Abstract
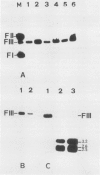
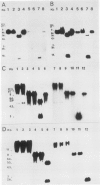
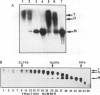
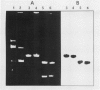
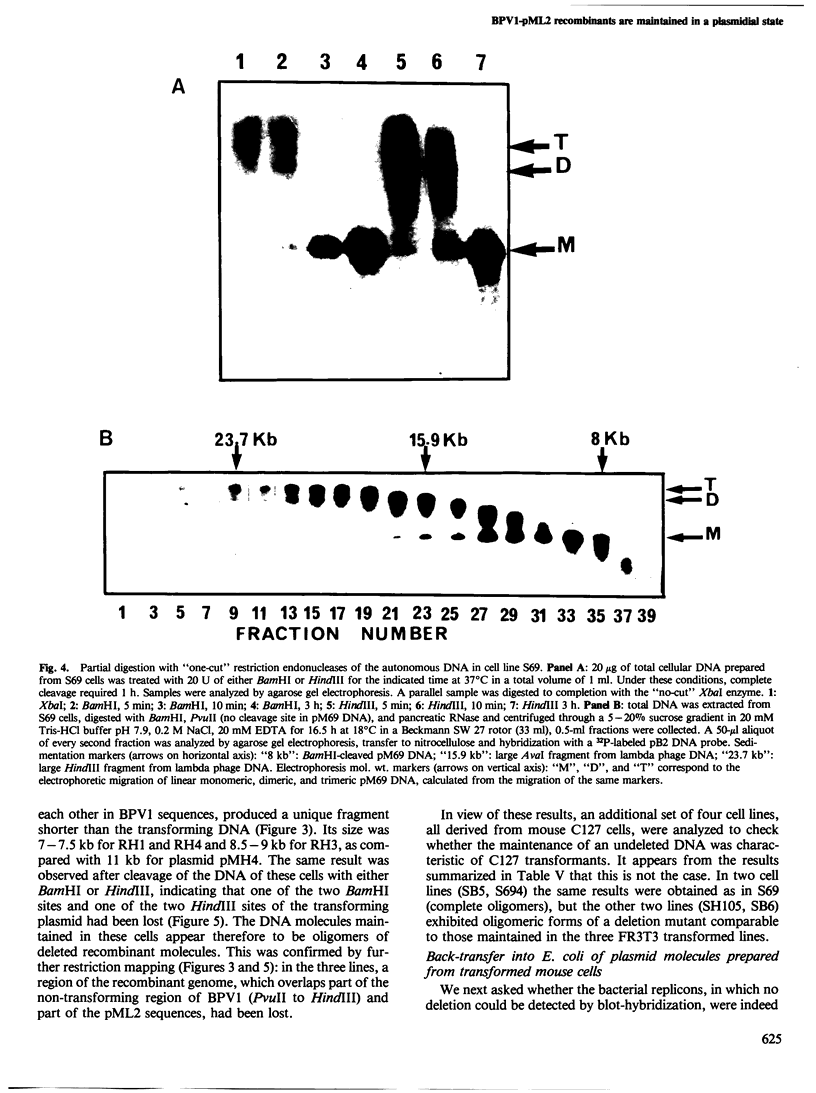
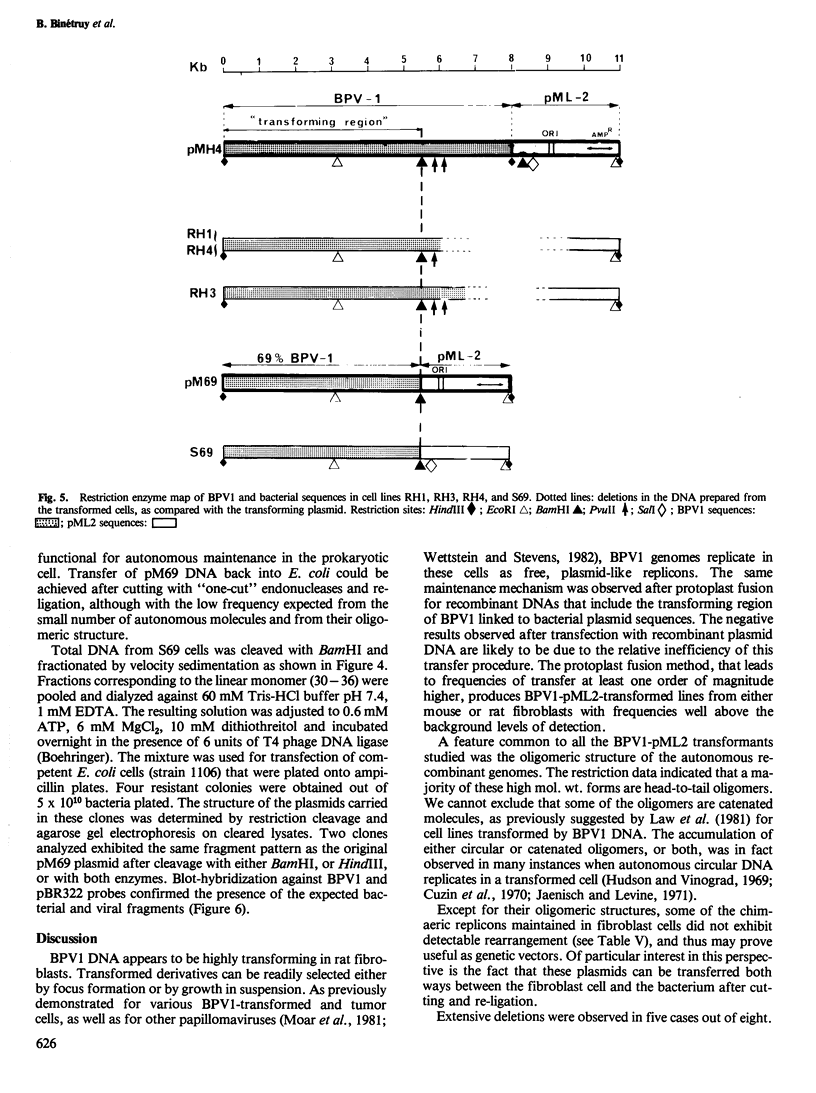
Transformed cells obtained after transfecting FR3T3 rat fibroblasts with DNA of bovine papilloma virus type 1 ( BPV1 ) maintained only free copies of the viral genome. Transfection with BPV1 DNA inserted in a bacterial plasmid (pBR322 or pML2 ) did not produce transformants at a detectable rate, unless the viral sequences had been first excised from the plasmid. In contrast, transfer of the same plasmids by polyethylene glycol-induced fusion of bacterial protoplasts with FR3T3 rat or C127 mouse cells led to significant transformation frequencies. A total of eight cell lines were studied, three rat and five mouse transformants, obtained with various BPV1 - pML2 recombinants. In all cell lines, both BPV1 and plasmid sequences were maintained as non-integrated molecules, predominantly as oligomeric forms of the transforming DNA. In the three rat transformants and in two of the mouse lines, parts of the non-transforming viral region and some bacterial sequences were deleted. In the remaining three mouse lines, the monomeric repeat was a non-rearranged plasmid molecule which could be re-established as a plasmid in Escherichia coli after cleavage with "one-cut" restriction endonucleases and circularization of the molecule.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amtmann E., Müller H., Sauer G. Equine connective tissue tumors contain unintegrated bovine papilloma virus DNA. J Virol. 1980 Sep;35(3):962–964. doi: 10.1128/jvi.35.3.962-964.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colbère-Garapin F., Horodniceanu F., Kourilsky P., Garapin A. C. A new dominant hybrid selective marker for higher eukaryotic cells. J Mol Biol. 1981 Jul 25;150(1):1–14. doi: 10.1016/0022-2836(81)90321-1. [DOI] [PubMed] [Google Scholar]
- Cuzin F., Vogt M., Dieckmann M., Berg P. Induction of virus multiplication in 3T3 cells transformed by a thermosensitive mutant of polyoma virus. II. Formation of oligometric polyoma DNA molecules. J Mol Biol. 1970 Feb 14;47(3):317–333. doi: 10.1016/0022-2836(70)90305-0. [DOI] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. Transformation of rat cells by DNA of human adenovirus 5. Virology. 1973 Aug;54(2):536–539. doi: 10.1016/0042-6822(73)90163-3. [DOI] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Hudson B., Vinograd J. Sedimentation velocity properties of complex mitochondrial DNA. Nature. 1969 Jan 25;221(5178):332–337. doi: 10.1038/221332a0. [DOI] [PubMed] [Google Scholar]
- Jaenisch R., Levine A. DNA replication in SV40-infected cells. V. Circular and catenated oligomers of SV40 DNA. Virology. 1971 Jun;44(3):480–493. doi: 10.1016/0042-6822(71)90361-8. [DOI] [PubMed] [Google Scholar]
- Lancaster W. D. Apparent lack of integration of bovine papillomavirus DNA in virus-induced equine and bovine tumor cells and virus-transformed mouse cells. Virology. 1981 Jan 30;108(2):251–255. doi: 10.1016/0042-6822(81)90433-5. [DOI] [PubMed] [Google Scholar]
- Lancaster W. D., Olson C. Demonstration of two distinct classes of bovine papilloma virus. Virology. 1978 Sep;89(2):372–379. doi: 10.1016/0042-6822(78)90179-4. [DOI] [PubMed] [Google Scholar]
- Law M. F., Lowy D. R., Dvoretzky I., Howley P. M. Mouse cells transformed by bovine papillomavirus contain only extrachromosomal viral DNA sequences. Proc Natl Acad Sci U S A. 1981 May;78(5):2727–2731. doi: 10.1073/pnas.78.5.2727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowy D. R., Dvoretzky I., Shober R., Law M. F., Engel L., Howley P. M. In vitro tumorigenic transformation by a defined sub-genomic fragment of bovine papilloma virus DNA. Nature. 1980 Sep 4;287(5777):72–74. doi: 10.1038/287072a0. [DOI] [PubMed] [Google Scholar]
- Lowy D. R., Rands E., Scolnick E. M. Helper-independent transformation by unintegrated Harvey sarcoma virus DNA. J Virol. 1978 May;26(2):291–298. doi: 10.1128/jvi.26.2.291-298.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lusky M., Botchan M. Inhibition of SV40 replication in simian cells by specific pBR322 DNA sequences. Nature. 1981 Sep 3;293(5827):79–81. doi: 10.1038/293079a0. [DOI] [PubMed] [Google Scholar]
- Meneguzzi G., Chenciner N., Corallini A., Grossi M. P., Barbanti-Brodano G., Milanesi G. The arrangement of integrated viral DNA is different in BK virus-transformed mouse and hamster cells. Virology. 1981 May;111(1):139–153. doi: 10.1016/0042-6822(81)90660-7. [DOI] [PubMed] [Google Scholar]
- Moar M. H., Campo M. S., Laird H., Jarrett W. F. Persistence of non-integrated viral DNA in bovine cells transformed in vitro by bovine papillomavirus type 2. Nature. 1981 Oct 29;293(5835):749–751. doi: 10.1038/293749a0. [DOI] [PubMed] [Google Scholar]
- Mougneau E., Birg F., Rassoulzadegan M., Cuzin F. Integration sites and sequence arrangement of SV40 DNA in a homogeneous series of transformed rat fibroblast lines. Cell. 1980 Dec;22(3):917–927. doi: 10.1016/0092-8674(80)90569-3. [DOI] [PubMed] [Google Scholar]
- Mulligan R. C., Berg P. Selection for animal cells that express the Escherichia coli gene coding for xanthine-guanine phosphoribosyltransferase. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2072–2076. doi: 10.1073/pnas.78.4.2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Hare K., Benoist C., Breathnach R. Transformation of mouse fibroblasts to methotrexate resistance by a recombinant plasmid expressing a prokaryotic dihydrofolate reductase. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1527–1531. doi: 10.1073/pnas.78.3.1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rassoulzadegan M., Binetruy B., Cuzin F. High frequency of gene transfer after fusion between bacteria and eukaryotic cells. Nature. 1982 Jan 21;295(5846):257–259. doi: 10.1038/295257a0. [DOI] [PubMed] [Google Scholar]
- Sarver N., Gruss P., Law M. F., Khoury G., Howley P. M. Bovine papilloma virus deoxyribonucleic acid: a novel eucaryotic cloning vector. Mol Cell Biol. 1981 Jun;1(6):486–496. doi: 10.1128/mcb.1.6.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffner W. Direct transfer of cloned genes from bacteria to mammalian cells. Proc Natl Acad Sci U S A. 1980 Apr;77(4):2163–2167. doi: 10.1073/pnas.77.4.2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seif R., Cuzin F. Temperature-sensitive growth regulation in one type of transformed rat cells induced by the tsa mutant of polyoma virus. J Virol. 1977 Dec;24(3):721–728. doi: 10.1128/jvi.24.3.721-728.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wettstein F. O., Stevens J. G. Variable-sized free episomes of Shope papilloma virus DNA are present in all non-virus-producing neoplasms and integrated episomes are detected in some. Proc Natl Acad Sci U S A. 1982 Feb;79(3):790–794. doi: 10.1073/pnas.79.3.790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigler M., Sweet R., Sim G. K., Wold B., Pellicer A., Lacy E., Maniatis T., Silverstein S., Axel R. Transformation of mammalian cells with genes from procaryotes and eucaryotes. Cell. 1979 Apr;16(4):777–785. doi: 10.1016/0092-8674(79)90093-x. [DOI] [PubMed] [Google Scholar]