reverse primer; 16SIII (5′-CACAAATCCATCTCTGGA-3′) of the 16S rRNA gene (Inqaba Biotech, South Africa) were used to amplify the 572 bp PCR product. Amplifications were carried in 50 μl reaction volumes containing; 25 μl of Dream Taq Master Mix (Thermoscientific, USA), 15 μl of nuclease free water, 2.5 μl of each primer and 2.5 μl of the extracted bacterial DNA. The amplifications were done in 35 cycles with an initial denaturation at 95 °C for 5 min, a denaturation step of 95 °C for 2 min, primer annealing at 55 °C for 30 s and primer extension at 72 ° C for 1 min. Finally, an additional extension was done for 10 min at 72 °C. The PCR products were visualized on 2% agarose gel with ethidium bromide.
Gel extraction
The PCR products obtained were extracted using QIAquick gel extraction kit (Qiagen, Valencia CA, USA). Thirty microliters of the purified DNA of each sample was sequenced (Macrogen, Netherlands).
Phylogenetic analysis
The 16S rRNA sequences obtained were compared with known 16S rRNA sequences at National Center for Biotechnology Information (NCBI) database using BLASTn (Basic Local Alignment Search Tool) algorithm obtained from; https://blast.ncbi.nlm.nih.gov/Blast.cgi. Identification of the sequences at both the genus and species level was defined as a 16S rRNA sequence similarity of ≥ 99% with that of the prototype strain sequence in GenBank. The sequences together with reference sequences derived from the Genbank were aligned using CLUSTAL W. The topology distance and probability of phylogenetic tree were determined using Mr. Bayes software. The topological robustness of the trees was evaluated by a bootstrap analysis involving 10,000 replications. The tree was then visualized using fig tree software v. 1.3.1.
Salmonella antimicrobial susceptibility tests
Antibiotic susceptibility testing was tested using the Kirby-Bauer disk diffusion method according to the Clinical and Laboratory Standards Institute (CLSI) guidelines. Briefly, pure colonies, bacterial suspension were placed in test tubes and their turbidity adjusted to 0.5McFarland turbidity standards. The diluted bacterial suspensions were then transferred onto Mueller-Hinton agar plates using a sterile cotton swab and seeded uniformly. Antibiotic impregnated discs were then placed to the plate surfaces using sterile forceps. The plates were incubated aerobically at 37 °C for 24 h and susceptible E. coli (ATCC 25922) was used as a control. A total of 8 selected antibiotic disks were used which contained the commonly used antibiotics namely; tetracycline (100 μg), nitrofurantoin (200 μg), nalidixic acid (30 μg), streptomycin (25 μg), sulphamethoxazole (200 μg), cotrimaxazole (25 μg), gentamycin (15 μg) and ampicillin (25 μg). Zones of inhibitions were measured to determine whether the bacteria were susceptible, intermediate or resistant in comparison to CLSI critical points.
Results
Salmonella prevalence and serotypes
Salmonella was isolated in 31 (4%) out of the total 740 samples collected and this comprised of 7 (3.8%) pigs faeces, 4 (2.6%) cattle faeces, 7 (3.6%) chicken cloacal swabs and 13 (5.9%) eggs. The PCR product obtained was 572bp (Fig 1). According to the NCBI blast, 2 serovars of Salmonella were identified: 18 out of 21 samples were identified to be S. Typhimurium (85.8%) while 3 out of 21 (14.2%) of the samples were identified to be S. Enteritidis. All the cattle and chicken positive samples contained S. Typhimurium, 25% (¼) of the positive pig samples and 16.7% (1/6) of the positive egg samples contained as S. Enteritidis while 75% (¾) as of the pig samples and 83.3% (5/6) of the egg samples were identified to be S. Typhimurium.
Fig. 1.
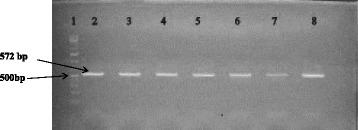
Agarose gel analysis of PCR (572 bp) of Salmonella isolates. Lane 1: 100 bp ladder; Lane 2: Positive control; Lanes 3, 4, 5, 8: S. Typhimurium; Lane 7: S. Enteritidis
Antibiotic resistance profiles of Salmonella isolates
Antimicrobial resistance was identified in 40% of Salmonella isolates whereas 20% were resistant to: sulphamethoxazole cotrimoxazole, streptomycin sulphamethoxazole and tetracycline sulphamaethoxazole. In addition, intermediate resistance was observed against nitrofurantoin (84%), ampicillin (76%), sulphamethoxazole (52%), streptomycin (40%), tetracycline (36%), gentamycin (28%), co-trimoxazole (20%) and nalidixic acid (12%) (Fig. 2).
Fig. 2.
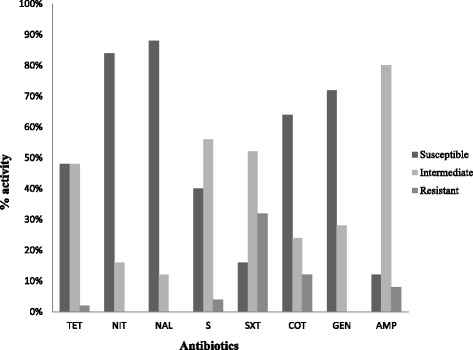
Percentage activity of Salmonella isolated from eggs and fecal matter of cows, pigs and chicken to various antibiotics. The activity was grouped as susceptible, intermediate or resistant to the following drugs: tetracycline (TET), nitrofurantoin (NIT), nalidixic acid (NAL), streptomycin (S), sulphamethoxazole (SXT), cotrimoxazole (CoT), gentamycin (GEN) and ampicillin (AMP)
Phylogenetic analysis
S. Typhimurium and S. Enteritidis were identified and formed two clades in the phylogenetic tree (Fig. 3). Escherichia coli was used to be the root the tree where S. Enteritidis clade showed a 68% probability from the majority S. Typhimurium clade. A 91% probability between the S. Choleraesuis and S. Paratyphi that were used as reference sequences in this analysis was observed. The branch length it appears that more variation has occurred in S. Typhimurium human isolate (S. Tm NR074800.1) than the S. Typhimurium field samples used in this study.
Fig. 3.
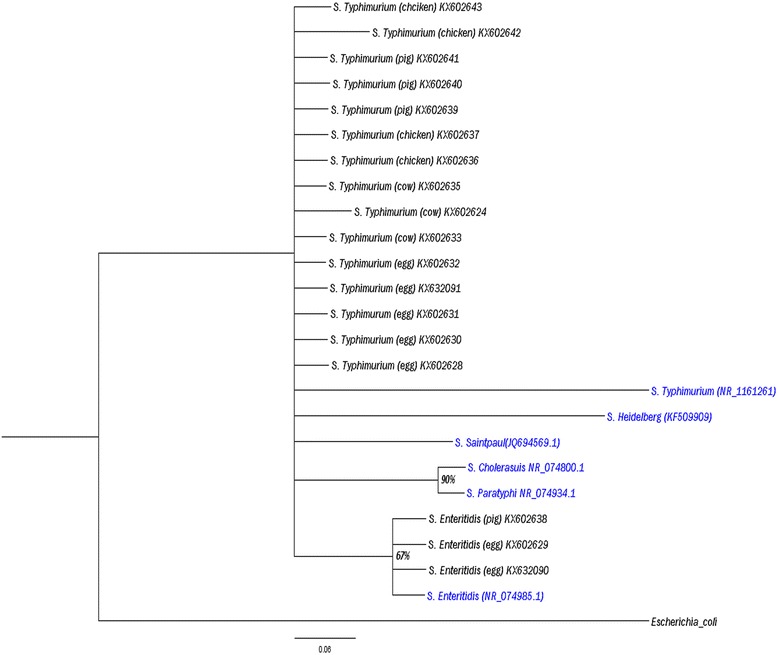
A phylogenetic tree based on 16S rRNA sequences of Salmonella isolates. The phylogeny was inferred by Bayesian method using the Markov Chain Monte Carlo (MCMC) method from an alignment performed using Bioedit. The Phylogenetic tree was visualized using Fig Tree v. 1.3.1. Numbers at the nodes show percentage of posterior probabilities indicating topological robustness
Discussion
The study aimed to determine the prevalence of Salmonella, the genetic relatedness of the serovars as well as determine their antibiotic resistance patterns. The study findings show that Salmonella was detected in the faecal matter of seemingly healthy animals and animal products meant for human consumption as well as in animal products. Before the animals are slaughtered a certified veterinarian inspects the health status of the animal by checking the skin fur, faecal matter, physical appearance and other clinical parameters. The prevalence of Salmonella was pigs (3.8%), cows (2.6%), chicken (3.6%) and eggs (5.9%) that are meant for human consumption. Out of the positive samples isolated, two serovars were detected: S. Enteritidis and S. Typhimurium. Resistance was detected in 40% of the samples.
The prevalence of Salmonella in eggs (5.9%) was higher in this study than in the Ethiopia study which established a prevalence of 4.69% [18]. The presence of Salmonella in eggs in Kenya therefore is a concern because several outbreaks have been attributed to consumption of contaminated eggs in other parts of the world [20]. Most food-borne S. Enteritidis infections are associated with the consumption of raw eggs and foods containing raw eggs such as homemade ice cream, mayonnaise and others egg products [18, 21]. There are two methods of contamination: on the outer shell and internally. Internal contamination can be as a result of the contamination through the eggshell or direct contamination of egg contents before oviposition [22]. The detection of Salmonella in eggs demonstrates that improvements need to be made in controlling Salmonella transmission in farms.
The prevalence of Salmonella in chicken in this study was 3.6%. Salmonella contamination rates for chicken reported in literature vary from 0.8 to 11% in Ethiopia [17, 23, 24] and Nigeria [25, 26]. The results of this study are comparable to results obtained in Tanzania [27]. The lower prevalence of 0.8% in Aragaw et al. [23] could be due to the fact that pre enrichment was not done. Pre enrichment helps to proliferate or regenerate cells thus increasing their viability when cultured on a solid medium [28]. The differences in prevalence could also be due to the geographical region, the type of chicken screened whether local indigenous or the exotic breeds. This study corroborates the work done in an Ethiopian study [17] where there was a higher prevalence of Salmonella in the indigenous chicken 71.4% compared to the grade chicken 28.6%. The levels of Salmonella in poultry can vary depending on the method of isolation, country, the nature of the production system and the specific control measures in place [12].
The prevalence of Salmonella in cows was lower in this study compared to studies done in Ethiopia [19]. This could be due to differences in environment, geographical distribution as well as husbandry practices. In the above studies a higher prevalence has been observed amongst dairy cattle compared to beef cattle [19].
The levels of Salmonella found in pigs in this study (3.8%) is comparable to those obtained by in Korea [29] but is much lower than previously reported in Kenya [16] and Burkina Faso [12]. The disparity in the prevalence for the Kenyan study could be due to better husbandry practices that have caused a reduction in the Salmonella prevalence in the pigs.
In this current study, two [2] serovars were identified: S. Typhimurium and S. Enteritidis. These two serovars are most commonly associated with food products and are the major causes of Salmonellosis in humans worldwide [30, 31]. The serovars identified in this study are contrary to the study done in Kenya where S. Heidelberg, S. Agona and S. Saintpaul were the most common isolated serovars in pigs [16]. In cows S. Typhimurium and Newport were the most isolated in Ethiopia [32] while in another Ethiopian study S. Anatum and S. Newport were the most commonly isolated [33]. In eggs S. Enteritidis was the isolated serovar in Ethiopia whereas in Australia S. Typhimurium is the most isolated serovar [30]. These results highlight the complexity of the global epidemiology of Salmonella as the frequency and occurrence of different serovars changes over time in countries and regions. Shifts in prevalence may follow introduction of the strain through animal feed and livestock trade [34].
Genotypic identification methods are emerging as an alternative or complement to established phenotypic identification procedures. For bacteria, 16S rRNA gene sequence analysis is a widely accepted tool for molecular identification [35]. From the phylogenetic tree (Fig. 2) the two serovars: S. Typhimurium and S. Enteritidis formed two distinct clades. From the branch length it appears that more variation has occurred in S. Typhimurium human isolate than the S. Typhimurium field samples used in this study.
Antibiotic resistance is the evolutionary response by bacteria to the strong selective pressure that results from exposure to antibiotics [36]. The Salmonella isolates in this study were susceptible to most of the easily accessible and cheaper drugs such as tetracycline while resistance was observed against sulphamethoxazole and cotrimoxazole. This could be an indicator of the acquisition of the resistance genes for those drugs due to the indiscriminate use of these 2 drugs at recommended doses or at sub therapeutic doses in feed additives to promote growth creating on farm selection of antimicrobial resistant strains [6]. Two of the isolates were resistant to sulphamethoxazole and not to cotrimoxazole which is a combination of sulphamethoxazole and trimethoprim (a folic acid analogue). Cotrimoxazole works by inhibiting 2 steps in the enzymatic pathway for bacterial folate synthesis. The isolates therefore seem to have not acquired the trimethoprim resistance, dhfr genes that encode altered dihydrofolate reductases that reduced affinity for the antimicrobial agent, allowing folic acid biosynthesis to occur in the presence of trimethoprim [37]. There was also a high percentage of isolates that were intermediately resistant to the panel of antibiotics tested. As compared to a previous study done by Kariuki et al. [21] where all the isolates from animals were susceptible to the commonly used drugs, our study findings could be indicative of increasing resistance towards the commonly used drugs and a cause of concern in the treatment of NTS. The detection of resistance in the samples in this study shows that there could be an indicator of the increased use of the antibiotics at sub-therapeutic levels or prophylactic doses which may promote on-farm selection of antimicrobial resistant strains.
Conclusion
The study showed that animal and animal products carry Salmonella. Isolation of Salmonella Typhimurium and Salmonella Enteritidis that exhibit resistance or intermediate sensitivity to commonly used antibiotics from seemingly healthy animals and eggs poses a significant public health threat because it is indicative that there is presence of zoonotic organisms that have the potential of entering the food-chain especially in Kenya. The emergence of antimicrobial resistant Salmonella strains is a problem and prudent use of antibiotics in animal husbandry and human therapy should be encouraged to help conserve the limited options of antibiotics available. Continual monitoring and surveillance to detect drug resistant Salmonella strains should be done and this should guide the administration of effective antibiotics accordingly.
Acknowledgments
We’d like to thank the National Council for Science and Technology (NACOSTI) for awarding us a grant to carry out this study. We’d also like to thank the personnel at the slaughterhouses as well as the poultry traders for their assistance.
Funding
This study was funded by the National Council for Science and Technology (NACOSTI) Kenya.
Availability of data and materials
The sequences used in this publication are available in GenBank.
Authors’ contributions
The concept and design of the study were performed by DN, AN, GJ, PK, JK. DN and RK participated in data collection. AN, GJ, PK, JK supervised the data analysis and interpretation. The manuscript was prepared by DN and critically revised by ON and AN. All authors approved and read the manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
Permission to conduct this study was issued by the Department of Veterinary Services, Nairobi and the various slaughterhouse authorities. The traders in the markets provided consent for cloacal swabs to be taken. Swabbing was carried out by a veterinary public health technician.
Abbreviations
- AMP
Ampicillin
- CoT
Cotrimoxazole
- GEN
Gentamycin
- NAL
Nalidixic acid
- NIT
Nitrofurantoin
- NTS
Non typhoidal Salmonella
- S
Streptomycin
- SXT
Sulphamethoxazole
- TET
Tetracycline
Contributor Information
Diana Nyabundi, Email: dnyabundi@yahoo.com.
Nyamongo Onkoba, Email: bwonkoba@gmail.com.
Rinter Kimathi, Email: reemykim@gmail.com.
Atunga Nyachieo, Email: anyachieo@yahoo.com.
Gerald Juma, Email: gjumaagengo@gmail.com.
Peter Kinyanjui, Email: pwkinyash@yahoo.com.
Joseph Kamau, Email: kamauvet@yahoo.com.
References
- 1.Card R, Vaughan K, Bagnall M, Spiropoulos J, Cooley W, Strickland T, Davies R, Anjum MF. Virulence characterisation of salmonella enterica isolates of differing antimicrobial resistance recovered from UK livestock and imported meat samples. Front Microbiol. 2016;7:1–11. doi: 10.3389/fmicb.2016.00640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Majowicz SE, Musto J, Scallan E, Angulo FJ, Kirk M, O’Brien SJ, Jones TF, Fazil A, Hoekstra RM. The global burden of nontyphoidal Salmonella gastroenteritis. Clin Infect Dis. 2010;50(6):882–9. doi: 10.1086/650733. [DOI] [PubMed] [Google Scholar]
- 3.Kemal J. A review on the public health importance of bovine salmonellosis. J Vet Sci Technol. 2014;5(2):175. doi: 10.4172/2157-7579.1000175. [DOI] [Google Scholar]
- 4.Uzzau S, Brown DJ, Wallis T, Rubino S, Leori G, Bernard S, Casadesus J, Olsen JE. Host adapted serotypes of Salmonella enterica. Epidemiol Infect. 2000;125(2):229–55. doi: 10.1017/S0950268899004379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Loongyai W, Promphet K, Kangsukul N, Noppha R, Egg A. Detection of Salmonella in Egg Shell and Egg Content from Different Housing Systems for Laying Hens. International Journal of Biological, Biomolecular, Agricultural, Food and Biotechnological Engineering. 2010; 4(5):232-4 .
- 6.Kariuki S, Revathi G, Kariuki N, Kiiru J, Mwituria J, Muyodi J, Hart CA. Invasive multidrug-resistant non-typhoidal Salmonella infections in Africa: Zoonotic or anthroponotic transmission? J Med Microbiol. 2006;55(5):585–91. doi: 10.1099/jmm.0.46375-0. [DOI] [PubMed] [Google Scholar]
- 7.Kariuki S, Dougan G. Antibacterial resistance in sub-Saharan Africa: an underestimated emergency. Ann N Y Acad Sci. 2014;1323(1):43–55. doi: 10.1111/nyas.12380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kingsley RA, Msefula CL, Thomson NR, Kariuki S, Holt KE, Gordon MA, Harris D, Clarke L, Whitehead S, Sangal V, Marsh K, Achtman M, Molyneux ME, Cormican M, Parkhill J, MacLennan CA, Heydermann RS, Dougan G. Epidemic multiple drug resistant Salmonella Typhimurium causing invasive disease in sub-Saharan Africa have a distinct genotype Epidemic multiple drug resistant Salmonella Typhimurium causing invasive disease in sub-Saharan Africa have a distinct genotype. Genome Res. 2009;19(12):2279–87. [DOI] [PMC free article] [PubMed]
- 9.Gordon MA, Graham SM, Walsh AL, Wilson L, Phiri A, Molyneux E, Ziljistra EE, Heyderman RS, Molyneux ME. Epidemics of invasive Salmonella enterica serovar enteritidis and S. enterica Serovar typhimurium infection associated with multidrug resistance among adults and children in Malawi. Clin Infect Dis. 2008;46(7):963–9. doi: 10.1086/529146. [DOI] [PubMed] [Google Scholar]
- 10.Forshell LP, Wierup M. Salmonella contamination: a significant challenge to the global marketing of animal food products. Rev Sci Tech. 2006;25(2):541–54. [PubMed] [Google Scholar]
- 11.Threlfall EJ. Antimicrobial drug resistance in Salmonella: problems and perspectives in food- and water-borne infections. (Special issue: Pathogenic microbes in water and food) FEMS Microbiol Rev. 2002;26(2):141–8. doi: 10.1111/j.1574-6976.2002.tb00606.x. [DOI] [PubMed] [Google Scholar]
- 12.Kagambèga A, Lienemann T, Aulu L, Traoré AS, Barro N, Siitonen A, Haukka K. Prevalence and characterization of Salmonella enterica from the feces of cattle, poultry, swine and hedgehogs in Burkina Faso and their comparison to human Salmonella isolates. BMC Microbiol. 2013;13(1):253. doi: 10.1186/1471-2180-13-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kithuka JM, Maingi N, Njeruh FM, Ombui JN. The prevalence and economic importance of bovine fasciolosis in Kenya--an analysis of abattoir data. Onderstepoort J Vet Res. 2002;69(4):255–62. [PubMed] [Google Scholar]
- 14.Kikuvi GM, Ombui JN, Mitema ES, Schwarz S, Kikuvi Antimicrobial Resistance in Salmonella serotypes isolated from slaughter animals in Kenya. East Afr Med J. 2007;84(5):233–9. doi: 10.4314/eamj.v84i5.9531. [DOI] [PubMed] [Google Scholar]
- 15.Daniel WW. Biostatistics: a foundation for analysis in the health sciences. 7. New York: John Wiley & Sons; 1999. [Google Scholar]
- 16.Kikuvi GM, Ombui JN, Mitema ES. Serotypes and antimicrobial resistance profiles of Salmonella isolates from pigs at slaughter in Kenya. J Infect Dev Ctries. 2010;4(4):243–8. doi: 10.3855/jidc.446. [DOI] [PubMed] [Google Scholar]
- 17.Endris M, Taddesse F, Geloye M, Degefa T, Jibat T. Sero and media culture prevalence of Salmonellosis in local and exotic chicken, Debre Zeit, Ethiopia. African J Microbiol Res. 2013;7(12):1041–4. [Google Scholar]
- 18.Bayu Z, Asrade B, Kebede N, Sisay Z, Bayu Y. Identification and characterization of Salmonella species in whole egg purchased from local markets in Addis Ababa, Ethiopia. J Vet Med Anim Heal. 2013;5(5):133–7. [Google Scholar]
- 19.Addis Z, Kebede N, Worku Z, Gezahegn H, Yirsaw A, Kassa T. Prevalence and antimicrobial resistance of Salmonella isolated from lactating cows and in contact humans in dairy farms of Addis Ababa: a cross sectional study. BMC Infect Dis. 2011;11(1):222. doi: 10.1186/1471-2334-11-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Andino A, Hanning I. Salmonella enterica : survival, colonization, and virulence differences among serovars. Sci World J. 2015;2015(Table 3):1–16. doi: 10.1155/2015/520179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kariuki S, Revathi G, Gakuya F, Yamo V, Muyodi J, Hart CA. Lack of clonal relationship between non-typhi Salmonella strain types from humans and those isolated from animals living in close contact. FEMS Immunol Med Microbiol. 2002;33:165–71. doi: 10.1111/j.1574-695X.2002.tb00587.x. [DOI] [PubMed] [Google Scholar]
- 22.Gantois I, Ducatelle R, Pasmans F, Haesebrouck F, Gast R, Humphrey TJ, Van Immerseel F. Mechanisms of egg contamination by Salmonella Enteritidis: review article. FEMS Microbiol Rev. 2009;33(4):718–38. doi: 10.1111/j.1574-6976.2008.00161.x. [DOI] [PubMed] [Google Scholar]
- 23.Aragaw K, Terefe L, Abera M. Prevalence of Salmonella infection in intensive poultry farms in Hawassa and isolation of Salmonella species from sick and dead chickens. Ethiop Vet J. 2010;14(2):115–24. [Google Scholar]
- 24.Menghistu HT, Rathore R, Dhama K, Agarwal RK. Isolation, identification and polymerase chain reaction (PCR) detection of salmonella species from field materials of poultry origin. Intl J Microbiol Res. 2011;2(2):135–42. [Google Scholar]
- 25.Fashae K, Ogunsola F, Aarestrup FM, Hendriksen RS. Antimicrobial susceptibility and serovars of Salmonella from chickens and humans in Ibadan, Nigeria. J Infect Dev Ctries. 2010;4(08):484–94. doi: 10.3855/jidc.909. [DOI] [PubMed] [Google Scholar]
- 26.Raufu I, Hendriksen RS, Ameh JA, Aarestrup FM. Occurrence and characterization of Salmonella Hiduddify from chickens and poultry meat in Nigeria. Foodborne Pathog Dis. 2009;6(4):425–30. doi: 10.1089/fpd.2008.0150. [DOI] [PubMed] [Google Scholar]
- 27.Mdegela RH, Yongolo MG, Minga UM, Olsen JE. Molecular epidemiology of Salmonella gallinarum in chickens in Tanzania. Avian Pathol. 2000;29(5):457–63. doi: 10.1080/030794500750047216. [DOI] [PubMed] [Google Scholar]
- 28.Zadernowska A, Chajecka W. Detection of Salmonella spp.Presence in Food. Salmonella -A Danger Foodborne Pathog [Internet]. 2012;21.
- 29.Kim ES, Hooper DC. Clinical importance and epidemiology of quinolone resistance. Infect Chemother. 2014;46(4):226–38. doi: 10.3947/ic.2014.46.4.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Whiley H, Ross K. Salmonella and eggs: from production to plate. Int J Environ Res Public Health. 2015;12(3):2543–56. doi: 10.3390/ijerph120302543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.De Medici D, Croci L, Delibato E, Di Pasquale S, Filetici E, Toti L. Evaluation of DNA extraction methods for use in combination with SYBR green I real-time PCR to detect Salmonella enterica serotype enteritidis in poultry. Appl Environ Microbiol. 2003;69(6):3456–61. doi: 10.1128/AEM.69.6.3456-3461.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alemu S, Zewde BM. Prevalence and antimicrobial resistance profiles of Salmonella enterica serovars isolated from slaughtered cattle in Bahir Dar, Ethiopia. Trop Anim Health Prod. 2012;44(3):595–600. doi: 10.1007/s11250-011-9941-y. [DOI] [PubMed] [Google Scholar]
- 33.Sibhat B, Molla ZB, Zerihun A, Muckle A, Cole L, Boerlin P, Wilkie E, Perets A, Mistry K, Gebreyes WA. Salmonella serovars and antimicrobial resistance profiles in beef cattle, slaughterhouse personnel and slaughterhouse environment in Ethiopia. Zoonoses Public Health. 2011;58(2):102–9. doi: 10.1111/j.1863-2378.2009.01305.x. [DOI] [PubMed] [Google Scholar]
- 34.Hendriksen RS, Vieira AR, Karlsmose S, Lo Fo Wong DM, Jensen AB, Wegener HC, Aarestrup FM. Global monitoring of salmonella serovar distribution from the world health organization global foodborne infections network country data bank: results of quality assured laboratories from 2001 to 2007. Foodborne Pathog Dis. 2011;8(8):887–900. doi: 10.1089/fpd.2010.0787. [DOI] [PubMed] [Google Scholar]
- 35.Bosshard PP, Zbinden R, Abels S, Böddinghaus B, Altwegg M, Böttger EC. 16S rRNA gene sequencing versus the API 20 NE system and the VITEK 2 ID-GNB card for identification of nonfermenting Gram-negative bacteria in the clinical laboratory. J Clin Microbiol. 2006;44(4):1359–66. doi: 10.1128/JCM.44.4.1359-1366.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wright GD. Q&A: antibiotic resistance: where does it come from and what can we do about it? BMC Biol. 2010;8:123. doi: 10.1186/1741-7007-8-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Foley SL, Lynne AM. Food animal-associated salmonella challenges: pathogenicity and antimicrobial resistance. J Anim Sci. 2007;86(No 14, Sup 2008):E173–87. doi: 10.2527/jas.2007-0447. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The sequences used in this publication are available in GenBank.


